Origin and Hydrocarbon Generation of γ-Alkylbutyrolactones in Permian Shales
Abstract
:1. Introduction
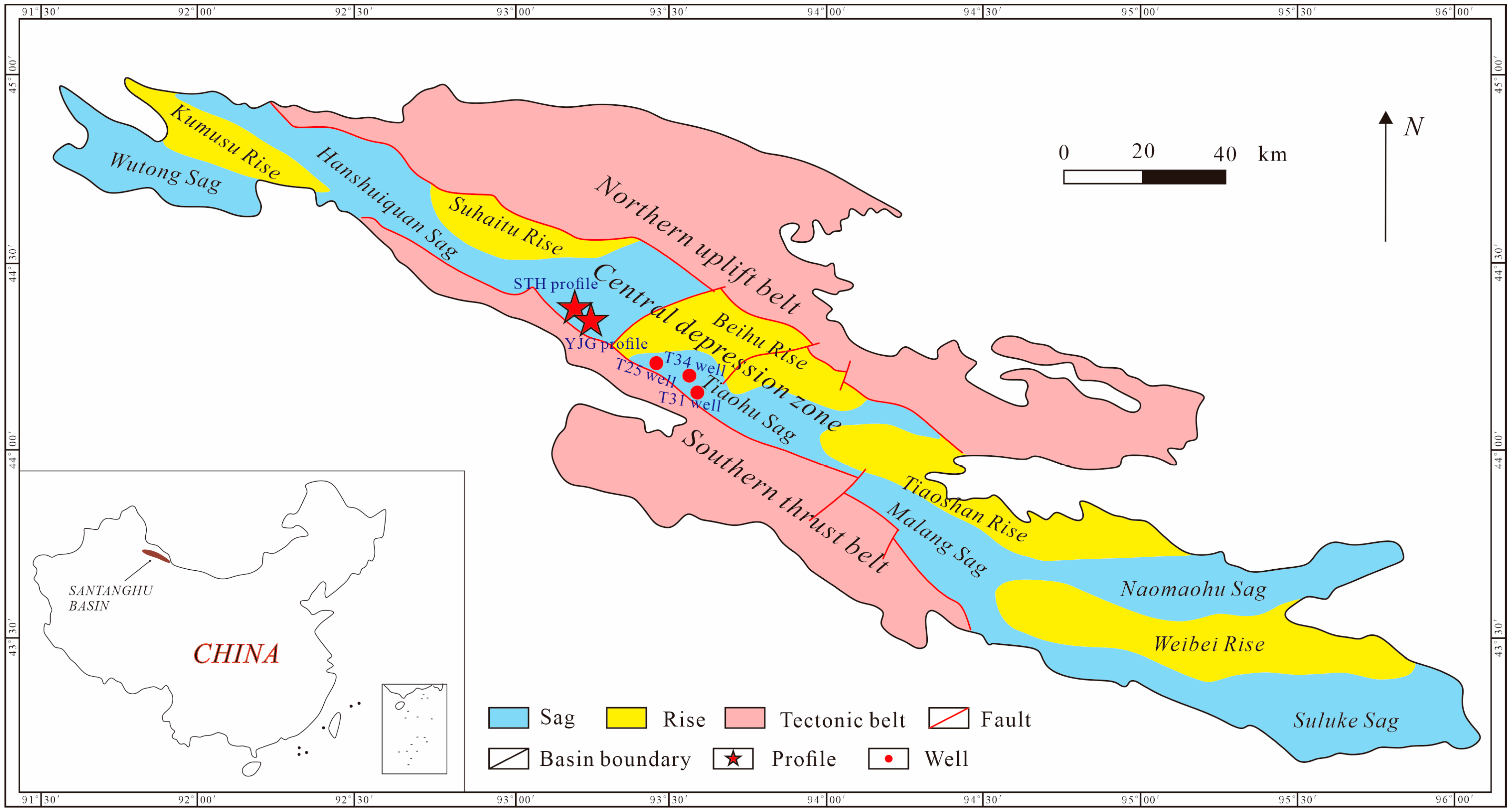
2. Geological Setting
3. Samples and Analytical Methods
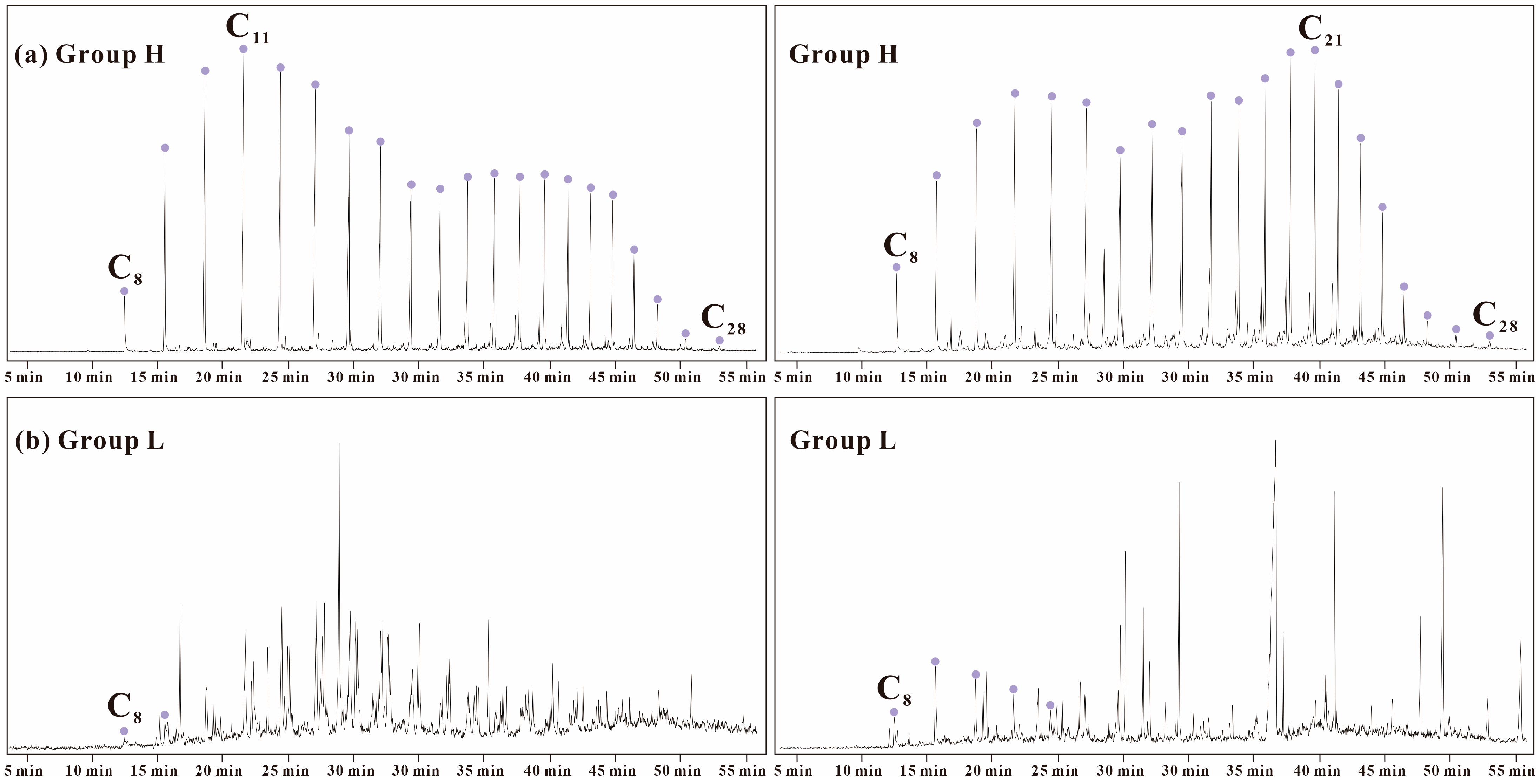
3.1. Samples
3.2. Organic Carbon Content, Rock-Eval Pyrolysis, and Vitrinite Reflectance
3.3. Molecular Geochemistry
3.4. Carbon Isotope Analysis of Individual Hydrocarbons
3.5. Thermal Simulation Experiment
3.6. Carbon Isotope Analysis of Carbon Dioxide (CO2)
3.7. Pyrolysis Gas Chromatography Mass Spectrometry (Py–GC–MS)
4. Results and Discussions
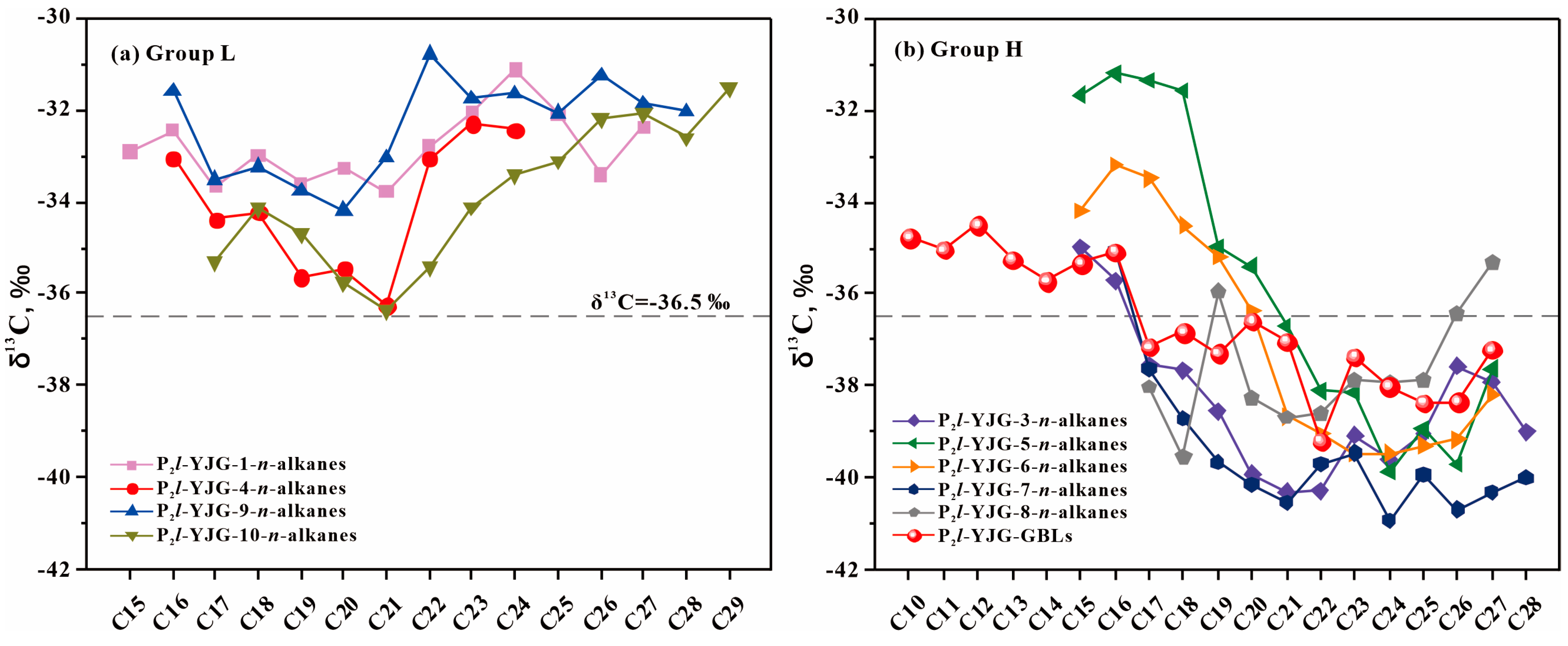
4.1. Source and Depositional Environment of GBLs
4.2. Contribution of GBLs to Source Rock Quality
4.3. Evaluation of Hydrocarbon Generation Capacity of GBLs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.S.; Huang, Z.L.; Li, T.J.; Guo, X.B.; Xu, X.F.; Chen, X. Environmental response to volcanic activity and its effect on organic matter enrichment in the Permian Lucaogou Formation of the Malang Sag, Santanghu Basin, Northwest China. Palaeogeogr. Palaeoclim. Palaeoecol. 2020, 560, 110024. [Google Scholar] [CrossRef]
- Xiao, D.S.; Xu, X.F.; Kang, J.L.; Zhang, Y.T. Characteristics and genesis of tuffaceous shale oil lithofacies: A case study of Lucaogou Formation in Santanghu Basin. Pet. Res. 2024, 9, 37–47. [Google Scholar]
- Liu, B.; Song, Y.; Zhu, K.; Su, P.; Ye, X.; Zhao, W.C. Mineralogy and element geochemistry of salinized lacustrine organic-rich shale in the middle Permian Santanghu Basin: Implications for paleoenvironment, provenance, tectonic setting and shale oil potential. Mar. Pet. Geol. 2020, 120, 104569. [Google Scholar]
- Wei, D.F. Potential resources and exploration direction of shale oil and gas in Xinjiang. Geophys. Prospect. Pet. 2024, 63, 1087–1099. [Google Scholar]
- Wang, Z.D.; Liang, M.L.; Qian, Y.; Wang, Z.Y.; Li, X.B.; Li, Z.P. The oxygen-bearing geolipids in the Lucaogou shale of Upper Permian, Santanghu Basin, China. Org. Geochem. 2016, 102, 59–66. [Google Scholar]
- Cheng, B.; Xu, J.B.; Liang, Y.G.; Deng, Q.; Tian, Y.K.; Liao, Z.W. Determination and geochemical implication of multiple series of long-chain oxygen-bearing compounds trapped in kerogen in the Lucaogou Formation, Santanghu Basin, NW China. Org. Geochem. 2018, 121, 68–79. [Google Scholar]
- Zhang, T.; Wang, Z.D.; Wang, X.F.; Liu, P.; Wang, Y.L.; Wu, Y.D. Characterization of oxygen-bearing geolipids in the Upper Permian Lucaogou shale, Santanghu Basin, NE China, using stepwise pyrolysis and hydrous pyrolysis. Mar. Pet. Geol. 2021, 126, 104926. [Google Scholar]
- Hertz, H.S.; Andresen, B.D.; Djujricic, M.V.; Biemann, K.; Saban, M.; Vitorovic, D. The isolation and identification of gamma-lactones in the acidic fraction in Aleksinac (Yugoslavia) shale bitumen. Geochim. Cosmochim. Acta 1973, 37, 1687–1695. [Google Scholar]
