Fluorescence-Based Detection of Picric Acid Using Vortex-Assisted Liquid–Liquid Microextraction: An Innovative Analytical Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Applied Instruments
2.2. Methods
2.2.1. Samples Preparation
2.2.2. Procedure of Calibration and PA Determination with VALLME-FLD
2.3. Computational Details
3. Results and Discussion
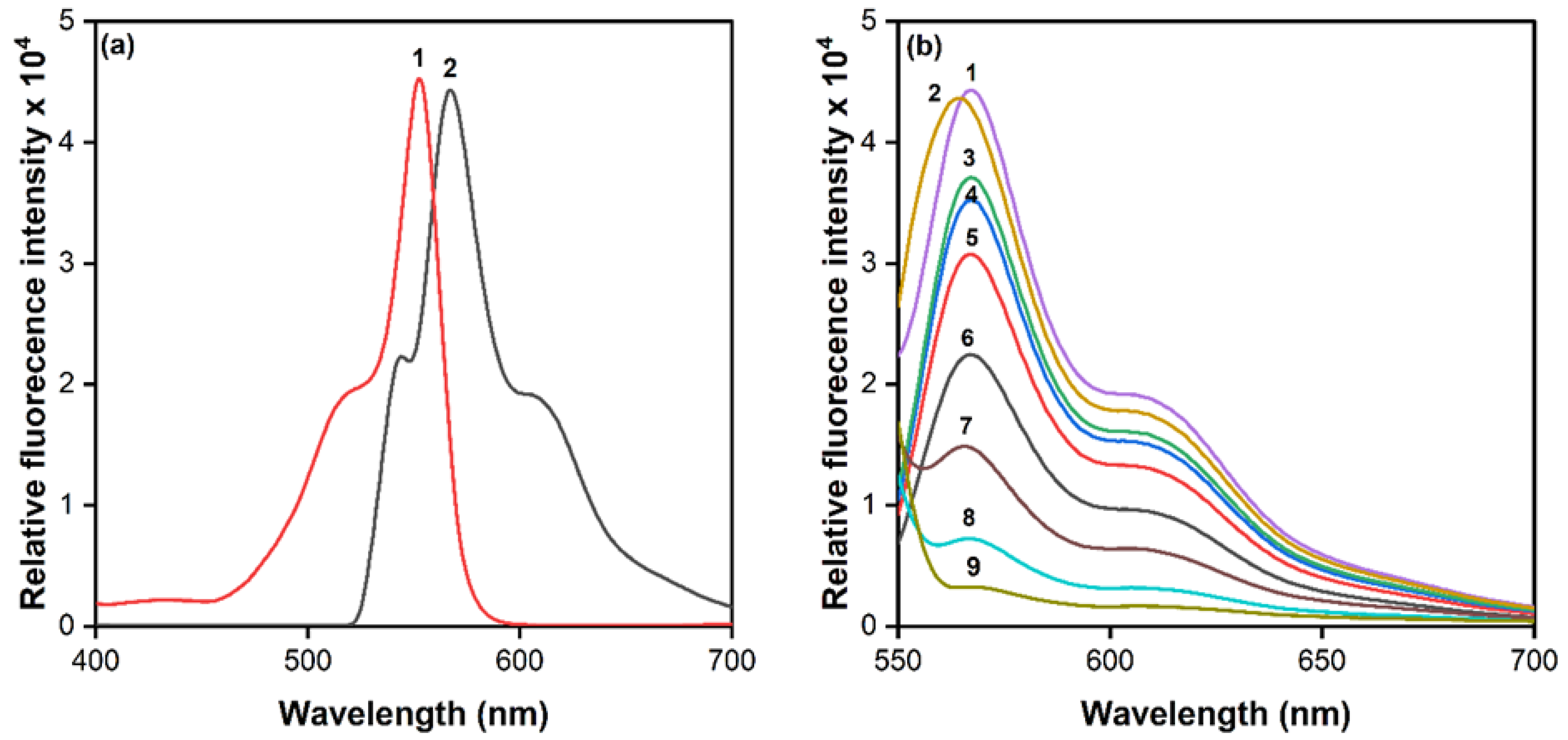
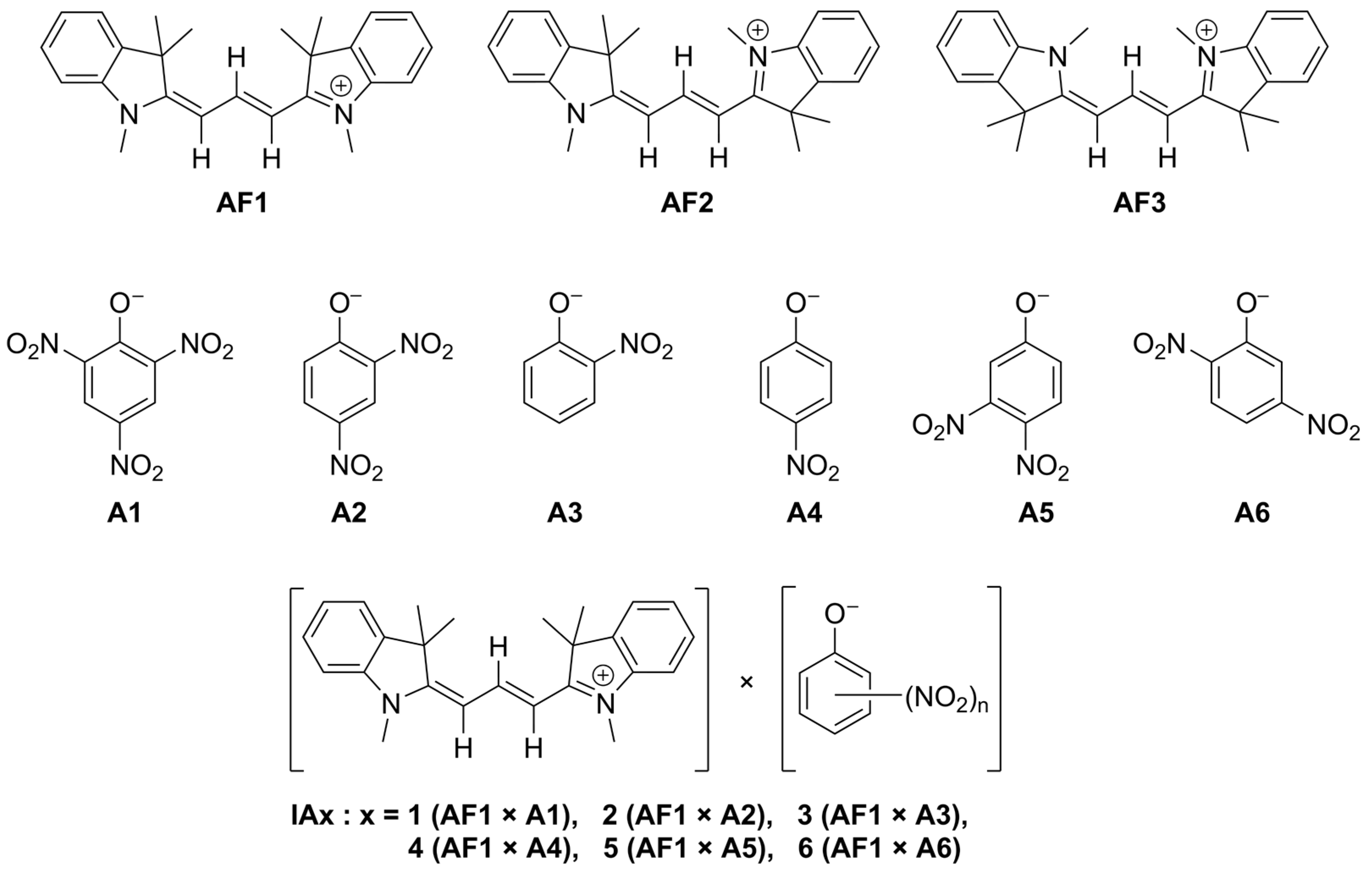
3.1. Reaction Chemistry
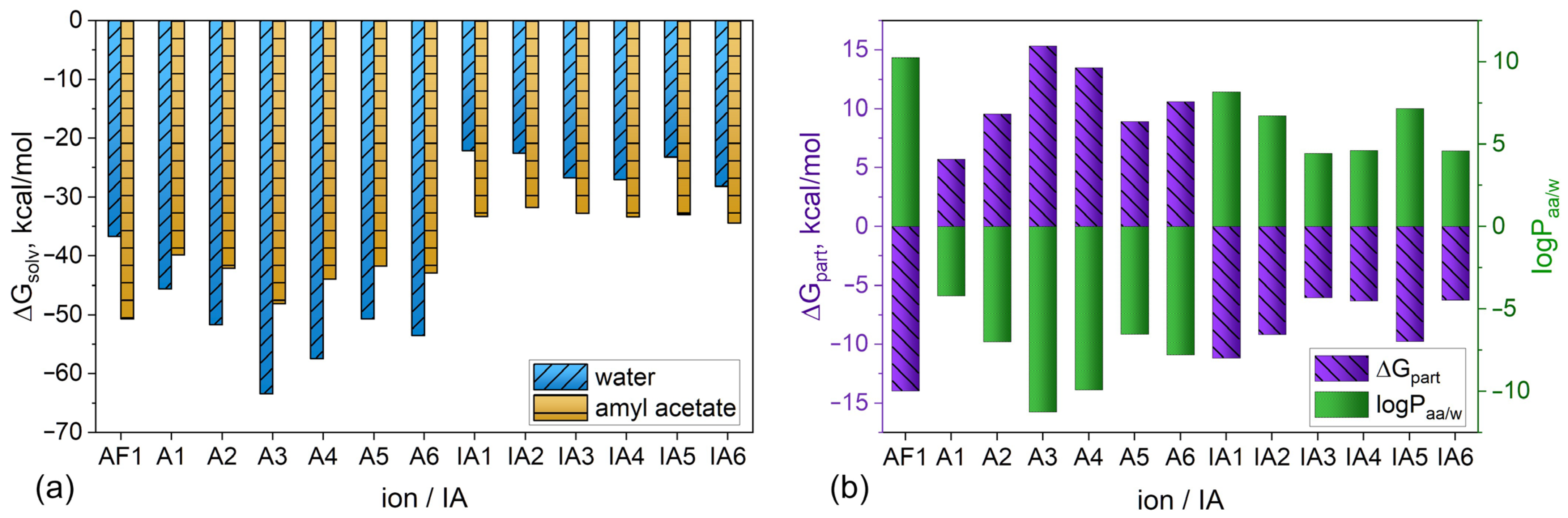
3.2. Theoretical Explanation of the Mechanism of Picrate Extraction
3.3. Investigation of the Experimental Conditions
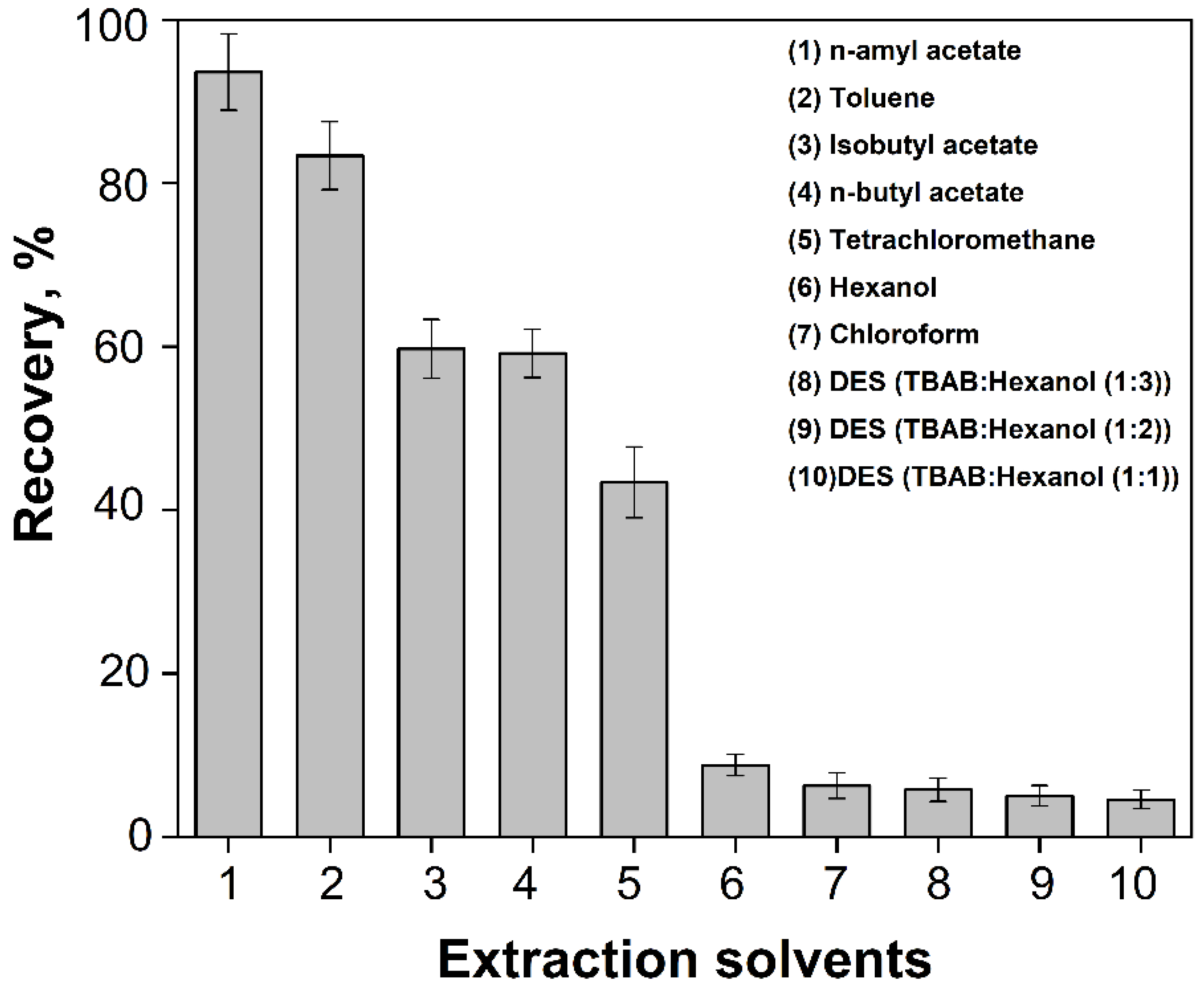
3.3.1. Effect of Type of Extraction Solvents
3.3.2. Effect of AF Concentration
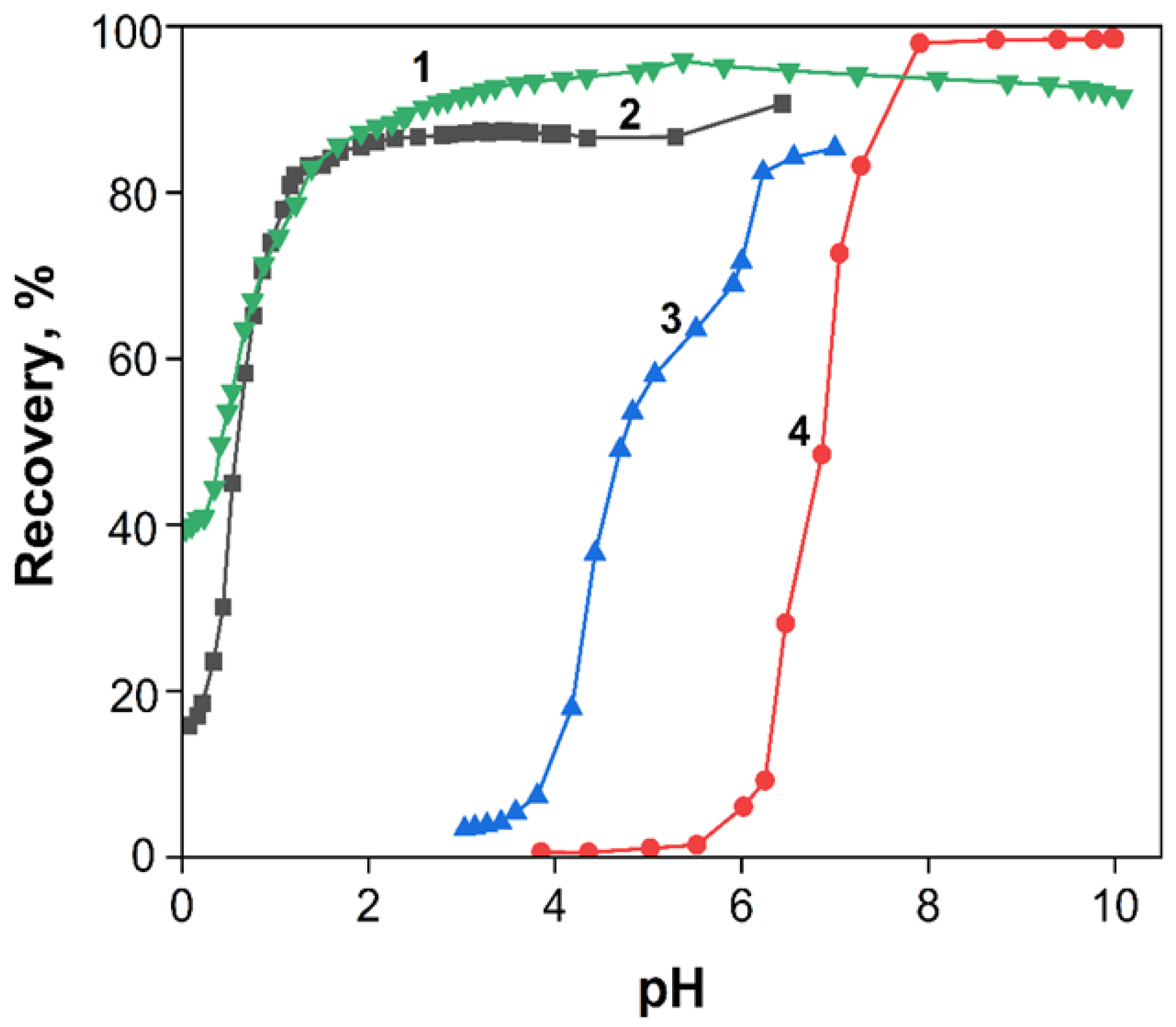
3.3.3. Effect of pH and Buffer Volume
3.3.4. Effect of Extraction and Vortexing Time
3.3.5. Effect of the Stability of the Extracts
3.4. Interference Study
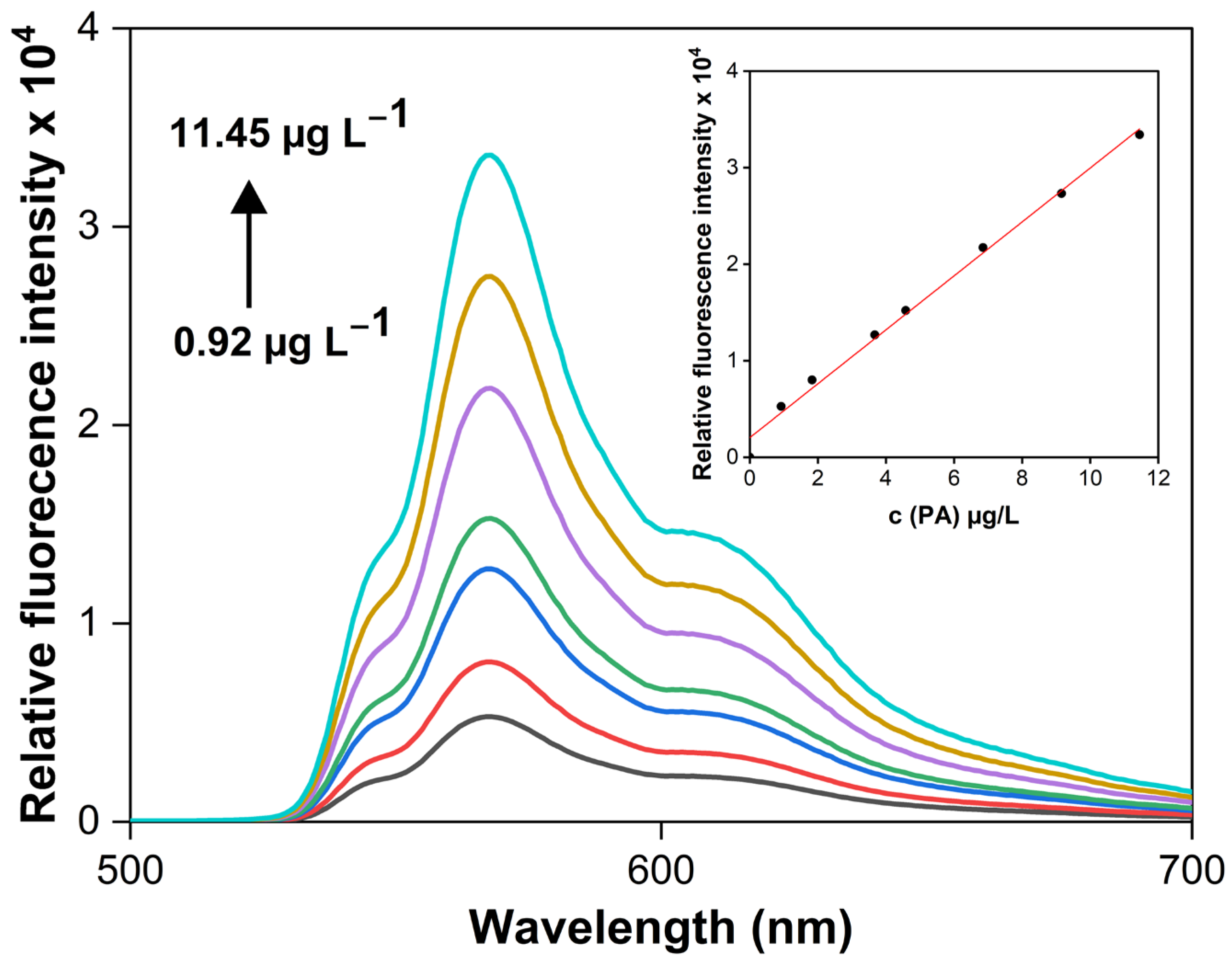
3.5. Analytical Characteristics of the VALLME-FLD Method and Analytical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez, G.V.; Perez, A.L. Organic acids without a carboxylic acid functional group. J. Chem. Educ. 2000, 77, 910. [Google Scholar] [CrossRef]
- Roy, B.; Bar, A.K.; Gole, B.; Mukherjee, P.S. Fluorescent tris-imidazolium sensors for picric acid explosive. J. Org. Chem. 2013, 78, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed]
