Impact of Heterogeneity in Low-Permeability Reservoirs on Self-Diverting Acid Wormhole Formation and Acidizing Parameter Optimization
Abstract
:1. Introduction
2. Mathematical Models
- (1)
- Pressure equation:The self-diverting acid is considered an incompressible fluid, which means the pressure equation can be expressed as follows [29,30]:Here, K is the permeability, is the mobility, and P is the pressure field.
- (2)
- Velocity equation of the self-diverting acid:The velocity equation of the self-diverting acid is formulated based on Darcy’s law [14]:where represents the velocity field of the self-diverting acid.
- (3)
- Self-diverting acid concentration Equation ():Based on the acidizing (TSC) model, the scalar transport equation for the self-diverting acid can be expressed as [29,30]:where is the porosity, is the cup-mixing mass concentration of the self-diverting acid concentration (), is the effective dispersion tensor for the self-diverting acid concentration (), and is the interfacial area of the rock.
- (4)
- Ca2+ concentration equation:
- (5)
- SDVA concentration equation:
- (6)
- Self-diverting acid temperature equation:The fluid within the pores and the rock are assumed to undergo dynamic heat transfer processes. As a result, the acid and the rock each have distinct temperature equations. Below, we present the temperature equation for the acid [28,29,30]:where is the density of acid, is the specific heat capacity of the self-diverting acid, is the acid temperature field, is the acid conductivity, is the convective heat transfer coefficient, and is the rock temperature field.
- (7)
- Rock temperature equation:The heat exchange between rock and acid takes place via thermal conduction, eliminating the presence of a convective term. Additionally, as the acid dissolves the rock, heat is released, leading to the following equation for the rock [28,29,30]:where is the density of rock, is the specific heat capacity of rock, is the rock conductivity, and is the acid–rock molar reaction heat.
- (8)
- Chemical reaction process of self-diverting acid and rock:The reaction equation is as follows:where is the reaction rate at the interface between fluid and solid in rock pores. The interaction between HCl and CaCO3 is modeled as an irreversible first-order reaction. Consequently, the formula used to compute the chemical reaction terms is expressed as follows:where is the mass transfer coefficient, is the reaction rate constant, R is the gas constant, is the, m is the asymptotic Sherwood number, is the ratio of the pore length to pore diameter, is the pore Reynolds number, and is the average pore radius.
- (9)
- Rock quantity update:During the process of dissolving rocks, the transformation of acid can change the pore structure. Based on the TSC model, the updating of rock physical quantities can be divided into the Darcy scale and the pore scale. The updated calculation formula for quantity at the Darcy scale is [14]:where is the dissolving power of the acid. The updated calculation formula for quantity at the poro scale is [14]:and correspond to the initial permeability and porosity, respectively. These values remain unchanged throughout the calculation process, serving as fixed constants. Moreover, and indicate the effective diffusion coefficients in the x and y directions. , , and represent structural constants related to the pore characteristics.