- Shimoyama, A.; Komiya, M.; Harada, K. Low molecular weight monocarboxylic acid and γ-lactones in Neogene sediments of the Shinjo Basin. Geochem. J. 1991, 25, 421–428. [Google Scholar]
- Lyu, J.; Nam, P.W.; Lee, S.J.; Lee, K.G. Volatile compounds isolated from rice beers brewed with three medicinal plants. J. Inst. Brew. 2013, 119, 271–279. [Google Scholar] [CrossRef]
- Schütt, J.; Schieberle, P. Quantitation of nine lactones in dairy cream by stable isotope dilution assays based on novel syntheses of carbon-13 labeled γ-lactones and deuterium labeled δ-lactones in combination with GC-GC-TOF-MS. J. Agric. Food Chem. 2017, 65, 10534–10541. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.G.; Zheng, F.P.; Liu, Y.P. Flavours and Fragrances; China Petrochemical Press: Beijing, China, 2000; pp. 100–104. [Google Scholar]
- Sachse, V.F.; Heim, S.; Jabour, H.; Kluth, O.; Schumann, T.; Aquit, M.; Littke, R. Organic geochemical characterization of Santonian to Early Campanian organic matter-rich marls (Sondage No. 1 cores) as related to OAE3 from the Tarfaya Basin, Morocco. Mar. Pet. Geol. 2014, 56, 290–304. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Li, C.; Song, Z.Y.; Zhang, X.L. Organic Geochemical Characteristics and Hydrocarbon Significance of the Permian System Around the Bogda Mountain, Junggar Basin, Northwest China. Sustainability 2025, 17, 347. [Google Scholar] [CrossRef]
- Li, Z.; Ma, X.Q.; Li, A.F.; Zhang, C.W. A novel potential source of β-carotene: Eustigmatos cf. polyphem (Eustigmatophyceae) and pilot β-carotene production in bubble column and flat panel photobioreactors. Bioresour. Technol. 2012, 117, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ari, M.A.I.; Nton, M.E.; Harouna, M. Biomarker geochemistry and oil-oil correlation from reservoir unit oils of the Sokor-1 Formation, Fana low uplift, Termit Basin, southeastern Niger. J. Afr. Earth Sci. 2025, 223, 105522. [Google Scholar] [CrossRef]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.S.; Kim, Y.S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food. Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Li, E.T.; Jin, J.; Chen, J.; Wang, M.; Mi, J.L.; Gao, X.W. Study on biomarkers and carbon isotopic compositions of monomer hydrocarbons in asphaltene pyrolysis products from biodegraded heavy oil. Geochimica 2019, 48, 284–292. [Google Scholar]
- Zhang, Y.D.; Sun, Y.G.; Liu, Q. Distribution and carbon isotope composition of pregnane in carbonate-evaporitic rocks from the Bonan Sag, Bohai Bay Basin, Eastern China: Insights into sources and associated lake environments. Org. Geochem. 2021, 151, 104127. [Google Scholar] [CrossRef]
- Kosakowski, P.; Kotarba, M.J.; Piestrzyński, A.; Shogenova, A. Petroleum source rock evaluation of the Alum and Dictyonema Shales (Upper Cambrian-Lower Ordovician) in the Baltic Basin and Podlasie Depression (eastern Poland). Int. J. Earth Sci. 2016, 106, 1–19. [Google Scholar] [CrossRef]
