Technological Challenges of Spirulina Powder as the Functional Ingredient in Gluten-Free Rice Crackers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plan of the Experiment
2.3. Analysis of Chemical Composition
2.4. Baking Method
2.5. Determination of Color
2.6. Rheological Determination of the Dough
2.7. Textural Determination
2.8. Sensory Analysis of Crackers
2.9. Total Polyphenolic Contents
2.10. HPLC Analysis of Phenolic Compounds
2.11. Antioxidant Capacity Tests
2.11.1. DPPH● Assay
2.11.2. Reducing Power (RP)
2.11.3. ABTS●+ Method
3. Results and Discussion
3.1. Chemical Composition of Raw Materials
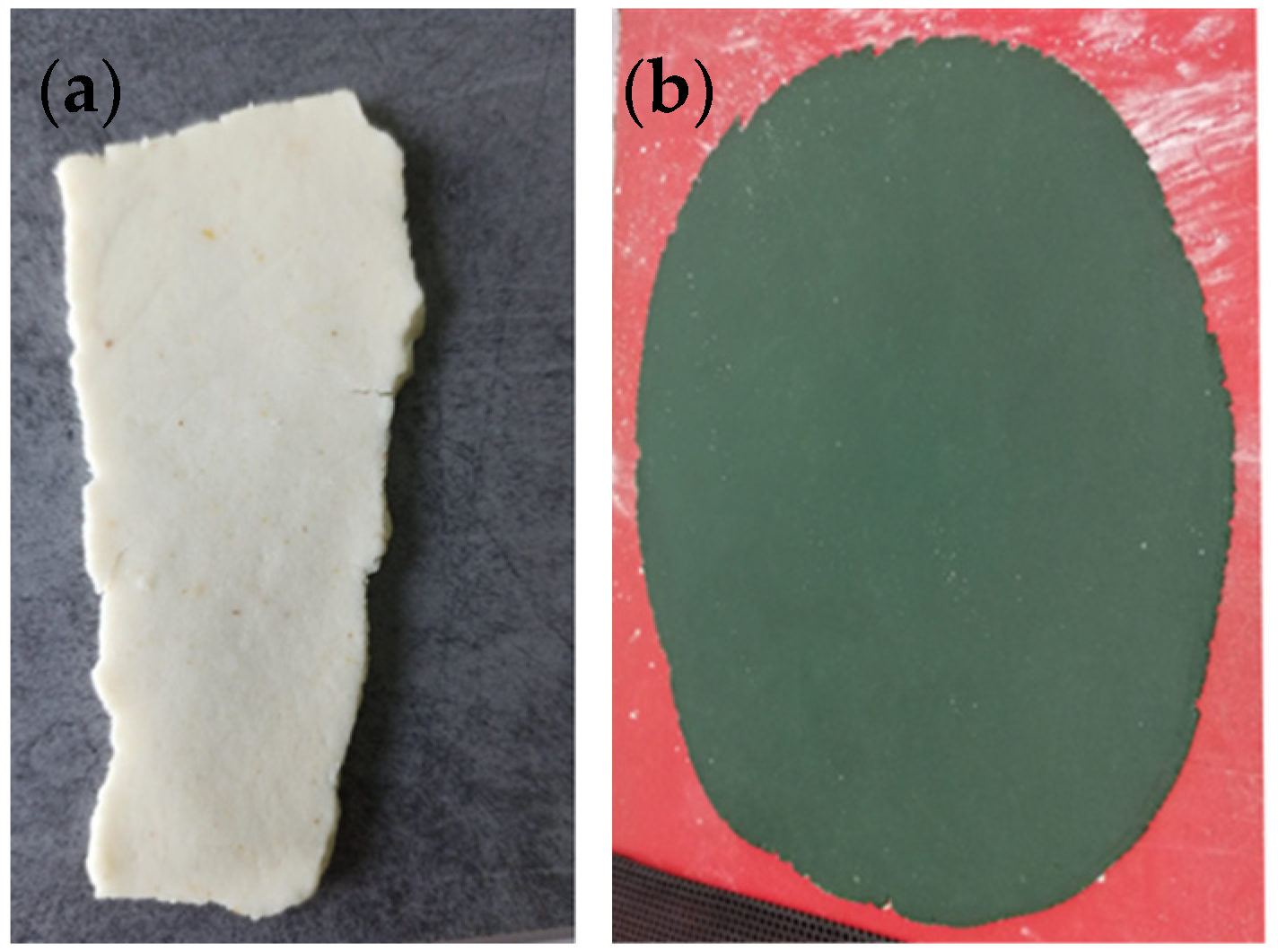
3.2. Color of Dough for Crackers
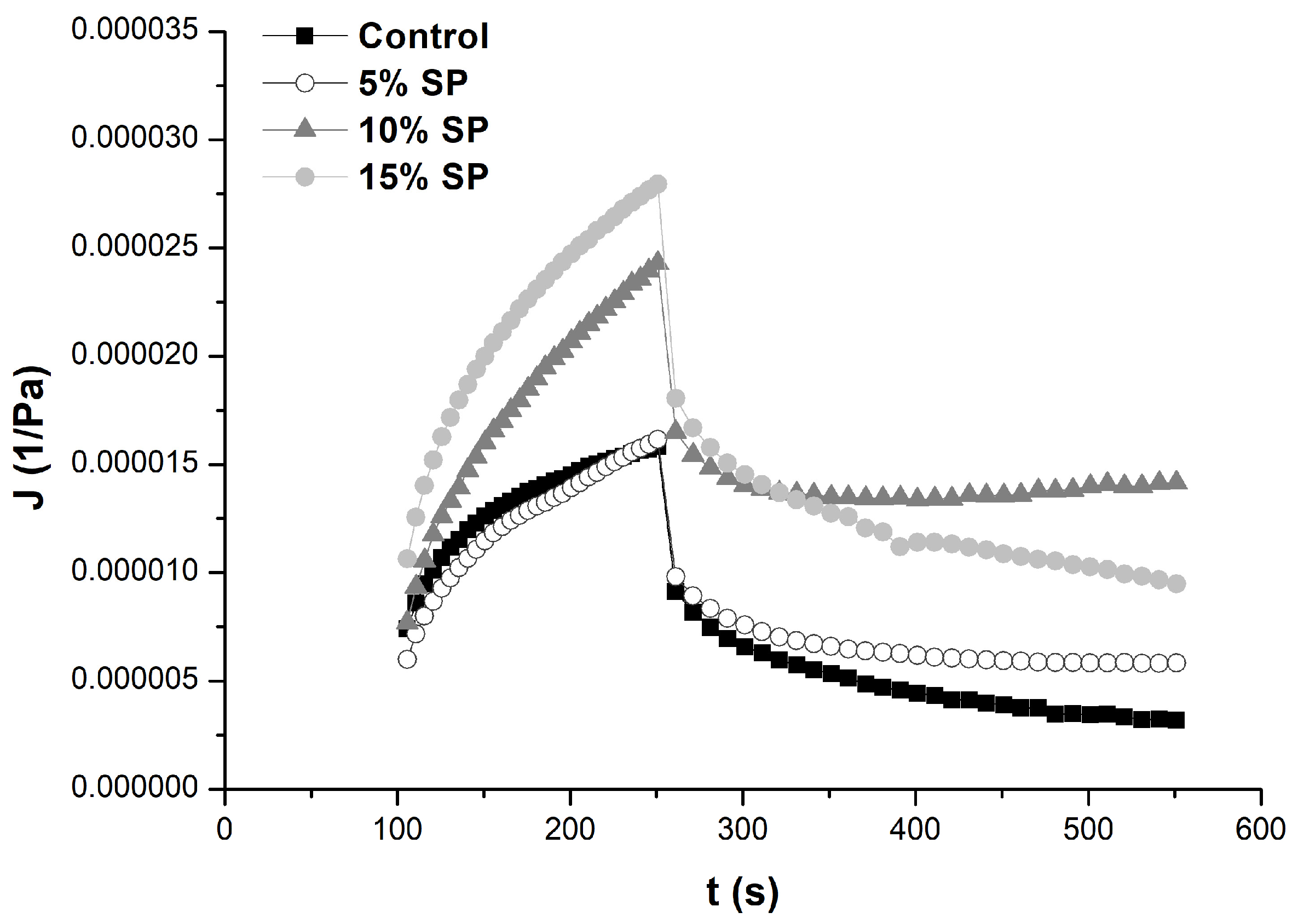
3.3. Rheological Properties of Dough
3.4. Textural Properties of Dough
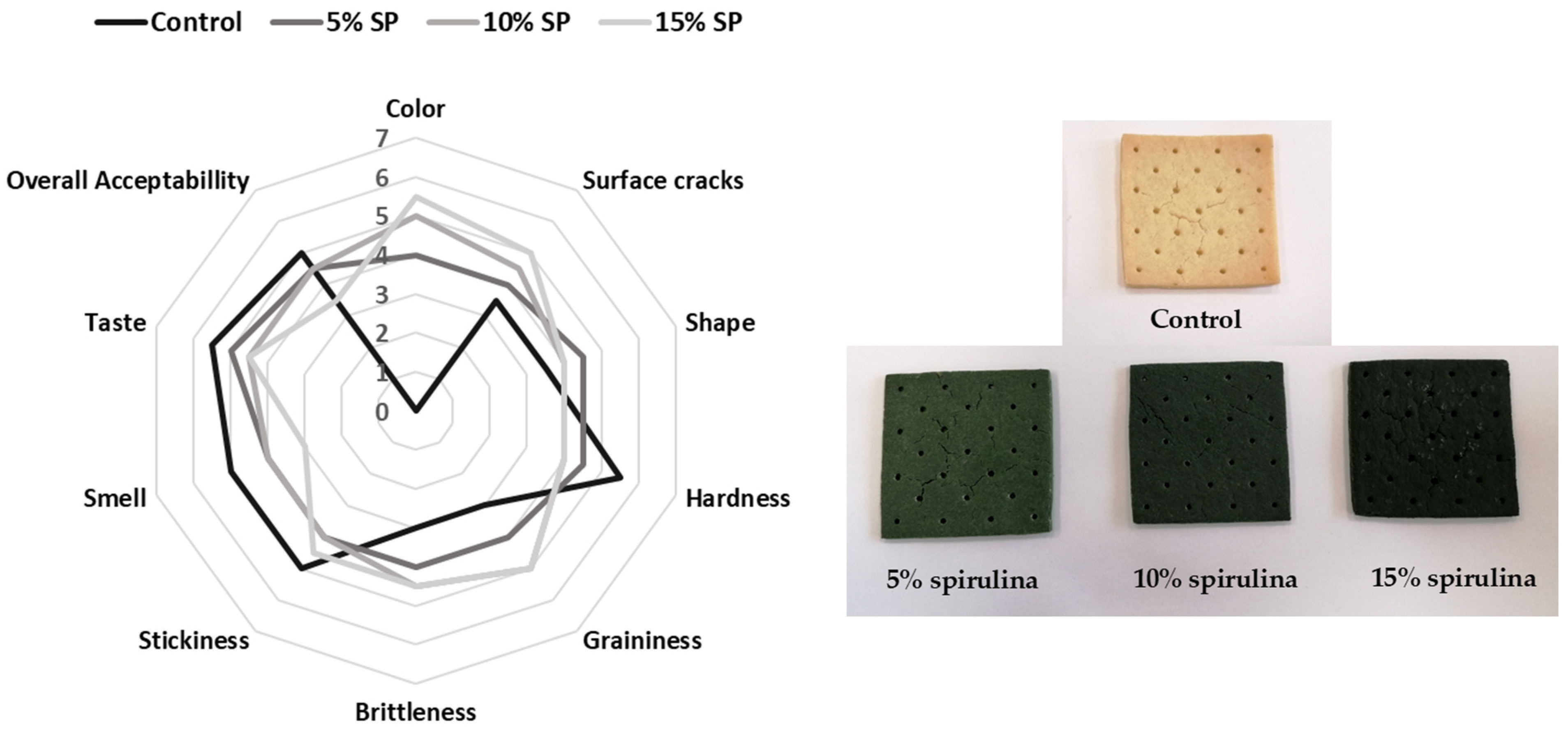
3.5. Sensory Evaluation of Crackers
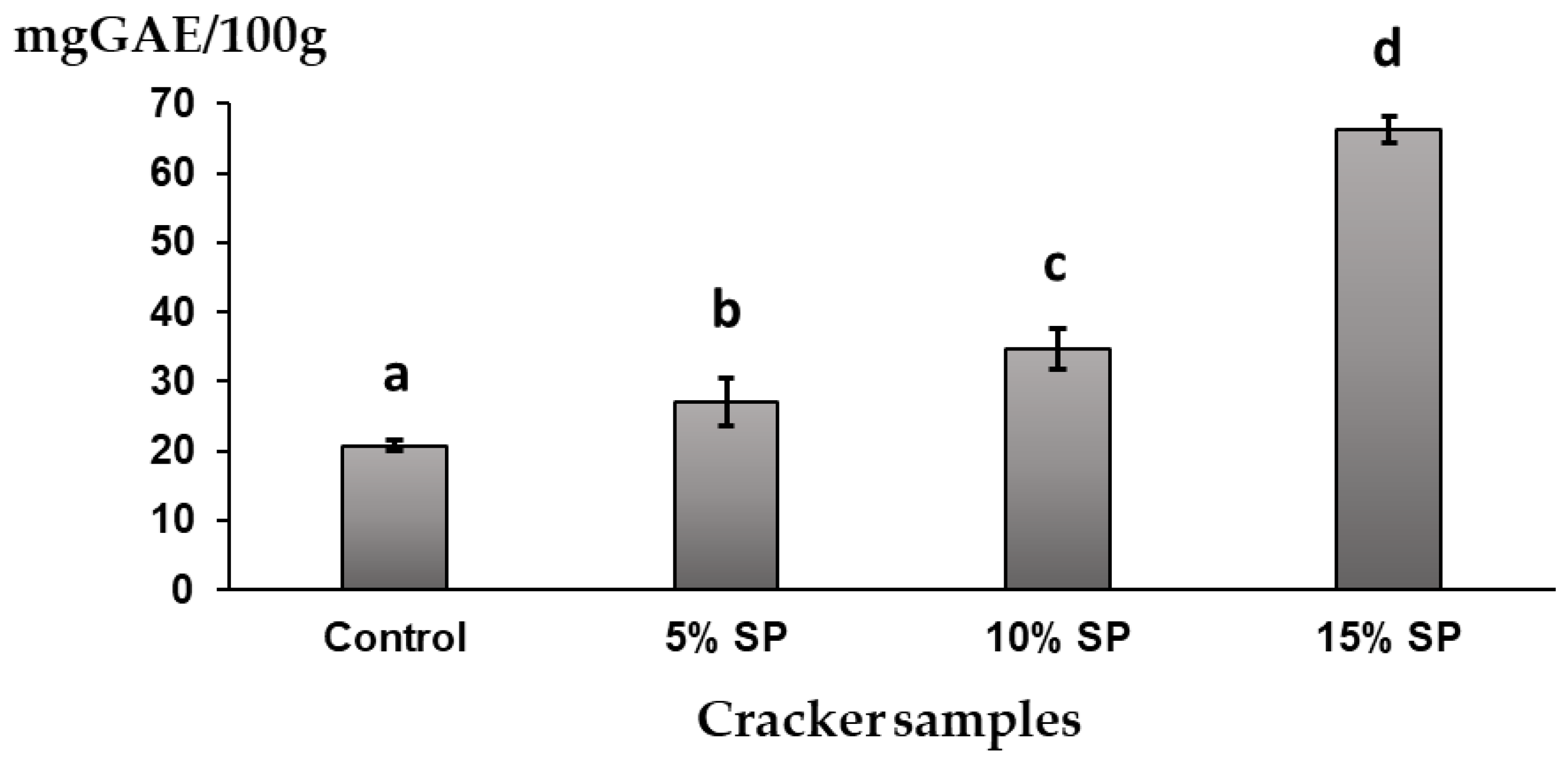
3.6. Polyphenolics and Antioxidant Capacity of the Crackers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El–Hadidy, G.S.; Shaban, H.H.; Mospah, W.M. Gluten-Free Crackers Preparation. J. Food Res. 2022, 11, 47–56. [Google Scholar] [CrossRef]
- Aaron, L.; Torsten, M.; Patricia, W. Autoimmunity in celiac disease: Extra–intestinal manifestations. Autoimmun. Rev. 2019, 18, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.A. Celiac Disease Review: Background and Emphasis on the Important Role of Health Education in the Prevention and Control of Symptom. Sch. J. Appl. Med. Sci. 2024, 4, 431–442. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Wang, W.; Li, Y. Advanced properties of gluten–free cookies, cakes, and crackers: A review. Trends Food Sci. Technol. 2020, 103, 200–213. [Google Scholar] [CrossRef]
- Turk Aslan, S.; Isik, F. Effects of pseudocereal flours addition on chemical and physical properties of gluten–free crackers. Food Sci. Technol. 2022, 42, e52521. [Google Scholar] [CrossRef]
- Gómez, M. Gluten–free bakery products: Ingredients and processes. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2022; Volume 99, pp. 189–238. [Google Scholar] [CrossRef]
- Khairuddin MA, N.; Lasekan, O. Gluten–free cereal products and beverages: A review of their health benefits in the last five years. Foods 2021, 10, 2523. [Google Scholar] [CrossRef]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Pestorić, M.; Šimurina, O.; Čanadanović–Brunet, J. Quality assessment of gluten-free crackers based on buckwheat flour. LWT–Food Sci. Technol. 2011, 44, 694–699. [Google Scholar] [CrossRef]
- Tomić, J.; Škrobot, D.; Popović, L.; Dapčević-Hadnađev, T.; Čakarević, J.; Maravić, N.; Hadnađev, M. Gluten–Free crackers based on chickpea and pumpkin seed press cake flour: Nutritional, functional and sensory properties. Food Technol. Biotechnol. 2022, 60, 488–498. [Google Scholar] [CrossRef]