- (10)
- Viscosity of the self-diverting acid update:As the chemical reaction between acid and rock progresses, the viscosity of the spent acid of self-diverting acid can change. Based on Liu’s experiment, the updated formula for the viscosity of the spent acid is [24]:where and are the initial and max viscosity of self-diverting acid, respectively. In this paper, the max viscosity is 350 mPa·s (0.35 Pa·s). and are the max concentration of Ca2+ and SDVA, and the max values are 2.109 and 0.06, respectively. b, c, , and are both empirical coefficients with the value are 1, 1, 0.0173, 1.1 and 2, respectively. The pH value can be calculated by the equilibrium equation of acid concentration:
3. Numerical Methods
3.1. Discretition Method
- (1)
- Pressure equation:
- (2)
- Self-diverting acid concentration equation ():
- (3)
- Ca2+ concentration equation:
- (4)
- SDVA concentration equation:
- (5)
- Self-diverting acid temperature equation:
- (6)
- Rock temperature equation:
3.2. Numerical Discretization Method and Test Parameters
3.3. Model Validation
4. Results and Analysis
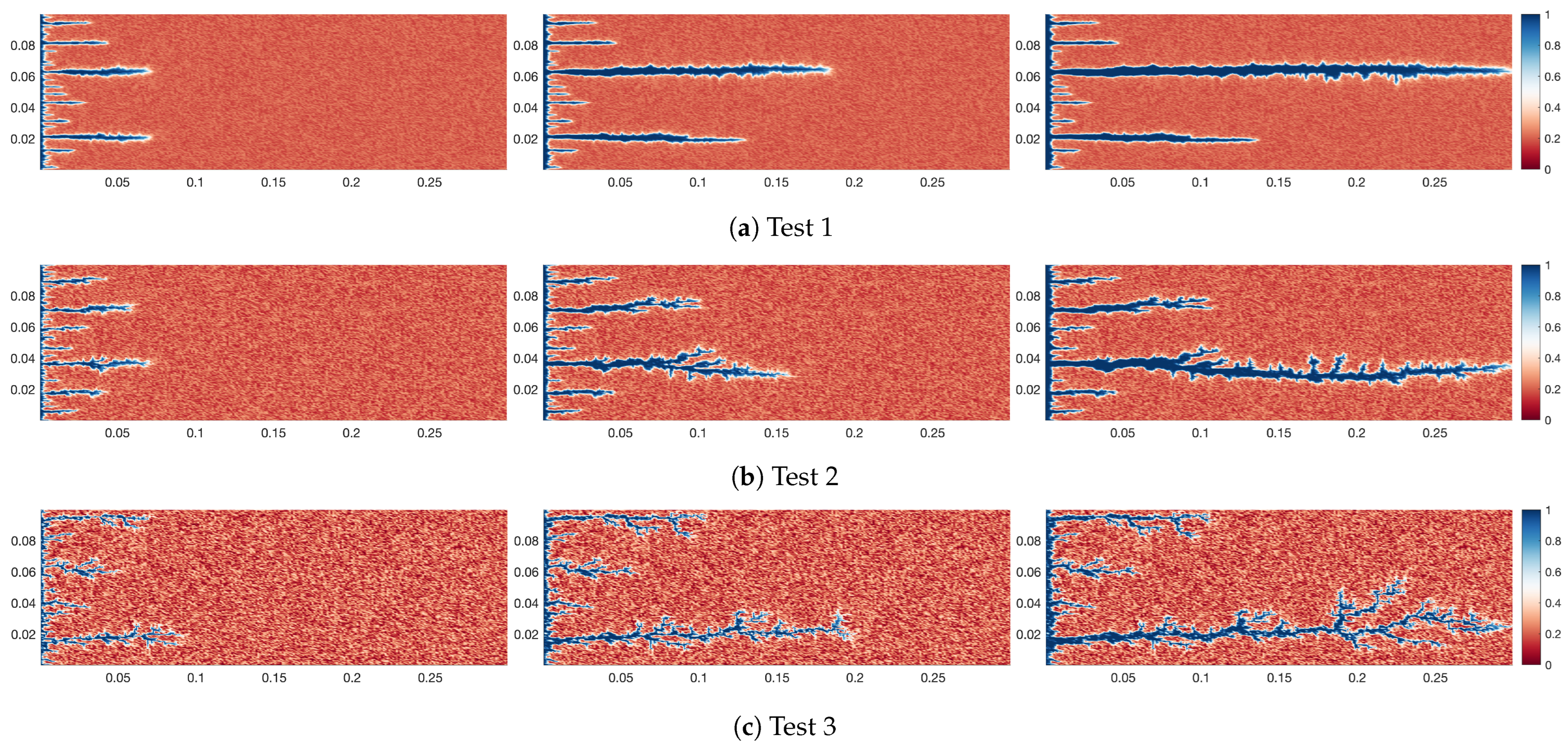
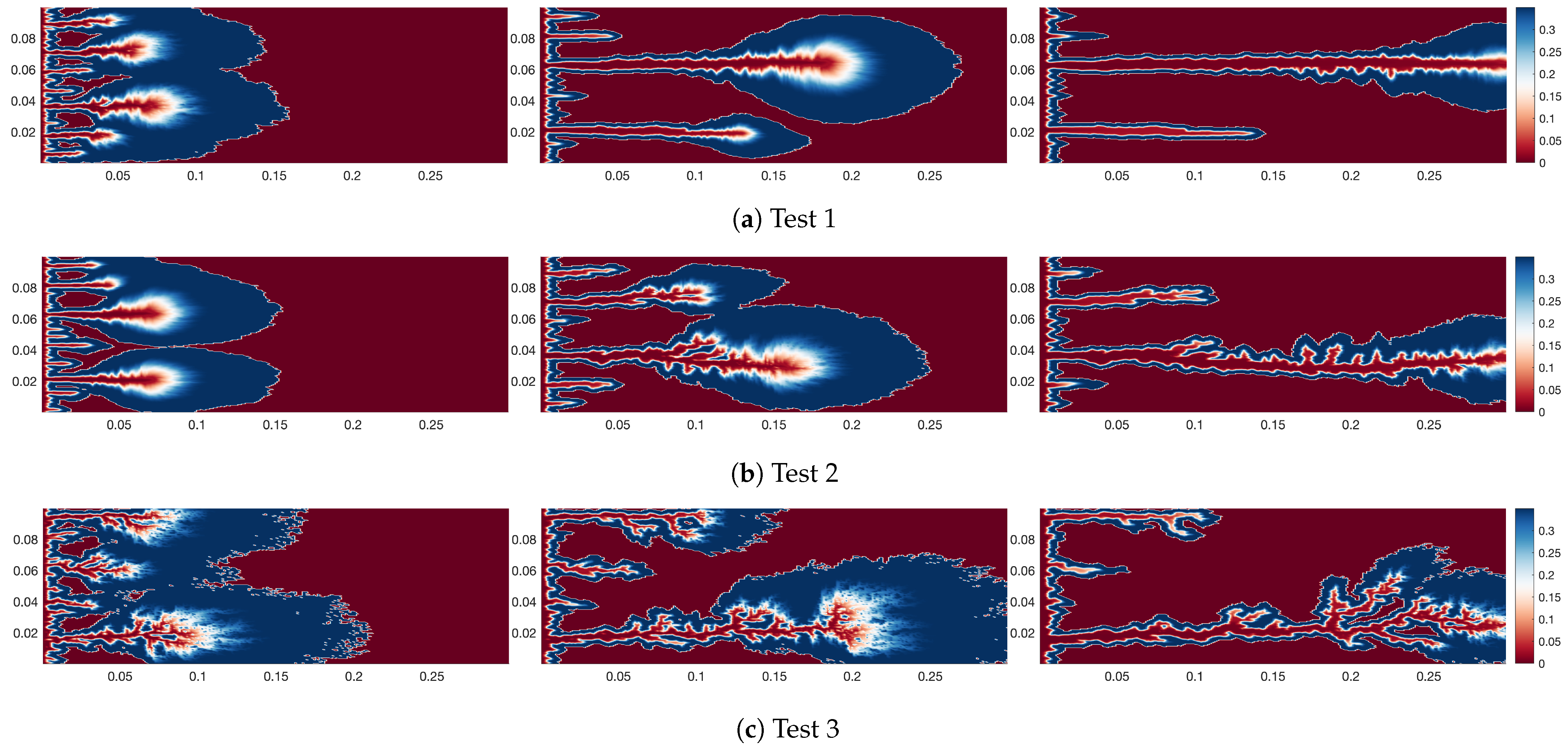
4.1. Effect of Different Heterogeneity Intensity on Wormhole Morphology of Self-Diverting
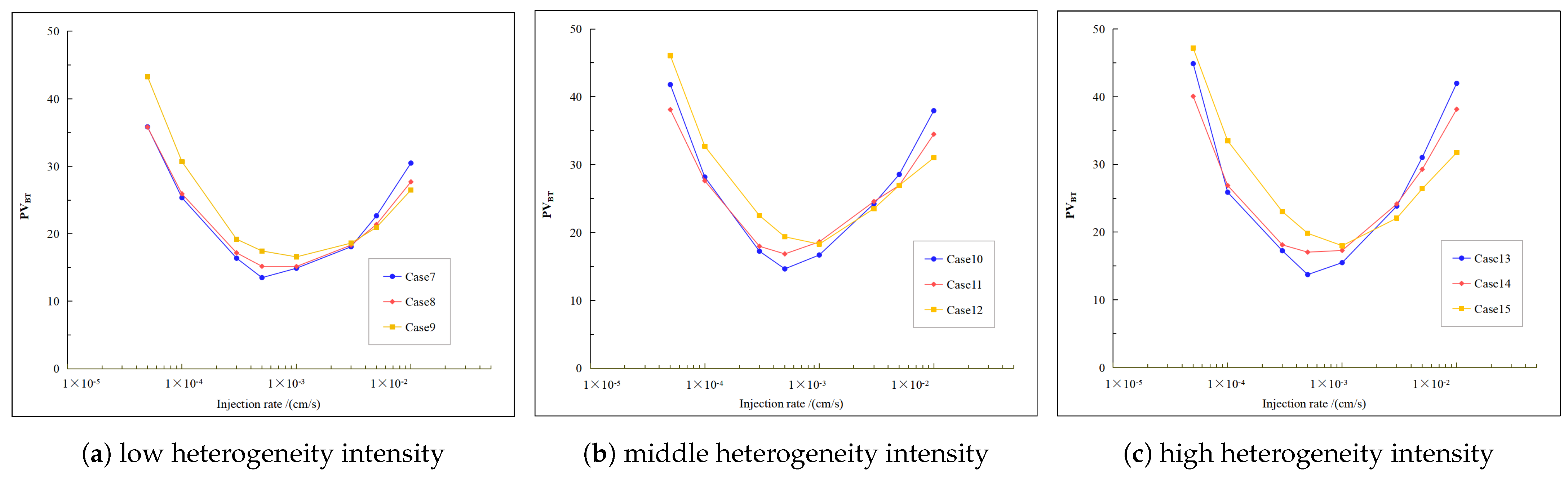
4.2. Influence of Varying Heterogeneity Intensity on the Wormhole Breakthrough Curve
4.3. Influence of Varying Injection Temperatures on the Breakthrough Curve
5. Conclusions
- (1)
- The heterogeneity intensity significantly affects the wormhole morphology. At low heterogeneity intensity, the wormhole morphology approaches a straight line. At moderate non-uniform strength, the shape of the wormhole can undergo some bending. At high heterogeneity intensity, the wormhole appears tree-like.
- (2)
- The impact of heterogeneity intensity on wormhole morphology primarily originates from the distribution of high-viscosity zones. At low intensities, no clear dominant pathways exist within the rock, causing the spent acid to advance relatively uniformly and create a consistent high-viscosity region. As heterogeneity intensity increases, the dominance of specific pathways becomes more pronounced, leading to more uneven progression of the spent acid. This non-uniform advancement generates irregular high-viscosity areas, which ultimately contribute to the complex morphology of wormholes.
- (3)
- The stronger the heterogeneity, the longer the flow distance of acid in the rock, and the higher the . Although the efficiency of rock breakthrough has slowed down, the improvement effect of porosity inside the rock has become stronger.
- (4)
- With the increase in heterogeneity intensity, the optimal injection rate of self-steering acid remains unchanged. In particular, under strong heterogeneous pore conditions, the self-diverting acid can produce more branching wormholes, which leads to a wider area of acidizing, but the difference in values at larger and smaller acid injection rates is not significant. In order to enhance the effect of self-steering acid, it is recommended to appropriately reduce the acid injection rate.