- Ahoure, N.D.; Egoran, B.A.; Kouadio, G.R.N.; Sehi, Z.S.; Oura, E.L.; Digbehi, Z.B. Geochemical Analysis of Albian-Maastrichtian Formations in the Offshore Basin of the Abidjan Margin: Rock-Eval Pyrolysis Study. Open J. Geol. 2024, 14, 805–822. [Google Scholar] [CrossRef]
- Grundman, G.; Behar, F.; Malo, M.; Baudin, F.; Lorant, F. Evaluation of hydrocarbon potential of the Paleozoic (Cambrian–Devonian) source rocks of the Gaspé Peninsula, Québec, Canada: Geochemical characterization, expulsion efficiency, and erosion scenario. Am. Assoc. Pet. Geol. Bull. 2012, 96, 729–751. [Google Scholar] [CrossRef]
- Ercegovac, M.; Kostic, A. Organic facies and palynofacies: Nomenclature, classification and applicability for petroleum source rock evaluation. Int. J. Coal. Geol. 2006, 68, 70–78. [Google Scholar]
- Jiang, H.; Pang, X.; Shi, H.; Yu, Q.; Cao, Z.; Yu, R.; Chen, D.; Long, Z.; Jiang, F. Source rock characteristics and hydrocarbon expulsion potential of the Middle Eocene Wenchang formation in the Huizhou depression, Pearl River Mouth basin, south China sea. Mar. Pet. Geol. 2015, 67, 635–652. [Google Scholar]
- Hakimi, M.H.; Abdullah, W.H. Thermal maturity history and petroleum generation modelling for the Upper Jurassic Madbi source rocks in the Marib-Shabowah Basin, western Yemen. Mar. Pet. Geol. 2015, 59, 202–216. [Google Scholar] [CrossRef]
- Guo, X.B.; Zhou, L.F.; Shi, B.H.; Li, Y.; Lin, S.Y.; Li, Y.X.; Sun, J.B.; Liu, G.; Yin, J.T.; Zhang, C.L. Pyrolytic hydrocarbon generation characteristics of the Chang 7 shale based on different experimental methods: Implications for shale oil and gas in the Ordos Basin. Geol. J. 2024, 59, 2175–2189. [Google Scholar] [CrossRef]
- Li, C.M.; Zeng, J.H.; Liu, S.N.; Dong, Y.Y.; Wang, M.Y. How did submarine volcanic eruptions interact with organic matter to favour hydrocarbon generation in the Bohai Bay Basin, China? Int. Geol. Rev. 2024, 66, 2345–2364. [Google Scholar]
- Liu, P.; Wang, X.F.; Liu, C.J.; Lin, Y.; Guo, R.L.; Liu, W.H. Intramolecular carbon isotopic rollover in propane from natural gas reservoirs of the Santanghu Basin: Insights into chemical structure of kerogen. Org. Geochem. 2024, 188, 104740. [Google Scholar]
- Sun, Z.M.; Xiong, B.X.; Li, Y.L.; He, H.Q. An assessment of hydrocarbon potential of Mesozoic–Cenozoic basins in the north part of Kashen depression of Tarim basin. Pet. Geol. Exp. 2001, 23, 23–26. [Google Scholar]
- Jiao, X.; Liu, Y.Q.; Yang, W.; Li, H.; Meng, Z.Y.; Zhao, M.R.; Li, Z.X. Microcrystalline dolomite in a middle Permian volcanic lake: Insights on primary dolomite formation in a non-evaporitic environment. Sedimentology 2023, 70, 48–77. [Google Scholar]
- Liu, X.W.; Zhen, J.J.; Yang, X.; Liu, Y.H. Paleozoic structural evolution Santanghu basin and its surrounding and prototype basin recovery. Nat. Gas Geosci. 2010, 21, 947–954. [Google Scholar]
- GB/T 19145-2022; Determination for Total Organic Carbon in Sedimentary Rock. China Standards Press: Beijing, China, 2022.