- Ren, Y.; Yakubov, G.E.; Linter, B.R.; Foster, T.J. Development of a separated–dough method and flour/starch replacement in gluten free crackers by cellulose and fibrillated cellulose. Food Funct. 2021, 12, 8425–8439. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Soltanizadeh, N.; Mirmoghtadaee, P.; Banavand, P.; Mirmoghtadaie, L.; Shojaee-Aliabadi, S. Gluten–free products in celiac disease: Nutritional and technological challenges and solutions. J. Res. Med. Sci. 2018, 23, 109. [Google Scholar] [CrossRef]
- Schirrmeister, B.E.; Antonelli, A.; Bagheri, H.C. The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 2011, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina–From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Čabarkapa, I.; Rakita, S.; Popović, S.; Tomičić, Z.; Spasevski, N.; Vulić, J.; Đuragić, O. Characterization of organic Spirulina spp. and Chlorella vulgaris as one of the most nutrient–dense food. J. Food Saf. Food Qual. 2022, 73, 75–108. [Google Scholar] [CrossRef]
- Ali, A. Bioactive properties of spirulina: A review. Microb. Bioact. 2021, 4, 134–142. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Maciel, G.M.; Fernandes ID, A.A.; Pedro, A.C.; Rubio FT, V.; Branco, I.G.; Haminiuk, C.W.I. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Üseyin Bozkurt, H. Influence of incorporated Spirulina platensis on the growth of microflora and physicochemical properties of ayran as a functional food. Algal Res. 2019, 44, 101710. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández–Sevilla, J.M.; González–López, C.; Acién–Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- AlFadhly, N.K.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and technological advancements in the possible food applications of Spirulina and their health benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Ramírez–Rodrigues, M.M.; Estrada–Beristain, C.; Metri–Ojeda, J.; Pérez–Alva, A.; Baigts–Allende, D.K. Spirulina platensis protein as sustainable ingredient for nutritional food products development. Sustainability 2021, 13, 6849. [Google Scholar] [CrossRef]
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.H. Spirulina platensis, a super food? J. Cell Biotechnol. 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Marjanović, B.; Benković, M.; Jurina, T.; Sokač Cvetnić, T.; Valinger, D.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Bioactive Compounds from Spirulina spp.—Nutritional Value, Extraction, and Application in Food Industry. Separations 2024, 11, 257. [Google Scholar] [CrossRef]
- Spínola, M.P.; Mendes, A.R.; Prates, J.A. Chemical composition, bioactivities, and applications of Spirulina (Limnospira platensis) in food, feed, and medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The true value of Spirulina. J. Agricult. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.; Mendes, M.A. Chlorella and spirulina microalgae as sources of functional foods. Nutraceut Food Suppl. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Ghaeni, M.; Roomiani, L.; Moradi, Y. Evaluation of Carotenoids and Chlorophyll as Natural Resources for Food in Spirulina Microalgae. Appl. Food Biotechnol. 2015, 2, 39–44. [Google Scholar] [CrossRef]