- Wyman, J.F.; Serve, M.P.; Hobson, D.W.; Lee, L.H.; Uddin, D.E. Acute toxicity, distribution, and metabolism of 2,4,6-trinitrophenol (picric acid) in Fischer 344 rats. J. Toxicol. Environ. Health 1992, 37, 313–327. [Google Scholar] [CrossRef]
- Bauri, K.; Saha, B.; Mahanti, J.; De, P. A nonconjugated macromolecular luminogen for speedy, selective and sensitive detection of picric acid in water. Polym. Chem. 2017, 8, 7180–7187. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, X.; Jing, N.; Zhang, Y. Hydrothermal synthesis of carbon quantum dots as fluorescent probes for the sensitive and rapid detection of picric acid. Anal. Methods 2018, 10, 2775–2784. [Google Scholar] [CrossRef]
- Tanwar, A.S.; Meher, N.; Adil, L.R.; Iyer, P.K. Stepwise elucidation of fluorescence based sensing mechanisms considering picric acid as a model analyte. Analyst 2020, 14, 4753–4767. [Google Scholar] [CrossRef]
- Ashbrook, P.C.; Houts, T.A. Targeting chemicals for waste minimization. Chem. Health Saf. 2000, 7, 41. [Google Scholar] [CrossRef]
- Sivaraman, G.; Vidya, B.; Chellappa, D. Rhodamine based selective turn-on sensing of picric acid. RSC Adv. 2014, 4, 30828–30831. [Google Scholar] [CrossRef]