- SY/T 5214-2012; Determination Method of Vitrinite Reflectance in Sedimentary Rocks. Petroleum Industry Press: Beijing, China, 2012.
- Si, W.X.; Zhang, S.B. The Soxhlet extraction method is often used to extract soluble organic matter from source rocks. Its principle is to extract the organic matter from the samples through solvent reflux. Inn. Mong. Petrochem. Ind. 2010, 36, 112–113. [Google Scholar]
- Liao, J.; Wang, J.; Lu, H.; Sheng, G.Y.; Peng, P.A.; Hsu, C.S. Solvent effect in Soxhlet extraction of source rocks. Org. Geochem. 2025, 200, 104917. [Google Scholar]
- GB/T 18606-2017; The Test Method for Biomarkers in Sediment and Crude Oil by GC-MS. China Standards Press: Beijing, China, 2017.
- GB/T 18340.2-2010; Organic Geochemical Analysis Method for Geological Samples—Part 2: Determination of Organic Carbon Stable Isotopic Component—Isotopic Mass Spectrometry. China Standards Press: Beijing, China, 2010.
- Guenther, F.; Aichner, B.; Siegwolf, R.; Xu, B.Q.; Yao, T.D.; Gleixner, G. A synthesis of hydrogen isotope variability and its hydrological significance at the Qinghai–Tibetan Plateau. Quat. Int. 2013, 313, 3–6. [Google Scholar]
- Rodrigues, B.; Duarte, L.V.; Filho, J.G.M.; Santos, L.G.; de Oliveira, A.D. Evidence of terrestrial organic matter deposition across the early Toarcian recorded in the northern Lusitanian Basin, Portugal. Int. J. Coal Geol. 2016, 168, 35–45. [Google Scholar]
- Wang, Z.; Zhong, W.; Wang, X.J.; Du, Y.Y.; Li, T.H.; Xue, J.B.; Quan, M.Y. Organic Matter Source Traced by n-Alkane Records Derived from Sediments of Barkol Lake in Eastern Xinjiang (NW China) and Its Response to Moisture Variability in the Past 8800 Years. Geochem. Int. 2024, 62, 419–433. [Google Scholar]
- Qiao, J.Q.; Baniasad, A.; Zieger, L.; Zhang, C.; Luo, Q.; Littke, R. Paleo-depositional environment, origin and characteristics of organic matter of the Triassic Chang 7 Member of the Yanchang Formation throughout the mid-western part of the Ordos Basin, China. Int. J. Coal Geol. 2021, 237, 103636. [Google Scholar]
- Sikes, E.L.; Uhle, M.E.; Nodder, S.D.; Howard, M.E. Sources of organic matter in a coastal marine environment: Evidence from n-alkanes and their δ13C distributions in the Hauraki Gulf, New Zealand. Mar. Chem. 2009, 113, 149–163. [Google Scholar]
- Hu, X.; Zhu, L.; Wang, Y.; Wang, J.; Peng, P.; Ma, Q.; Hu, J.; Lin, X. Climatic significance of n-alkanes and their compound-specific δD values from lake surface sediments on the Southwestern Tibetan Plateau. Chin. Sci. Bull. 2014, 59, 3022–3033. [Google Scholar]
- Ficken, K.J.; Li, B.; Swain, D.L.; Eglinton, G. An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Org. Geochem. 2000, 31, 745–749. [Google Scholar]