- Gantar, M.; Simović, D.; Djilas, S.; Gonzalez, W.W.; Miksovska, J. Isolation, characterization and antioxidative activity of C–phycocyanin from Limnothrix sp. strain 37–2–1. J. Biotechnol. 2012, 159, 21–26. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Gouveia, L.; Raymundo, A.; Batista, A.P.; Sousa, I.; Empis, J. Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur. Food Res. Technol. 2006, 222, 362–367. [Google Scholar] [CrossRef]
- O’Shea, N.; Gallagher, E. Evaluation of novel–extruding ingredients to improve the physicochemical and expansion characteristics of a corn–puffed snack–containing pearled barley. Eur. Food Res. Technol. 2019, 245, 1293–1305. [Google Scholar] [CrossRef]
- Batista, A.P.; Nunes, M.C.; Raymundo, A.; Gouveia, L.; Sousa, I.; Cordobés, F.; Guerrero, A.; Franco, J.M. Microalgae biomass interaction in biopolymer gelled systems. Food Hydrocolloid 2011, 25, 817–825. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of new microalgae-based sourdough “crostini”: Functional effects of Arthrospira platensis (spirulina) addition. Sci. Rep. 2019, 9, 19433. [Google Scholar] [CrossRef] [PubMed]
- Tańska, M.; Konopka, I.; Ruszkowska, M. Sensory, physico–chemical and water sorption properties of corn extrudates enriched with spirulina. Plant Food Hum. Nutr. 2017, 72, 250–257. [Google Scholar] [CrossRef]
- Almeida, L.M.; da Silva Cruz, L.F.; Machado, B.A.; Nunes, I.L.; Costa, J.A.; de Souza Ferreira, E.; Lemos, P.V.; Druzian, J.I.; de Souza, C.O. Effect of the addition of Spirulina sp. biomass on the development and characterization of functional food. Algal Res. 2021, 58, 102387. [Google Scholar] [CrossRef]
- Sofia, R.N.; Pernilla, S.; Susanne, N.; Jietse, S.J.; Monica, L. Green color drives rejection of crackers added with algae in children but not in adults. Food Qual. Prefer. 2025, 127, 105461. [Google Scholar] [CrossRef]
- Van den Burg, S.W.; Dagevos, H.; Helmes RJ, K. Towards sustainable European seaweed value chains: A triple P perspective. ICES J. Mar. Sci. 2021, 78, 443–450. [Google Scholar] [CrossRef]
- Hosseinkhani, N.; McCauley, J.I.; Ralph, P.J. Key challenges for the commercial expansion of ingredients from algae into human food products. Algal Res. 2022, 64, 102696. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Fradinho, P.; Niccolai, A.; Soares, R.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Effect of Arthrospira platensis (spirulina) incorporation on the rheological and bioactive properties of gluten–free fresh pasta. Alg. Res. 2020, 45, 101743. [Google Scholar] [CrossRef]
- Villaró–Cos, S.; Sánchez JL, G.; Acién, G.; Lafarga, T. Research trends and current requirements and challenges in the industrial production of spirulina as a food source. Trend Food Sci. Technol. 2024, 143, 104280. [Google Scholar] [CrossRef]