- Saleem, A.; Rafi, N.; Qasim, S.; Ashraf, U.; Virk, N. Synthesis of Picric Acid at Domestic Scales. Int. J. Innov. Sci. Technol. 2019, 1, 62–78. [Google Scholar] [CrossRef]
- Ferreira de Sousa, P.V.; de Moraes Guimarães, Y.; Pinto, G.C.; Fernando de Oliveira, A.; Alberto da Silva, A.; Lopes, R.P. Study of Cu NPs reactivity for compounds with different chemical structures: Black reactive dye 5, picric acid and 2,4-D herbicide. Chemosphere 2019, 235, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.R.; Maheria, K.C.; Parikh, J.K. Highly efficient micellar extraction of toxic picric acid into novel ionic liquid: Effect of parameters, solubilization isotherm, evaluation of thermodynamics and design parameters. J. Hazard. Mater. 2015, 300, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Udhayakumari, D.; Velmathi, S.; Venkatesa, P.; Wu, S.P. A pyrene-linked thiourea as a chemosensor for cations and simple fluorescent sensor for picric acid. Anal. Methods 2015, 7, 1161–1166. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Kong, F.; Yu, L. Design and preparation of poly(tannic acid) nanoparticles with intrinsic fluorescence: A sensitive detector of picric acid. J. Chem. Eng. 2021, 416, 1385–8947. [Google Scholar] [CrossRef]
- Hussain, S.; Malik, A.H.; Afroz, M.A.; Iyer, P.K. Ultrasensitive detection of nitroexplosive—Picric acid via a conjugated polyelectrolyte in aqueous media and solid support. Chem. Commun. 2015, 51, 7207–7210. [Google Scholar] [CrossRef]