- Barham, A.; Ismail, M.; Hermana, M.; Abidin, N.S. Biomarker characteristics of Montney source rock, British Columbia, Canada. Heliyon 2021, 7, 83–95. [Google Scholar]
- Sohail, J.; Mehmood, S.; Jahandad, S.; Ehsan, M.; Abdelrahman, K.; Ali, A.; Qadri, S.M.T.; Fnais, M. Geochemical Evaluation of Paleocene Source Rocks in the Kohat Sub-Basin, Pakistan. ACS Omega 2024, 9, 14123–14141. [Google Scholar] [CrossRef]
- Gao, G.; Yang, S.R.; Zhang, W.W.; Wang, Y.; Gang, W.Z.; Lou, G.Q. Organic geochemistry of the lacustrine shales from the Cretaceous Taizhou Formation in the Gaoyou Sag, Northern Jiangsu Basin. Mar. Pet. Geol. 2018, 89, 594–603. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.Q.; Hao, F.; Zhang, Z.; Yang, X.Z.; Chen, Y.Q.; Zhang, K.; Wang, X.X.; Cong, F.Y. The generation mechanism of deep natural gas in Tabei uplift, Tarim Basin, Northwest China: Insights from instantaneous and accumulative effects. Pet. Sci. 2024, 21, 3804–3814. [Google Scholar] [CrossRef]
- Wenger, L.M.; Davis, C.L.; Isaksen, G.H. Multiple Controls on Petroleum Biodegradation and Impact on Oil Quality. SPE Annu. Tech. Conf. Exhib. 2002, 5, 375–383. [Google Scholar] [CrossRef]
- Head, I.M.; Jones, D.M.; Larter, S.R. Biological activity in the deep subsurface and the origin of heavy oil. Nature 2003, 426, 344–352. [Google Scholar] [CrossRef]
- Onojake, M.C.; Nkanta, N.E.; Osakwe, J.O.; Akpuluma, D.A.; Ohenhen, I.; Osuji, L.C. Organic geochemical evaluation of crude oils from some producing fields in the Niger Delta basin, Nigeria. J. Pet. Explor. Prod. Technol. 2024, 14, 1799–1811. [Google Scholar] [CrossRef]
- Lai, H.F.; Lu, Q.P.; Yang, Z. Origin and microbial degradation of thermogenic hydrocarbons within the sandy gas hydrate reservoirs in the Qiongdongnan Basin, northern South China Sea. Mar. Pet. Geol. 2024, 165, 106871. [Google Scholar] [CrossRef]
- Liu, S.J.; Gao, G.; Shi, X.Y.; Gang, W.Z.; Xiang, B.L.; Wang, M.; Zhao, W.Z. Geochemical constraints on the hydrocarbon generation and expulsion in source rocks with different primary organic matter compositions: A case study on the Lucaogou Formation in the Jimusaer Sag, Junggar Basin, Northwest China. Org. Geochem. 2025, 202, 104952. [Google Scholar] [CrossRef]
- Sachsenhofer, R.F.; Hentschke, J.; Bechtel, A.; Coric, S.; Gross, D.; Horsfield, B.; Rachetti, A.; Soliman, A. Hydrocarbon potential and depositional environments. of Oligo-Miocene rocks in the Eastern Carpathians (Vrancea Nappe, Romania). Mar. Pet. Geol. 2015, 68, 269–290. [Google Scholar] [CrossRef]
- Mani, D.; Patil, D.J.; Dayal, A.M.; Prasad, B.N. Thermal maturity, source rock potential and kinetics of hydrocarbon generation in Permian shales from the Damodar valley basin, eastern India. Mar. Pet. Geol. 2015, 66, 1056–1072. [Google Scholar] [CrossRef]