- Nejatian, M.; Yazdi, A.P.G.; Saberian, H.; Bazsefidpar, N.; Karimi, A.; Soltani, A.; Assadpour, E.; Toker, O.S.; Jafari, S.M. Application of Spirulina as an innovative ingredient in pasta and bakery products. Food Biosci. 2024, 62, 105170. [Google Scholar] [CrossRef]
- Donato, N.R.; de Melo Queiroz, A.J.; Feitosa, R.M.; de Figueirêdo RM, F.; dos Santos Moreira, I.; de Lima, J.F. Production of cookies enriched with Spirulina platensis biomass. Agricult. Eng. 2019, 7, 323–342. [Google Scholar] [CrossRef]
- El Nakib, D.M.; Ibrahim, M.M.; Mahmoud, N.S.; Abd El Rahman, E.N.; Ghaly, A.E. Incorporation of Spirulina (Athrospira platensis) in traditional Egyptian cookies as a source of natural bioactive molecules and functional ingredients: Preparation and sensory evaluation of nutrition snack for school children. Eur. J. Nutr. Food Saf. 2019, 9, 372–397. [Google Scholar] [CrossRef]
- Ak, B.; Avsaroglu, E.; Isik, O.; Özyurt, G.; Kafkas, E.; Etyemez, M. Nutritional and physicochemical characteristics of bread enriched with microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016, 6, 30–38. [Google Scholar]
- Rabitti, N.S.; Bayudan, S.; Laureati, M.; Neugart, S.; Schouteten, J.J.; Apelman, L.; Dahlstedt, S.; Sandvik, P. Snacks from the sea: A cross-national comparison of consumer acceptance for crackers added with algae. Eur. Food Res. Technol. 2024, 250, 2193–2209. [Google Scholar] [CrossRef]
- Hussein, A.M.; Mostafa, S.; Ata, S.M.; Hegazy, N.A.; Abu-Reidah, I.M.; Zaky, A.A. Effect of Spirulina Microalgae Powder in Gluten-Free Biscuits and Snacks Formulated with Quinoa Flour. Processes 2025, 13, 625. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). International, Official Methods of Analysis of the Association of Official Analytical Chemists International, 17th ed.; Association of Official Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Da Silva Gorgônio, C.M.; Aranda DA, G.; Couri, S. Morphological and chemical aspects of Chlorella pyrenoidosa, Dunaliella tertiolecta, Isochrysis galbana and Tetraselmis gracilis microalgae. Nat. Sci. 2013, 5, 783–791. [Google Scholar] [CrossRef]
- Agrawal, N.; Hidame, P.; Gurla, S. Estimation of total carbohydrate in flour of different types of grain. Int. J. Res. Biosci. Technol. 2011, 3, 36–40. [Google Scholar]
- Petrović, J.; Rakić, D.; Fišteš, A.; Pajin, B.; Lončarević, I.; Tomović, V.; Zarić, D. Defatted wheat germ application: Influence on cookies’ properties with regard to its particle size and dough moisture content. Food Sci. Technol. Int. 2017, 23, 597–607. [Google Scholar] [CrossRef]
- Simić, S.; Petrović, J.; Rakić, D.; Pajin, B.; Lončarević, I.; Jozinović, A.; Fišteš, A.; Nikolić, S.; Blažić, M.; Miličević, B. The influence of extruded sugar beet pulp on cookies’ nutritional, physical and sensory characteristics. Sustainability 2021, 13, 5317. [Google Scholar] [CrossRef]