- (5)
- The optimal injection rate of self-steering acid increases with increasing temperature. At a low injection rate, more wormholes can be produced, and lower temperature promotes this phenomenon, and the effect is more obvious with the increase in heterogeneity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McLeod, H., Jr. Matrix acidizing. J. Pet. Technol. 1984, 36, 2055–2069. [Google Scholar] [CrossRef]
- Rae, P.; Di Lullo, G. Matrix acid stimulation—A review of the state-of-the-art. In Proceedings of the SPE European Formation Damage Conference and Exhibition, The Hague, The Netherlands, 13–14 May 2003; p. SPE-82260-MS. [Google Scholar]
- Nasr-El-Din, H.A.; Samuel, M. Lessons learned from using viscoelastic surfactants in well stimulation. SPE Prod. Oper. 2007, 22, 112–120. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H. Optimum conditions for wormhole formation in carbonate porous media: Influence of transport and reaction. SPE J. 1999, 4, 196–205. [Google Scholar] [CrossRef]
- Aidagulov, G.; Gwaba, D.; Kayumov, R.; Sultan, A.; Aly, M.; Qiu, X.; Almajed, H.; Abbad, M. Effects of pre-existing fractures on carbonate matrix stimulation studied by large-scale radial acidizing experiments. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 18–21 March 2019; p. D032S067R001. [Google Scholar]
- Dong, C.; Hill, A.; Zhu, D. Acid etching patterns in naturally fractured formations. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999; p. SPE-56531. [Google Scholar]
- Al-Mutairi, S.H.; Al-Obied, M.A.; Al-Yami, I.S.; Shebatalhamd, A.M.; Al-Shehri, D.A. Wormhole propagation in tar during matrix acidizing of carbonate formation. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 15–17 February 2012; p. SPE-151560. [Google Scholar]
- Gomaa, A.M.; Cutler, J.; Boles, J.; Sun, H. An effective acid placement technique to stimulate high-temperature sandstone and carbonate formations. In Proceedings of the IPTC 2013: International Petroleum Technology Conference, Beijing, China, 26–28 March 2013; p. cp-350. [Google Scholar]
- Sibarani, T.T.; Ziauddin, M.; Nasr-El-Din, H.A.; Zakaria, A.S. The impact of pore structure on carbonate stimulation treatment using ves-based HCl. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Brisbane, Australia, 23–25 October 2018; p. D032S009R011. [Google Scholar]
- Chang, F.; Qu, Q.; Frenier, W. A novel self-diverting-acid developed for matrix stimulation of carbonate reservoirs. In Proceedings of the SPE International Conference on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001; p. SPE-65033. [Google Scholar]
- Sarma, D.K.; Rao, Y.; Mandal, B.; Bhargava, P.K. Application of self-diverting acid system for stimulation of multilayered wells in carbonate reservoir: A case study. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 28–30 March 2012; p. SPE-154554. [Google Scholar]
- Cesin, S.; Fayard, A.; Camarillo, J.; Lian, E.; Flores, J.; Flores, P.T.; Mendo, A.R. Innovative combination of dynamic underbalanced perforating with emulsified acid and non-damaging viscoelastic surfactant-based fluids boost productivity in low-permeability naturally fractured carbonates. In Proceedings of the SPE Brasil Offshore, Macaé, Brazil, 14–17 June 2011; p. SPE-143735. [Google Scholar]
- Li, Q.; Li, Q.; Cao, H.; Wu, J.; Wang, F.; Wang, Y. The crack propagation behaviour of CO2 fracturing fluid in unconventional low permeability reservoirs: Factor analysis and mechanism revelation. Processes 2025, 13, 159. [Google Scholar] [CrossRef]