- Elyasi, S. Petroleum source-rock potential of the Piranj oil field, Zagros basin. Mar. Pet. Geol. 2016, 78, 448–454. [Google Scholar]
- Mahbobipour, H.; Kamali, M.R.; Solgi, A. Organic geochemistry and petroleum potential of Early Cretaceous Garau Formation in central part of Lurestan zone, northwest of Zagros, Iran. Mar. Pet. Geol. 2016, 77, 991–1009. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.G. n-Alkane distributions and concentrations in algae, submerged plants and terrestrial plants from the Qinghai-Tibetan Plateau. Org. Geochem. 2016, 99, 10–22. [Google Scholar]
- Liu, H.; Liu, Z.H.; Zhao, C.; Liu, W.G. n-alkyl lipid concentrations and distributions in aquatic plants and their individual δD variations. Sci. China Earth Sci. 2019, 62, 1441–1452. [Google Scholar]
- Bobrovskiy, I.; Poulton, S.W.; Hope, J.M.; Brocks, J.J. Impact of aerobic reworking of biomass on steroid and hopanoid biomarker parameters recording ecological conditions and thermal maturity. Geochim. Cosmochim. Acta 2024, 364, 114–128. [Google Scholar]
- Bailey, W.J.; Bird, C.N. Pyrolysis of esters. 27. Pyrolysis of lactones. J. Org. Chem. 1977, 42, 3895–3899. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Pyrolysis of various derivatives of carboxylic acids. Tech. Instrum. Anal. Chem. 2010, 28, 579–627. [Google Scholar]
- Bond, J.Q.; Alonso, D.M.; West, R.M.; Dumesci, J.A. Gamma-valerolactone ring-opening and decarboxylation over SiO2/Al2O3 in the presence of water. Langmuir 2010, 26, 16291–16298. [Google Scholar] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesci, J.A. Integrated catalytic conversion of gamma-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar]
- Bond, J.Q.; Jungong, C.S.; Chatzidimitriou, A. Microkinetic analysis of ring-opening and decarboxylation of cvalerolactone over silica alumina. J. Catal. 2016, 344, 640–656. [Google Scholar]
- Huang, X.Y.; Pancost, R.D.; Xue, J.T.; Gu, Y.S.; Evershed, R.P.; Xie, S.H. Response of carbon cycle to drier conditions in the mid-Holocene in central China. Nat. Commun. 2018, 9, 1369. [Google Scholar] [PubMed]

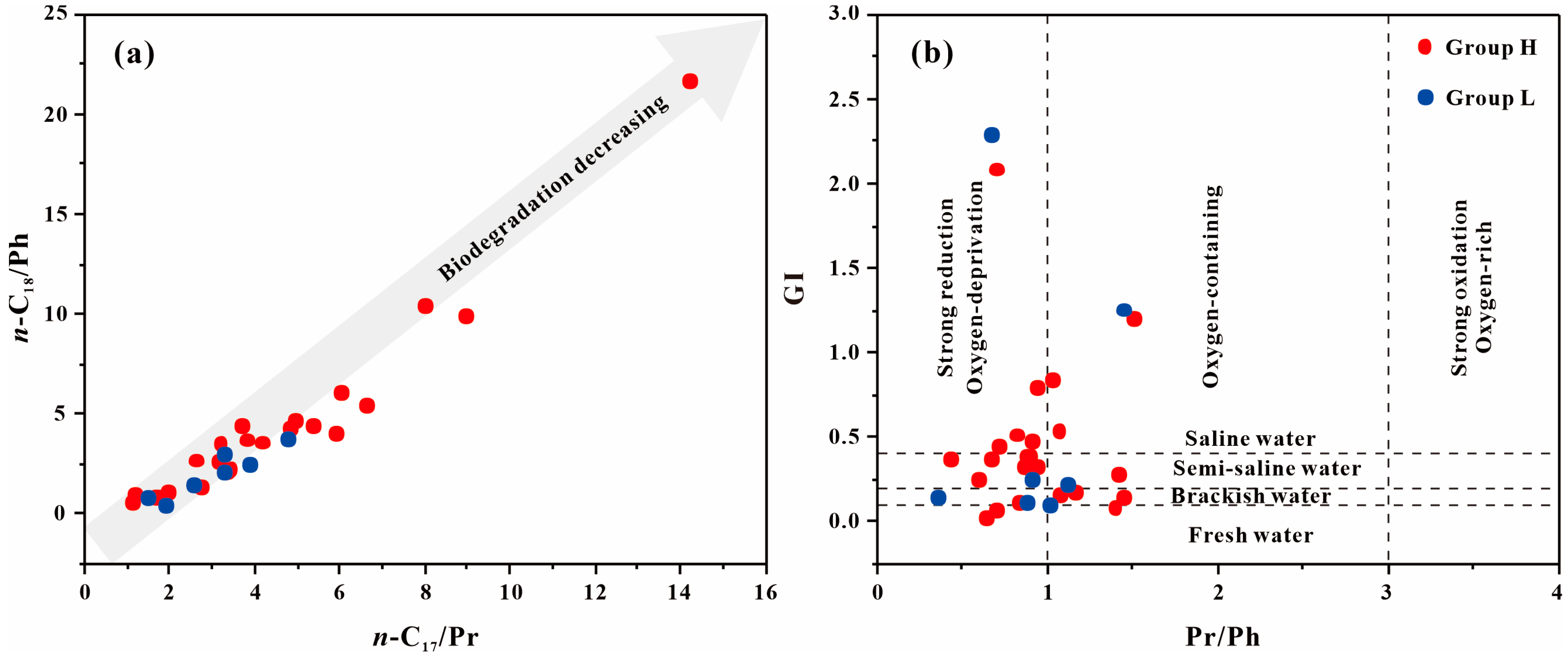
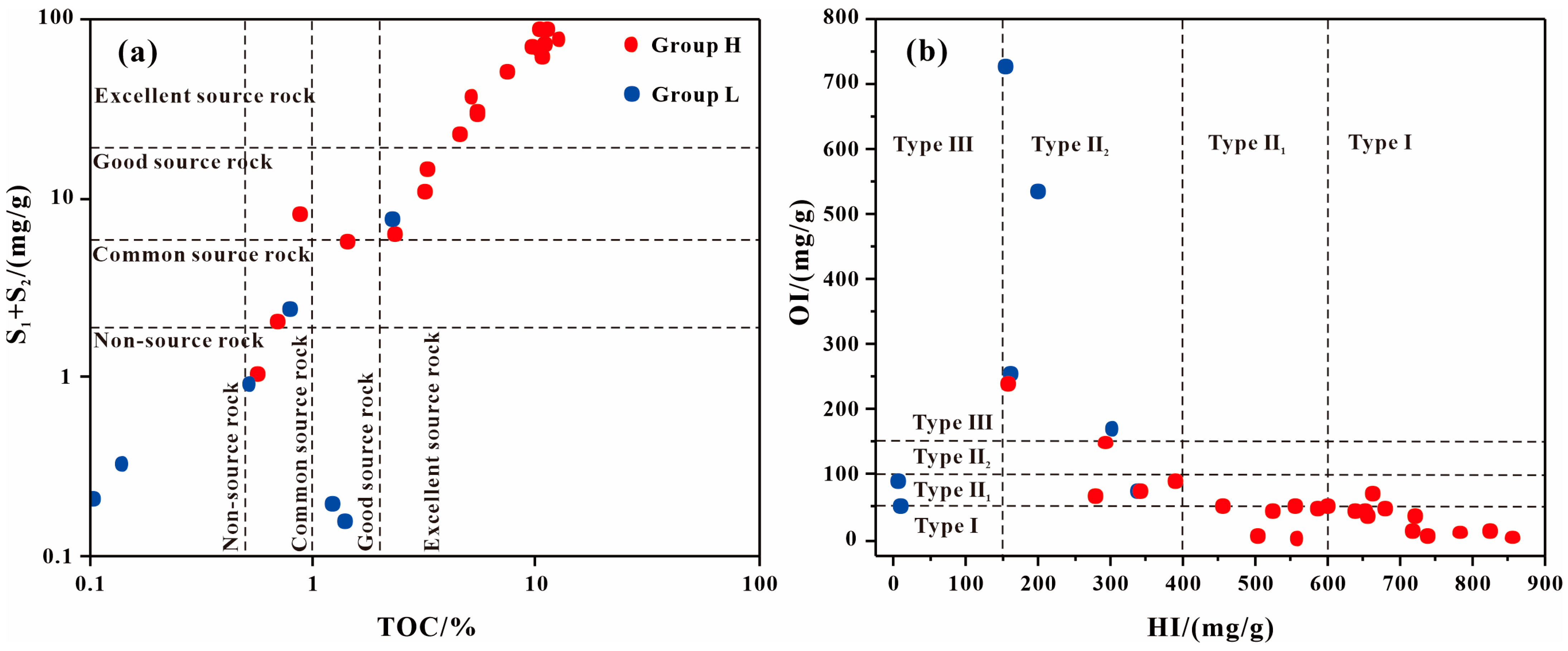

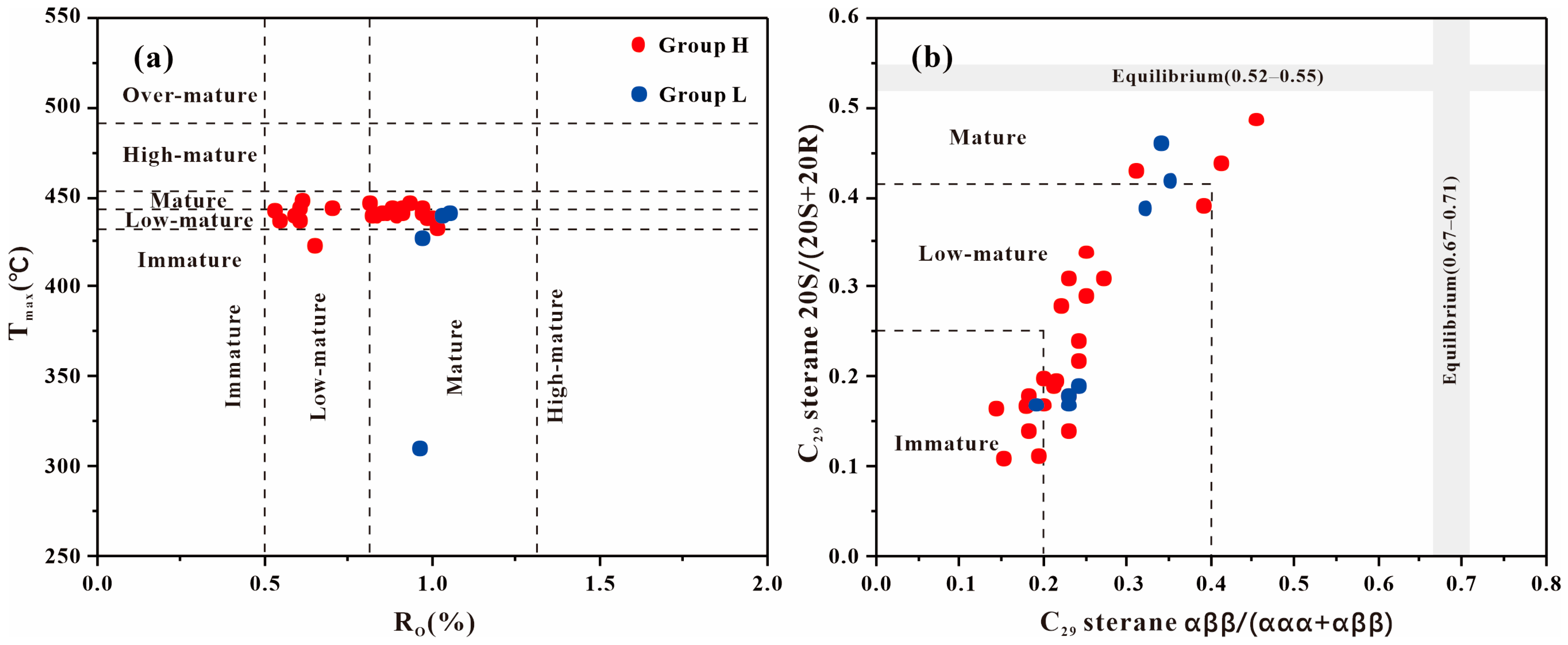
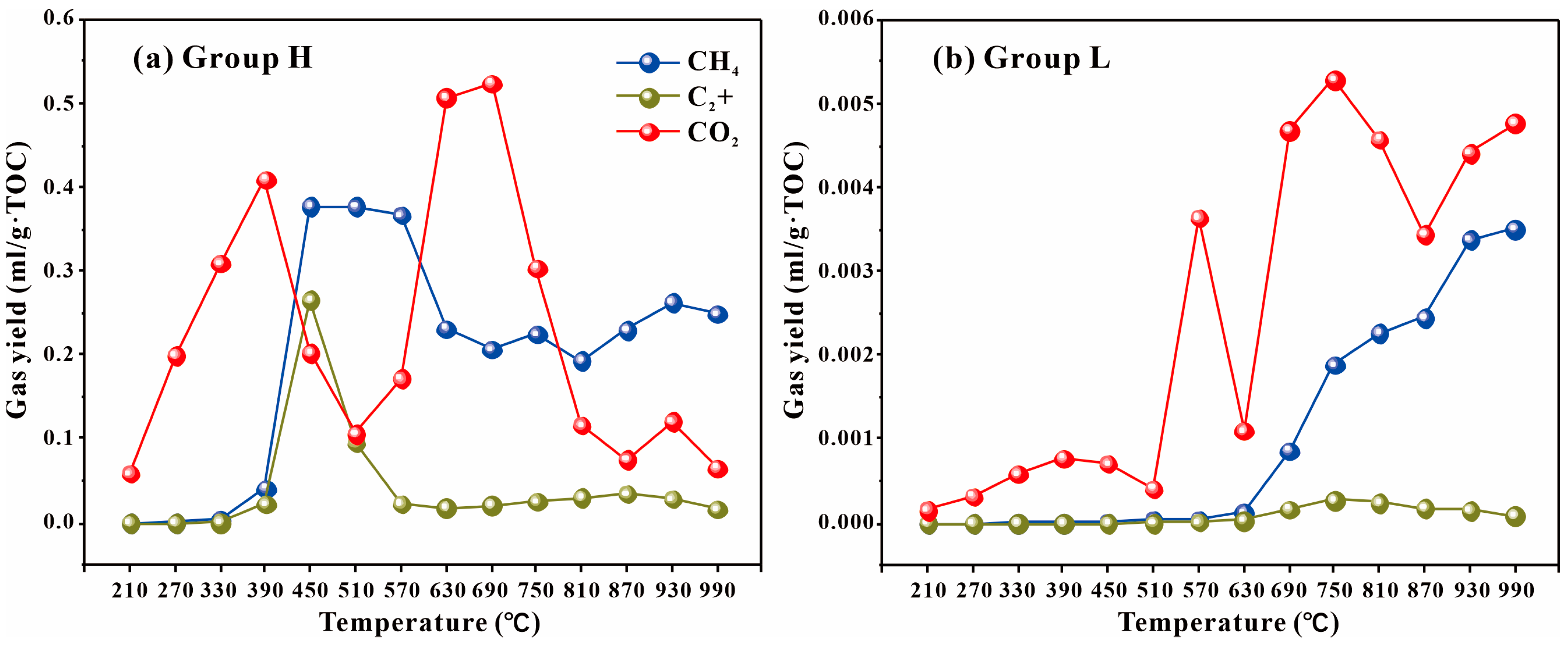


| Sample | Group | TOC | Ro | Tmax | S1 + S2 | S2/S3 | HI | OI | Paq | Pr/Ph | GI | n-C17/Pr | n-C18/Ph | C29 Sterane αββ/(ααα + αββ) | C29 Sterane S/(S + R) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | °C | mg/g | ||||||||||||
| STH-1 | H | 10.94 | 0.89 | 440 | 72.25 | 17.20 | 653.72 | 38.01 | 0.92 | 0.64 | 0.03 | 1.09 | 0.64 | 0.23 | 0.14 |
| STH-3 | H | 0.56 | 0.97 | 445 | 1.07 | 0.64 | 156.28 | 242.50 | 0.77 | 1.39 | 0.09 | 4.17 | 3.64 | 0.23 | 0.18 |
| STH-6 | H | 3.18 | 0.91 | 444 | 10.99 | 4.46 | 340.44 | 76.39 | 0.95 | 1.50 | 1.20 | 3.79 | 3.76 | 0.41 | 0.44 |