- CIE. International Commission on Illumination, Colorimetry: Official Recommendation of the International Commission on Illumination Publication CIE No. (E–1.31); Bureau Central de la CIE: Paris, France, 1976. [Google Scholar]
- Meriles, S.P.; Piloni, R.; Cáceres, G.V.; Penci, M.C.; Marín, M.A.; Ribotta, P.; Martínez, M.L. Compositional characteristics, texture, shelf–life and sensory quality of snack crackers produced from non–traditional ingredients. Int. J. Food Sci. Technol. 2022, 57, 4689–4696. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook: For Users of Rotational and Oscillation Rheometers; Vincentz Verlag: Hannover, Germany, 2002. [Google Scholar]
- Steffe, F.J. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: Dallas, TX, USA, 1996. [Google Scholar]
- Sozer, N. Rheological properties of rice pasta dough supplemented with proteins and gums. Food Hydrocolloid 2009, 23, 849–855. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C. Gluten–free doughs: Rheological properties, testing procedures–methods and potential problems. In Gluten–Free Food Science and Technology; Gallagher, E., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 52–83. [Google Scholar]
- Nikolić, I.; Pajin, B.; Lončarević, I.; Šubarić, D.; Jozinović, A.; Lončarić, A.; Petrović, J.; Šereš, Z.; Dokić, L.; Šoronja-Simović, D. Technological Characteristics of Wheat–Fiber–Based Fat Mimetics in Combination with Food Additives. Sustainability 2023, 15, 1887. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture, Viscosity and Food. In Food Texture and Viscosity: Concept and Measurement; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2002; Chapter 1. [Google Scholar]
- TA.HD. Texture Analyser Product Specification; Stable Micro Systems, Ltd.: Godalming Surrey, UK; Vienna, Austria, 2004. [Google Scholar]
- Choudhury, M.; Badwaik, L.S.; Borah, P.K.; Sit, N.; Deka, S.C. Influence of bamboo shoot powder fortification on physico-chemical, textural and organoleptic characteristics of biscuits. J. Food Sci. Technol. 2015, 52, 6742–6748. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kumar, D.; Badwaik, L.S. Rheological and textural properties of dough made out of de-oiled soya flour with application of different binding agents. J. Food Process. Eng. 2022, 45, e14027. [Google Scholar] [CrossRef]
- Dokić, L.; Nikolić, I.; Šoronja–Simović, D.; Šereš, Z.; Pajin, B.; Juul, N.; Maravić, N. Rheological properties of dough and sensory quality of crackers with dietary fibers. Int. J. Nutr. Food Eng. 2015, 9, 985–989. [Google Scholar]
- ISO 8589; Sensory Analysis–General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 8586–2; Sensory Analysis–General Guidance for the Selection, Training and Monitoring of Assessors–Part 2: Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 4121; Sensory Analysis–Guidelines for the Use of Quantitative Response Scales. International Organization for Standardization: Geneva, Switzerland, 2003.