- Xu, Y.; Li, B.; Li, W.; Zhao, J.; Sun, S.; Pang, Y. ICT-not-quenching near infrared ratiometric fluorescent detection of picric acid in aqueous media. Chem. Commun. 2013, 49, 4764–4766. [Google Scholar] [CrossRef]
- Wollin, K.M.; Dieter, H.H. Toxicological guidelines for monocyclic nitro-, amino- and aminonitroaromatics, nitramines, and nitrate esters in drinking water. Arch. Environ. Contam. Toxicol. 2005, 49, 18–26. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Z.; Li, J.; Shao, H.; Chen, L.; Yang, X. A molecular imprinting f luorescence sensor based on quantum dots and a mesoporous structure for selective and sensitive detection of 2, 4-dichlorophenoxyacetic acid. Sens. Actuators B 2017, 252, 934–943. [Google Scholar] [CrossRef]
- Rageh, A.H.; Ibrahim, M.; Said, M.I. Bifunctional Fe(III) metal-organic framework: A highly sensitive “turn-off” fluorescent probe and a precursor of magnetite nanoparticles for potential water decontamination. Solid State Sci. 2023, 140, 107202. [Google Scholar] [CrossRef]
- Fang, X.; Wang, S.; Wang, Q.; Gong, J.; Li, L.; Lu, H.; Xue, P.; Ren, Z.; Wang, X. A highly selective and sensitive fluorescence probe based on BODIPY-cyclen for hydrogen sulfide detection in living cells and serum. Talanta 2024, 268, 125339. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-H.; Zhang, W.; Ren, C.-X.; Xiong, Y.; Zhang, J.-Y.; He, J.; Huang, Y.; Tao, Z.; Xiao, X. A novel portable smart phone sensing platform based on a supramolecular fluorescence probe for quick visual quantitative detection of picric acid. Anal. Chim. Acta 2023, 1254, 341095. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Tiwari, P.; Mate, N.; Sharma, V.; Mobin, S.M. Photoactivatable carbon dots as a label-free fluorescent probe for picric acid detection and light-induced bacterial inactivation. J. Photochem. Photobiol. B 2022, 229, 112412. [Google Scholar] [CrossRef] [PubMed]
- Keerthana, P.; Cherian, A.R.; Sirimahachai, U.; Thadathil, D.A.; Varghese, A.; Hegde, G. Detection of picric acid in industrial effluents using multifunctional green fluorescent B/N-carbon quantum dots. J. Environ. Chem. Eng. 2022, 10, 107209. [Google Scholar] [CrossRef]
- Sreekumar, A.; Mohan, M.G.; Raksha, C.; Nair, A.R.; Manoj, P.; Janardhanan, J.A.; Sivan, A. Design, synthesis, and spectrofluorimetric analysis of a novel fluorene-based chemosensor for the selective detection of picric acid. J. Mol. Struct. 2024, 1312, 138499. [Google Scholar] [CrossRef]
- Arshad, M.; AT, J.R.; Joseph, V.; Joseph, A. Selective detection of picric acid in aqueous medium using a novel naphthaldehyde-based aggregation induced emission enhancement (AIEE) active “turn-off” fluorescent sensor. J. Lumin. 2023, 258, 119818. [Google Scholar] [CrossRef]