| STH-7 | H | 3.19 | 0.86 | 441 | 14.60 | 8.23 | 453.46 | 55.12 | 0.89 | 0.82 | 0.52 | 6.58 | 5.54 | 0.27 | 0.31 |
| STH-8 | H | 5.41 | 0.97 | 441 | 30.10 | 10.38 | 553.17 | 53.28 | 0.93 | 1.06 | 0.55 | 4.96 | 4.72 | 0.22 | 0.28 |
| STH-9 | H | 10.58 | 0.85 | 441 | 62.75 | 11.97 | 584.98 | 48.87 | 0.94 | 1.03 | 0.85 | 3.13 | 2.65 | 0.31 | 0.43 |
| STH-10 | H | 10.83 | 0.82 | 440 | 71.94 | 14.20 | 650.28 | 45.80 | 0.94 | 0.90 | 0.39 | 6.02 | 6.18 | 0.25 | 0.29 |
| STH-11 | H | 12.51 | 0.98 | 439 | 76.75 | 11.30 | 600.06 | 53.09 | 0.85 | 1.41 | 0.28 | 3.66 | 4.45 | 0.24 | 0.22 |
| STH-12 | H | 10.44 | 1.00 | 439 | 67.88 | 13.56 | 637.61 | 47.04 | 0.90 | 1.16 | 0.18 | 3.17 | 3.53 | 0.24 | 0.24 |
| STH-13 | H | 16.17 | 0.60 | 445 | 118.36 | 18.42 | 721.25 | 39.16 | 0.95 | 0.93 | 0.33 | 8.00 | 10.42 | 0.25 | 0.34 |
| STH-14 | H | 16.94 | 0.83 | 440 | 115.84 | 9.20 | 661.59 | 71.91 | 1.00 | 0.86 | 0.33 | 14.20 | 21.74 | 0.23 | 0.31 |
| STH-15 | H | 19.35 | 0.81 | 447 | 160.61 | 54.40 | 823.59 | 15.14 | 0.90 | 1.07 | 0.16 | 5.38 | 4.42 | 0.18 | 0.18 |
| YJG-3 | H | 11.11 | 0.53 | 443 | 87.51 | 58.29 | 781.49 | 13.41 | 0.93 | 0.94 | 0.80 | 5.90 | 4.07 | 0.20 | 0.20 |
| YJG-5 | H | 2.28 | 0.54 | 437 | 6.46 | 4.00 | 277.36 | 69.34 | 0.86 | 0.70 | 2.09 | 3.41 | 2.24 | 0.21 | 0.19 |
| YJG-6 | H | 0.68 | 0.88 | 444 | 2.07 | 1.93 | 292.30 | 151.29 | 0.85 | 0.91 | 0.48 | 3.30 | 2.10 | 0.15 | 0.11 |
| YJG-7 | H | 1.41 | 0.60 | 437 | 5.71 | 4.18 | 388.54 | 92.88 | 0.80 | 0.83 | 0.12 | 1.15 | 1.00 | 0.18 | 0.14 |
| YJG-8 | H | 7.41 | 0.59 | 440 | 51.32 | 13.94 | 677.22 | 48.59 | 0.91 | 0.87 | 0.39 | 2.58 | 2.74 | 0.20 | 0.17 |
| T25-1 | H | 5.43 | 0.61 | 449 | 30.71 | 11.76 | 522.87 | 5.16 | 0.93 | 1.45 | 0.15 | 8.96 | 9.90 | 0.39 | 0.39 |
| T34-1 | H | 0.85 | 1.01 | 433 | 8.32 | 107.86 | 556.01 | 44.45 | 0.91 | 0.42 | 0.38 | 4.80 | 4.36 | 0.45 | 0.49 |
| T31-1 | H | 5.07 | 0.65 | 424 | 37.18 | 45.34 | 715.61 | 15.78 | 0.89 | 0.71 | 0.45 | 1.64 | 0.94 | 0.14 | 0.11 |
| T31-2 | H | 9.45 | 0.70 | 445 | 70.11 | 112.19 | 736.44 | 6.56 | 0.84 | 0.59 | 0.25 | 1.70 | 0.91 | 0.19 | 0.20 |
| T31-3 | H | 4.47 | 0.91 | 441 | 22.78 | 62.42 | 502.39 | 8.05 | 0.88 | 0.69 | 0.07 | 1.95 | 1.11 | 0.21 | 0.17 |
| T31-4 | H | 10.22 | 0.93 | 447 | 88.17 | 150.59 | 854.63 | 5.68 | 0.91 | 0.66 | 0.38 | 2.73 | 1.37 | 0.18 | 0.17 |
| STH-2 | L | 0.78 | 1.03 | 440 | 2.44 | 1.75 | 299.92 | 171.75 | 0.63 | 1.01 | 0.11 | 1.48 | 0.85 | 0.24 | 0.19 |
| STH-4 | L | 0.51 | 1.05 | 441 | 0.92 | 0.62 | 159.07 | 257.27 | 0.96 | 0.88 | 0.12 | 3.27 | 3.04 | 0.23 | 0.17 |
| STH-5 | L | 2.23 | 0.97 | 428 | 7.82 | 4.45 | 335.89 | 75.44 | 0.63 | 0.35 | 0.15 | 1.87 | 0.44 | 0.19 | 0.17 |
| YJG-1 | L | 0.10 | - | 441 | 0.21 | 0.21 | 153.53 | 726.71 | 0.83 | 0.66 | 2.29 | 2.57 | 1.50 | 0.32 | 0.39 |