- Tumbas Šaponjac, V.; Čanadanović–Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S. Encapsulation of beetroot pomace extract: RSM optimization, storage and gastrointestinal stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Ćetković, G.; Čanadanović-Brunet, J.; Pajin, B.; Djilas, S.; Petrović, J.; Lončarević, I.; Stajčić, S.; Vulić, J. Sour cherry pomace extract encapsulated in whey and soy proteins: Incorporation in cookies. Food Chem. 2016, 207, 27–33. [Google Scholar] [CrossRef]
- Torres, P.; Osaki, S.; Silveira, E.; dos Santos, D.Y.; Chow, F. Comprehensive evaluation of Folin–Ciocalteu assay for total phenolic quantification in algae (Chlorophyta, Phaeophyceae, and Rhodophyta). Algal Res. 2024, 80, 103503. [Google Scholar] [CrossRef]
- Gironés–Vilaplana, A.; Mena, P.; Moreno, D.A.; García–Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Sci. Food Agric. 2014, 94, 1090–1100. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.A.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Vinčić, M. Chemical composition and potential bioactivity of strawberry pomace. RSC Adv. 2015, 5, 5397–5405. [Google Scholar] [CrossRef]
- Mena, P.; García–Viguera, C.; Navarro–Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Kraithong, S.; Lee, S.; Rawdkuen, S. Physicochemical and functional properties of Thai organic rice flour. J. Cereal Sci. 2018, 79, 259–266. [Google Scholar] [CrossRef]
- Paz, G.M.; King, J.M.; Prinyawiwatkul, W.; Tyus, C.M.; Aleman, R.J. High–protein rice flour in the development of gluten–free muffins. J. Food Sci. 2020, 85, 1397–1402. [Google Scholar] [CrossRef]
- Ronie, M.E.; Abdul Aziz, A.H.; Mohd Noor NQ, I.; Yahya, F.; Mamat, H. Characterisation of Bario rice flour varieties: Nutritional compositions and physicochemical properties. Appl. Sci. 2022, 12, 9064. [Google Scholar] [CrossRef]
- Bao, J.; Bergman, C.J. Rice flour and starch functionality. In Starch in Food; Woodhead Publishing: Sawston, UK, 2024; pp. 275–307. [Google Scholar] [CrossRef]
- Rathna Priya, T.S.; Eliazer Nelson, A.R.L.; Ravichandran, K.; Antony, U. Nutritional and functional properties of coloured rice varieties of South India: A review. J. Ethnic Food 2019, 6, 11. [Google Scholar] [CrossRef]
- Hussein, A.S.; Mostafa, S.; Fouad, S.; Hegazy, N.A.; Zaky, A.A. Production and evaluation of gluten–free pasta and pan bread from spirulina algae powder and quinoa flour. Processes 2023, 11, 2899. [Google Scholar] [CrossRef]
- Onacik–Gür, S.; Żbikowska, A.; Majewska, B. Effect of Spirulina (Spirulina platensis) addition on textural and quality properties of cookies. Ital. J. Food Sci. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Marzec, A.; Kramarczuk, P.; Kowalska, H.; Kowalska, J. Effect of type of flour and microalgae (Chlorella vulgaris) on the rheological, microstructural, textural, and sensory properties of vegan muffins. Appl. Sci. 2023, 13, 7632. [Google Scholar] [CrossRef]
- Lopes–Da–Silva, J.A.; Santos, D.M.; Freitas, A.; Brites, C.; Gil, A.M. Rheological and nuclear magnetic resonance (NMR) study of the hydration and heating of undeveloped wheat doughs. J. Agric. Food Chem. 2007, 55, 5636–5644. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N. Relationship of polymeric proteins and empirical dough rheology with dynamic rheology of dough and gluten from different wheat varieties. Food Hydrocolloid 2013, 33, 342–348. [Google Scholar] [CrossRef]
- Uribe–Wandurraga, Z.N.; Zhang, L.; Noort, M.W.; Schutyser, M.A.; García-Segovia, P.; Martínez-Monzó, J. Printability and physicochemical properties of microalgae–enriched 3D–printed snacks. Food Bioprocess Technol. 2020, 13, 2029–2042. [Google Scholar] [CrossRef]
- Amoriello, T.; Mellara, F.; Amoriello, M.; Ceccarelli, D.; Ciccoritti, R. Powdered seaweeds as a valuable ingredient for functional breads. Eur. Food Res. Technol. 2021, 247, 2431–2443. [Google Scholar] [CrossRef]
- Shahbazizadeh, S.; Khosravi-Darani, K.; Sohrabvandi, S. Fortification of Iranian traditional cookies with Spirulina platensis. Annu. Res. Rev. Biol. 2015, 7, 144–154. [Google Scholar] [CrossRef]
- Sanchez, M.; Bernal–Castillo, J.; Rozo, C.; Rodríguez, I. Spirulina (Arthrospira): An edible microorganism: A review. Univ. Sci. 2003, 8, 7–24. [Google Scholar]
- Wang, M.; Yin, Z.; Sun, W.; Zhong, Q.; Zhang, Y.; Zeng, M. Microalgae play a structuring role in food: Effect of Spirulina platensis on the rheological, gelling characteristics, and mechanical properties of soy protein isolate hydrogel. Food Hydrocolloid 2023, 136, 108244. [Google Scholar] [CrossRef]
- Filipčev, B.; Šimurina, O.; Bodroža–Solarov, M.; Brkljača, J. Dough rheological properties in relation to cracker–making performance of organically grown spelt cultivars. Int. J. Food Sci. Technol. 2013, 48, 2356–2362. [Google Scholar] [CrossRef]
- Dapčević–Hadnađev, T.; Tomić, J.; Škrobot, D.; Šarić, B.; Hadnađev, M. Processing strategies to improve the breadmaking potential of whole–grain wheat and non–wheat flours. Discov. Food 2022, 2, 11. [Google Scholar] [CrossRef]