- Panga, M.K.; Ziauddin, M.; Balakotaiah, V. Two-scale continuum model for simulation of wormholes in carbonate acidization. AIChE J. 2005, 51, 3231–3248. [Google Scholar]
- Kalia, N.; Balakotaiah, V. Effect of medium heterogeneities on reactive dissolution of carbonates. Chem. Eng. Sci. 2009, 64, 376–390. [Google Scholar] [CrossRef]
- Cohen, C.E.; Ding, D.; Quintard, M.; Bazin, B. From pore scale to wellbore scale: Impact of geometry on wormhole growth in carbonate acidization. Chem. Eng. Sci. 2008, 63, 3088–3099. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; Mou, J.; Zhou, F.; Shi, Y. Diverting mechanism of viscoelastic surfactant-based self-diverting acid and its simulation. J. Pet. Sci. Eng. 2013, 105, 91–99. [Google Scholar]
- Jia, C.; Huang, Z.; Sepehrnoori, K.; Yao, J. Modification of two-scale continuum model and numerical studies for carbonate matrix acidizing. J. Pet. Sci. Eng. 2021, 197, 107972. [Google Scholar] [CrossRef]
- de Paulo Ferreira, L.; Surmas, R.; Tonietto, S.N.; da Silva, M.A.P.; Peçanha, R.P. Modeling reactive flow on carbonates with realistic porosity and permeability fields. Adv. Water Resour. 2020, 139, 103564. [Google Scholar] [CrossRef]
- Maheshwari, P.; Balakotaiah, V. 3-D simulation of carbonate acidization with HCl: Comparison with experiments. In Proceedings of the SPE Oklahoma City Oil and Gas Symposium/Production and Operations Symposium, Oklahoma City, OK, USA, 23–26 March 2013; p. SPE-164517. [Google Scholar]
- Bekibayev, T.; Beisembetov, I.; Assilbekov, B.; Zolotukhin, A.; Zhapbasbayev, U.; Turegeldieva, K. Study of the impact of reduced permeability due to near-wellbore damage on the optimal parameters of the matrix acidizing in carbonate rocks. In Proceedings of the SPE Annual Caspian Technical Conference, Baku, Azerbaijan, 4–6 November 2015; p. SPE-177372. [Google Scholar]
- Liu, N.; Liu, M. Simulation and analysis of wormhole propagation by VES acid in carbonate acidizing. J. Pet. Sci. Eng. 2016, 138, 57–65. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Q.; Zhao, J.; Liao, Y.; Jiang, Y. Simulation and analysis of matrix stimulation by diverting acid system considering temperature field. J. Pet. Sci. Eng. 2018, 170, 932–944. [Google Scholar]
- Liu, P.; Xue, H.; Zhao, L.Q.; Fu, Y.; Luo, Z.; Qu, Z. Analysis and simulation of rheological behavior and diverting mechanism of in situ self-diverting acid. J. Pet. Sci. Eng. 2015, 132, 39–52. [Google Scholar] [CrossRef]
- Bulgakova, G.T.; Kharisov, R.Y.; Pestrikov, A.V.; Sharifullin, A.R. Experimental study of a viscoelastic surfactant-based in situ self-diverting acid system: Results and interpretation. Energy Fuels 2014, 28, 1674–1685. [Google Scholar]
- Mou, J.; Cai, X.; Liu, M.; Zhang, Y.; Zhang, S. The mechanism of leakoff reduction of clean self-diversion acid in acid fracturing. In Proceedings of the International Petroleum Technology Conference, Beijing, China, 26–28 March 2013; p. IPTC-16720. [Google Scholar]
- Mou, J.; Liu, M.; Zheng, K.; Zhang, S. Diversion conditions for viscoelastic-surfactant-based self-diversion acid in carbonate acidizing. SPE Prod. Oper. 2015, 30, 121–129. [Google Scholar]
- Kalia, N.; Glasbergen, G. Wormhole Formation in Carbonates Under Varying Temperature Conditions. In Proceedings of the 8th European Formation Damage Conference, Scheveningen, The Netherlands, 27–29 May 2009. [Google Scholar] [CrossRef]