| YJG-4 | L | 0.14 | - | 436 | 0.33 | 0.37 | 197.95 | 535.19 | 0.57 | 1.45 | 1.26 | 4.78 | 3.84 | 0.23 | 0.18 |
| YJG-9 | L | 1.19 | - | 433 | 0.20 | 0.18 | 9.21 | 51.93 | 0.78 | 1.11 | 0.22 | 3.27 | 2.22 | 0.35 | 0.42 |
| YJG-10 | L | 1.38 | 0.96 | 310 | 0.16 | 0.06 | 5.81 | 93.02 | 0.78 | 0.91 | 0.25 | 3.87 | 2.50 | 0.34 | 0.46 |
| δ13C | YJG-1 n-Alkanes | YJG-4 n-Alkanes | YJG-9 n-Alkanes | YJG-10 n-Alkanes | YJG-3 n-Alkanes | YJG-5 n-Alkanes | YJG-6 n-Alkanes | YJG-7 n-Alkanes | YJG-8 n-Alkanes | YJG GBLs |
|---|---|---|---|---|---|---|---|---|---|---|
| C10 | −34.8 | |||||||||
| C11 | −35 | |||||||||
| C12 | −34.5 | |||||||||
| C13 | −35.2 | |||||||||
| C14 | −35.7 | |||||||||
| C15 | −32.9 | −35 | −31.7 | −34.2 | −35.3 | |||||
| C16 | −32.4 | −33 | −31.6 | −35.7 | −31.2 | −33.2 | −35.1 | −35.1 | ||
| C17 | −33.7 | −34.4 | −33.5 | −35.3 | −37.5 | −31.4 | −33.5 | −37.6 | −38 | −37.2 |
| C18 | −33 | −34.2 | −33.2 | −34.1 | −37.7 | −31.6 | −34.5 | −38.7 | −39.5 | −36.8 |
| C19 | −33.6 | −35.6 | −33.8 | −34.7 | −38.5 | −35 | −35.2 | −39.7 | −36 | −37.3 |
| C20 | −33.3 | −35.5 | −34.2 | −35.8 | −39.9 | −35.4 | −36.4 | −40.1 | −38.3 | −36.6 |
| C21 | −33.8 | −36.3 | −33 | −36.4 | −40.3 | −36.7 | −38.7 | −40.5 | −38.7 | −37 |
| C22 | −32.8 | −33.1 | −30.8 | −35.4 | −40.3 | −38.1 | −39 | −39.7 | −38.6 | −39.2 |
| C23 | −32.1 | −32.3 | −31.8 | −34.1 | −39.1 | −38.2 | −39.5 | −39.5 | −37.9 | −37.4 |
| C24 | −31.1 | −32.4 | −31.7 | −33.4 | −39.6 | −39.9 | −39.5 | −40.9 | −37.9 | −38 |
| C25 | −32.1 | −32.1 | −33.1 | −39 | −38.9 | −39.3 | −39.9 | −37.9 | −38.4 | |
| C26 | −33.4 | −31.3 | −32.2 | −37.6 | −39.7 | −39.2 | −40.7 | −36.4 | −38.3 | |
| C27 | −32.4 | −31.9 | −32.1 | −37.9 | −37.6 | −38.2 | −40.3 | −35.3 | −37.2 | |
| C28 | −32 | −32.6 | −39 | −40 | ||||||
| C29 | −31.5 | −36.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, T.; Wang, Z.; Li, L.; Fu, Y.; Li, X.; Wang, X. Origin and Hydrocarbon Generation of γ-Alkylbutyrolactones in Permian Shales. Processes 2025, 13, 1011. https://doi.org/10.3390/pr13041011
Wang W, Zhang T, Wang Z, Li L, Fu Y, Li X, Wang X. Origin and Hydrocarbon Generation of γ-Alkylbutyrolactones in Permian Shales. Processes. 2025; 13(4):1011. https://doi.org/10.3390/pr13041011
Chicago/Turabian StyleWang, Wenjun, Ting Zhang, Zuodong Wang, Liwu Li, Yin Fu, Xiaobin Li, and Xiaofeng Wang. 2025. "Origin and Hydrocarbon Generation of γ-Alkylbutyrolactones in Permian Shales" Processes 13, no. 4: 1011. https://doi.org/10.3390/pr13041011
APA StyleWang, W., Zhang, T., Wang, Z., Li, L., Fu, Y., Li, X., & Wang, X. (2025). Origin and Hydrocarbon Generation of γ-Alkylbutyrolactones in Permian Shales. Processes, 13(4), 1011. https://doi.org/10.3390/pr13041011