- Duodu, K.G.; Taylor, J.R.N. The Quality of Breads Made with Non–Wheat Flours. In Breadmaking; Duodu, K.G., Taylor, J.R.N., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 754–782. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae biomass as an additional ingredient of gluten–free bread: Dough rheology, texture quality and nutritional properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- Putra HB, P.; Anandini, D.R.; Amani, Z.N.; Arifin, M.H. The Addition Effect of Spirulina platensis on the Physco-chemical and Hedonic Properties of Gluten–Free Cookies Based on Mocaf and Sorghum Composite Flour. Int. J. Res. Public. Rev. 2024, 5, 1070–1076. [Google Scholar] [CrossRef]
- Gün, D.; Çelekli, A.; Bozkurt, H. Nutritional and Sensory Optimization of Functional Crackers with the Incorporation of Arthrospira platensis. Preprints 2024. [Google Scholar] [CrossRef]
- Gün, D.; Çelekli, A.; Bozkurt, H.; Kaya, S. Optimization of biscuit enrichment with the incorporation of Arthrospira platensis: Nutritional and sensory approach. J. Appl. Phycol. 2022, 34, 1555–1563. [Google Scholar] [CrossRef]
- Hernández–López, I.; Abadias, M.; Prieto–Santiago, V.; Chic–Blanco, Á.; Ortiz–Solà, J.; Aguiló–Aguayo, I. Exploring the Nutritional Potential of Microalgae in the Formulation of Bakery Products. Foods 2023, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Sanjari, S.; Sarhadi, H.; Shahdadi, F. Investigating the effect of Spirulina platensis microalgae on textural and sensory properties of baguette bread. J. Nutrit. Food Secur. 2018, 3, 218–225. [Google Scholar] [CrossRef]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. In Phytochemicals: Source of Antioxidants and Role in Disease Prevention; Asao, T., Asaduzzaman, M., Eds.; BoD–Books on Demand: Norderstedt, Germany, 2018; Volume 7, pp. 49–74. [Google Scholar]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Inter. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Letras, P.; Oliveira, S.; Varela, J.; Nunes, M.C.; Raymundo, A. 3D printed gluten–free cereal snack with incorporation of Spirulina (Arthrospira platensis) and/or Chlorella vulgaris. Algal Res. 2022, 68, 102863. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as functional ingredients in savory food products: Application to wheat crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef]
- Ali, H. Nutritional value, amino acids of biscuits and cakes fortified with spirulina (Arthrospira platensis) powder. J. Home Econom. 2022, 23, 141–158. [Google Scholar]
- Rathnayake, H.A.; Navaratne, S.B.; Navaratne, C.M. Formulation of a Biologically–leavened Composite Cracker with Functional Properties. J. Culinar Sci. Technol. 2024, 22, 1025–1043. [Google Scholar] [CrossRef]
- Youssef, H.M. Assessment of gross chemical composition, mineral composition, vitamin composition and amino acids composition of wheat biscuits and wheat germ fortified biscuits. Food Nutr. Sci. 2015, 6, 845–853. [Google Scholar] [CrossRef]
- Seleet, R. The Analysis of Nutrients in Foods; Academic Press, Inc.: London, UK, 2010. [Google Scholar]
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbi, S. Nutritional, functional, textural and sensory evaluation of Spirulina enriched green pasta: A potential dietary and health supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Ashoush, I.S.; Mahdy, S.M. Nutritional evaluation of cookies enriched with different blends of Spirulina platensis and Moringa oleifera leaves powder. J. Food Dairy. Sci. 2019, 10, 53–60. [Google Scholar] [CrossRef]


| Components | WGRF ± SD (%) | SP ± SD (%) |
|---|---|---|
| Moisture | 9.50 ± 0.17 | 7.42 ± 0.14 |
| Carbohydrates | 76.25 ± 0.30 | 12.68 ± 0.15 |
| Proteins | 6.44 ± 0.14 | 66.77 ± 1.05 |
| Fat | 2.91 ± 0.24 | 1.35 ± 0.60 |
| Ash | 1.05 ± 0.05 | 6.22 ± 0.01 |
| Total dietary fibers | 3.80 ± 0.09 | 5.13 ± 0.07 |
| Dough Samples | Color Parameters | ||
|---|---|---|---|
| L* ± SD | a* ± SD | b* ± SD | |
| Control sample | 84.58 ± 0.27 d | 2.31 ± 0.14 c | 14.08 ± 0.29 c |
| 5% SP | 30.57 ± 0.49 c | −4.76 ± 0.07 d | −2.67 ± 0.03 b |
| 10% SP | 28.01 ± 0.07 b | −1.47 ± 0.10 b | −1.35 ± 0.06 a |
| 15% SP | 26.93 ± 0.39 a | −0.77 ± 0.06 a | −1.10 ± 0.07 a |
| Dough Samples | Creep Phase | ||||||
|---|---|---|---|---|---|---|---|
| J0·10−6 ± SD (1/Pa) | J0·10−6 ± SD (1/Pa) | η0·107 ± SD (Pas) | λ ± SD (s) | Jmax·10−5 ± SD (1/Pa) | R2 | ||
| Control sample | 7.41 ± 0.01 | 5.22 ± 0.34 | 1.58 ± 0.19 | 109.00 ± 0.00 | 1.58 ± 0.19 | 0.9909 | |
| 5% SP | 8.01 ± 0.11 | 5.33 ± 0.57 | 1.55 ± 0.18 | 109.03 ± 0.06 | 1.62 ± 0.18 | 0.9979 | |
| 10% SP | 7.70 ± 0.62 | 8.02 ± 0.73 | 1.03 ± 0.16 | 109.07 ± 0.06 | 2.43 ± 0.22 | 0.9993 | |
| 10% SP | 10.63 ± 0.48 | 9.22 ± 0.62 | 0.90 ± 0.17 | 109.00 ± 0.00 | 2.79 ± 0.21 | 0.9993 | |
| Recovery Phase | |||||||
| J0·10−6 ± SD (1/Pa) | J0·10−6 ± SD (1/Pa) | η0·107 ± SD (Pas) | λ ± SD(s) | Je/Jmax (%) | Jv/Jmax (%) | R2 | |
| Control sample | 9.16 ± 0.16 | 1.05 ± 0.05 | 1.72 ± 0.26 | 239.57 ± 0.06 | 59.94 ± 0.90 | 40.06 ± 0.19 | 0.9596 |
| 5% SP | 9.80 ± 0.06 | 1.93 ± 0.08 | 9.45 ± 0.08 | 239.60 ± 0.00 | 46.09 ± 0.88 | 53.91 ± 0.10 | 0.8573 |
| 10% SP | 16.48 ± 0.05 | 4.67 ± 0.17 | 3.89 ± 0.18 | 239.50 ± 0.00 | 58.21 ± 0.73 | 41.79 ± 0.75 | 0.9629 |
| 10% SP | 18.05 ± 0.13 | 3.13 ± 0.09 | 5.81 ± 0.25 | 239.57 ± 0.06 | 43.95 ± 0.33 | 56.05 ± 0.09 | 0.9463 |