- Chang, T.; Jiang, Y.; Li, Y.; Chen, X.; Kang, X.; Mo, W. Study on the effect of natural fractures and temperature on the wormhole morphology formed by two-phase acidizing in carbonate rocks. Phys. Fluids 2024, 36, 083333. [Google Scholar] [CrossRef]
- Chang, T.; Jiang, Y.; Zhao, H.; Chen, X.; Mo, W. Effect of two-phase viscosity difference and natural fractures on the wormhole morphology formed by two-phase acidizing with self-diverting acid in carbonate rocks. Phys. Fluids 2024, 36, 093623. [Google Scholar]
- Carman, P. Flow of Gases Through Porous Media; Academic Press Inc.: New York, NY, USA, 1956. [Google Scholar]
- Valdes-Parada, F.J.; Ochoa-Tapia, J.A.; Alvarez-Ramirez, J. Validity of the permeability carman–kozeny equation: A volume averaging approach. Phys. A Stat. Mech. Its Appl. 2009, 388, 789–798. [Google Scholar] [CrossRef]





| Operator | Symbol | Discrete Scheme | Accuracy |
|---|---|---|---|
| Temporal term | Backward Euler | ||
| Convective term | First-order upwind | ||
| Laplacian term | Center difference |
| Parameter | L [m] | H [m] | [kmol/m3] | [cm/s] |
| Value | 0.3 | 0.1 | 4.4178 (15 wt% HCL) | 1– |
| Parameter | [%] | K [m2] | [mPa·s] | [J/(kg·°C)] |
| Value | 0.01–0.5 | Calculated | 1 | 4180 |
| Parameter | [J/(kg·°C)] | [W/(m·°C)] | [kg/kmol] | [W/(m·°C)] |
| Value | 999 | 0.6508 | 50 | 5.2 |
| Parameter | [m2/m3] | [m2/s] | [kg/kmol] | [W/(m2·°C)] |
| Value | 5000 | 50 | 600 |
| Parameter | Symbol | Test 1 | Test 2 | Test 3 |
|---|---|---|---|---|
| Porosity of matrix [%] | [15, 25] | [10, 30] | [5, 40] | |
| Inject acid temperature [°C] | 60 | |||
| Inject velocity [cm/s] | ||||
| Parameter | Symbol | Test 4 | Test 5 | Test 6 |
|---|---|---|---|---|
| Porosity of matrix [%] | [15, 25] | [10, 30] | [5, 40] | |
| Inject acid temperature [°C] | 60 | |||
| Inject velocity [cm/s] | – | |||
| Parameter | Symbol | Test 7 | Test 8 | Test 9 |
| Porosity of matrix [%] | [15, 25] | |||
| Inject acid temperature [°C] | 20 | 60 | 100 | |
| Inject velocity [cm/s] | – | |||
| Parameter | Symbol | Test 10 | Test 11 | Test 12 |
| Porosity of matrix [%] | [10, 30] | |||
| Inject acid temperature [°C] | 20 | 60 | 100 | |
| Inject velocity [cm/s] | – | |||
| Parameter | Symbol | Test 13 | Test 14 | Test 15 |
| Porosity of matrix [%] | [5, 40] | |||
| Inject acid temperature [°C] | 20 | 60 | 100 | |
| Inject velocity [cm/s] | – | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Liu, C.; Liu, A.; Zhang, X.; Nie, F. Impact of Heterogeneity in Low-Permeability Reservoirs on Self-Diverting Acid Wormhole Formation and Acidizing Parameter Optimization. Processes 2025, 13, 1029. https://doi.org/10.3390/pr13041029
Luo J, Liu C, Liu A, Zhang X, Nie F. Impact of Heterogeneity in Low-Permeability Reservoirs on Self-Diverting Acid Wormhole Formation and Acidizing Parameter Optimization. Processes. 2025; 13(4):1029. https://doi.org/10.3390/pr13041029
Chicago/Turabian StyleLuo, Jun, Chunlin Liu, An Liu, Xuchen Zhang, and Fajian Nie. 2025. "Impact of Heterogeneity in Low-Permeability Reservoirs on Self-Diverting Acid Wormhole Formation and Acidizing Parameter Optimization" Processes 13, no. 4: 1029. https://doi.org/10.3390/pr13041029
APA StyleLuo, J., Liu, C., Liu, A., Zhang, X., & Nie, F. (2025). Impact of Heterogeneity in Low-Permeability Reservoirs on Self-Diverting Acid Wormhole Formation and Acidizing Parameter Optimization. Processes, 13(4), 1029. https://doi.org/10.3390/pr13041029