- Lv, W.; Song, Y.; Guo, R.; Liu, N.; Mo, Z. Metal-organic frame material encapsulated Rhodamine 6G: A highly sensitive fluorescence sensing platform for the detection of picric acid contaminants in water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 316, 124355. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P.; Soriano-Araújo, A.; Lobato, Z.I.P.; de Carvalho, S.M.; Leite, M.d.F. Water-soluble nanoconjugates of quantum dot-chitosan-antibody for in vitro detection of cancer cells based on “enzyme-free” fluoroimmunoassay. Mater. Sci. Eng. C 2015, 52, 61–71. [Google Scholar] [CrossRef]
- Martinis, E.M.; Castro Grijalba, A.; Pérez, M.B.; Llaver, M.; Wuilloud, R.G. Synergistic analytical preconcentration with ionic liquid–nanomaterial hybrids. TrAC 2017, 97, 333–344. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblinska, J.; Nowak, I. Modern approaches in dispersive liquid- liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Spietelun, A.; Marcinkowski, L.; de la Guardia, M.; Namiesnik, J. Green aspects, developments and perspectives of liquid phase microextraction techniques. Talanta 2014, 119, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.M.; Casado, N.; Porto-Figueira, P.; Camara, J.S. The potential of microextraction techniques for the analysis of bioactive compounds in food. Front. Nutr. 2022, 9, 825519. [Google Scholar] [CrossRef]