| Dough Sample | Hardness ± SD (g) | Resistance ± SD (g) | Extensibility ± SD (mm) |
|---|---|---|---|
| Control sample | 182.919 ± 2.827 c | 7.861 ± 0.454 a | 1.047 ± 0.203 a |
| 5% SP | 169.336 ± 5.143 b | 9.360 ± 0.905 b | 2.354 ± 0.362 b |
| 10% SP | 160.019 ± 3.466 ab | 10.619 ± 0.138 c | 3.214 ± 0.121 c |
| 15% SP | 158.293 ± 5.993 a | 12.163 ± 0.472 d | 3.760 ± 0.181 d |
| Variables | Tan δ | η0 | Jmax | Je/Jmax | Jv/Jmax | Hardness | Resistance | Extensibility |
|---|---|---|---|---|---|---|---|---|
| Tan δ | 1.0000 | −0.8445 | 0.7526 | −0.5793 | 0.5841 | −0.9213 | 0.9324 | 0.9866 |
| p = 0.001 | p = 0.005 | p = 0.048 | p = 0.046 | p = 0.000 | p = 0.000 | p = 0.000 | ||
| η0 | 1.0000 | −0.7306 | 0.2580 | −0.2605 | 0.7834 | −0.9017 | −0.8440 | |
| p = 0.007 | p = 0.418 | p = 0.414 | p = 0.003 | p = 0.000 | p = 0.001 | |||
| Jmax | 1.0000 | −0.2650 | 0.2794 | −0.6243 | 0.7813 | 0.7610 | ||
| p = 0.405 | p = 0.379 | p = 0.030 | p = 0.003 | p = 0.004 | ||||
| Je/Jmax | 1.0000 | −0.9953 | 0.6031 | −0.5719 | −0.5595 | |||
| p = 0.000 | p = 0.038 | p = 0.052 | p = 0.059 | |||||
| Jv/Jmax | 1.0000 | −0.6201 | 0.5766 | 0.5577 | ||||
| p = 0.031 | p = 0.050 | p = 0.060 | ||||||
| Hardness | 1.0000 | −0.8687 | −0.8623 | |||||
| p = 0.000 | p = 0.000 | |||||||
| Resistance | 1.0000 | 0.9187 | ||||||
| p = 0.000 | ||||||||
| Extensibility | 1.0000 |
| Cracker Samples | Color Parameters | |||
|---|---|---|---|---|
| L* ± SD | a* ± SD | b* ± SD | Firmness (g) | |
| Control sample | 75.41 ± 0.13 d | 1.61 ± 0.12 b | 25.92 ± 0.11 d | 232.20 ± 1.42 d |
| 5% SP | 30.77 ± 0.16 c | −6.05 ± 0.19 d | −7.39 ± 0.28 c | 199.09 ± 5.62 c |
| 10% SP | 27.64 ± 0.26 b | −3.18 ± 0.06 c | −3.91 ± 0.12 b | 166.62 ± 5.22 b |
| 15% SP | 24.90 ± 0.74 a | −1.05 ± 0.12 a | −2.31 ± 0.46 a | 114.67 ± 10.16 a |
| Variables | L* | a* | b* | Color |
|---|---|---|---|---|
| L* | 1.0000 | −0.3115 | 0.9945 | −0.9842 |
| p = 0.324 | p = 0.000 | p = 0.000 | ||
| a* | 1.0000 | −0.2213 | 0.1426 | |
| p = 0.489 | p = 0.658 | |||
| b* | 1.0000 | −0.9940 | ||
| p = 0.000 | ||||
| Color | 1.0000 |
| Cracker Samples | Control ± SD | 5% SP ± SD | 10% SP ± SD | 15% SP± SD |
|---|---|---|---|---|
| Gallic acid | 2.13 ± 0.10 a | 3.65 ± 0.21 b | 4.59 ± 0.25 c | 7.43 ± 0.34 d |
| Protocatechuic acid | 0.62 ± 0.02 a | 1.19 ± 0.98 b | 1.64 ± 0.92 b | 2.70 ± 1.02 c |
| Catechin | 9.61 ± 0.34 a | 14.40 ± 0.45 b | 18.27 ± 0.51 c | 39.79 ± 0.94 d |
| Epicatechin | 0.80 ± 0.11 a | 0.21 ± 0.00 b | 0.27 ± 0.00 b | 0.96 ± 0.02 a |
| Vanillic acid | 0 | 0 | 0 | 0 |
| Chlorogenic acid | 0 | 1.36 ± 0.05 a | 2.65 ± 0.09 b | 3.56 ± 0.14 c |
| Ferulic acid | 0.45 ± 0.02 b | 0.28 ± 0.01 a | 0 | 0 |
| Sinapic acid | 0.29 ± 0.01 a | 0.23 ± 0.01 a | 0.41 ± 0.02 b | 1.28 ± 0.04 c |
| Rutin | 0.11 ± 0.04 a | 0.07 ± 0.03 a | 0.38 ± 0.02 b | 1.29 ± 0.05 c |
| Quercetin | 0.01 ± 0.00 a | 0.48 ± 0.05 b | 0.69 ± 0.04 c | 1.92 ± 0.05 d |
| Apigenin | 0.08 ± 0.02 a | 0.28 ± 0.02 b | 0.26 ± 0.00 b | 1.88 ± 0.04 c |
| Total | 14.12 | 22.14 | 29.16 | 60.80 |
| Sample | DPPH● ± SD | RP ± SD | ABTS●+ ± SD |
|---|---|---|---|
| Control sample | 0.46 ± 0.04 a | 2.97 ± 0.81 a | 2.47 ± 1.43 a |
| 5% SP | 0.65 ± 0.05 b | 6.41 ± 0.92 b | 3.99 ± 0.15 b |
| 10% SP | 0.72 ± 0.02 b | 6.98 ± 0.42 b | 5.69 ± 0.68 c |
| 15% SP | 1.24 ± 0.07 c | 17.52 ± 0.51 c | 6.23 ± 0.64 d |
| Components | Control Cracker (%) | 5% SP (%) | 10% SP (%) | 15% SP (%) |
|---|---|---|---|---|
| Moisture | 4.95 ± 0.19 a | 4.80 ± 0.19 a | 4.62 ± 0.13 a | 4.56 ± 0.08 a |
| Carbohydrates | 66.45 ± 0.05 b | 61.11 ± 0.55 b | 55.50 ± 0.20 a | 48.58 ± 0.17 a |
| Proteins | 6.41 ± 0.01 a | 8.81 ± 0.95 b | 11.16 ± 0.22 c | 13.59 ± 0.40 d |
| Fat | 19.52 ± 0.43 a | 19.97 ± 0.60 a | 20.17 ± 1.20 a | 20.94 ± 0.25 a |
| Ash | 0.54 ± 0.05 a | 2.59 ± 0.11 b | 2.91 ± 0.10 b | 4.07 ± 0.09 c |
| Total dietary fibers | 2.05 ± 0.02 a | 2.61 ± 0.17 a | 5.58 ± 0.04 b | 8.01 ± 0.80 c |
| Energy value (kcal/100 g dw) | 467.12 | 459.41 | 448.17 | 437.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, I.; Lončarević, I.; Rakita, S.; Čabarkapa, I.; Vulić, J.; Takači, A.; Petrović, J. Technological Challenges of Spirulina Powder as the Functional Ingredient in Gluten-Free Rice Crackers. Processes 2025, 13, 908. https://doi.org/10.3390/pr13030908
Nikolić I, Lončarević I, Rakita S, Čabarkapa I, Vulić J, Takači A, Petrović J. Technological Challenges of Spirulina Powder as the Functional Ingredient in Gluten-Free Rice Crackers. Processes. 2025; 13(3):908. https://doi.org/10.3390/pr13030908
Chicago/Turabian StyleNikolić, Ivana, Ivana Lončarević, Slađana Rakita, Ivana Čabarkapa, Jelena Vulić, Aleksandar Takači, and Jovana Petrović. 2025. "Technological Challenges of Spirulina Powder as the Functional Ingredient in Gluten-Free Rice Crackers" Processes 13, no. 3: 908. https://doi.org/10.3390/pr13030908
APA StyleNikolić, I., Lončarević, I., Rakita, S., Čabarkapa, I., Vulić, J., Takači, A., & Petrović, J. (2025). Technological Challenges of Spirulina Powder as the Functional Ingredient in Gluten-Free Rice Crackers. Processes, 13(3), 908. https://doi.org/10.3390/pr13030908