- Psillakis, E. Vortex-assisted liquid-liquid microextraction revisited. TrAC 2019, 113, 332–339. [Google Scholar] [CrossRef]
- Guiñez, M.; Martinez, L.D.; Fernandez, L.; Cerutti, S. Dispersive liquid–liquid microextraction based on solidification of floating organic drop and fluorescence detection for the determination of nitrated polycyclic aromatic hydrocarbons in aqueous samples. Microchem. J. 2017, 131, 1–8. [Google Scholar] [CrossRef]
- Kakalejčikova, S.; Bazel, Y. A combination of vortex-assisted liquid–liquid microextraction with fluorescence detection: An innovative approach for a green and highly sensitive determination of sodium dodecyl sulfate in water samples and pharmaceuticals. Microchem. J. 2024, 199, 110226. [Google Scholar] [CrossRef]
- Zhang, J.; Dolg, M. ABCluster: The Artificial Bee Colony Algorithm for Cluster Global Optimization. Phys. Chem. Chem. Phys. 2015, 17, 24173–24181. [Google Scholar] [CrossRef]
- Zhang, J.; Dolg, M. Global Optimization of Rigid Molecular Clusters by the Artificial Bee Colony Algorithm. Phys. Chem. Chem. Phys. 2016, 18, 3003–3010. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended tight-binding quantum chemistry methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 11, e1493. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; People, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Zhukovetska, O.; Fizer, M.; Mariychuk, R.; Guzenko, O.; Snigur, D. Spectrophotometric and DFT study of Germanium (IV) interaction with 6,7-dihydroxy-2,4-diphenyl-1-benzopyran-1-ium bromide in solutions: Room-temperature cloud point extraction and its analytical application. J. Mol. Liq. 2024, 401, 124724. [Google Scholar] [CrossRef]
- Riley, K.E.; Tran, K.-A.; Lane, P.; Murray, J.S.; Politzer, P. Comparative analysis of electrostatic potential maxima and minima on molecular surfaces, as determined by three methods and a variety of basis sets. J. Comput. Sci. 2016, 17, 273–284. [Google Scholar] [CrossRef]
- Fizer, M.; Fizer, O. Theoretical study on charge distribution in cetylpyridinium cationic surfactant. J. Mol. Model. 2021, 27, 203. [Google Scholar] [CrossRef]
- Walker, M.; Harvey, A.J.A.; Sen, A.; Dessent, C.E.H. Performance of M06, M06-2X, and M06-HF density functionals for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J. Phys. Chem. A 2013, 117, 12590–12600. [Google Scholar] [CrossRef]
- Fizer, M.; Slivka, M.; Sidey, V.; Baumer, V.; Mariychuk, R. XRD, NMR, FT-IR and DFT structural characterization of a novel organic-inorganic hybrid perovskite-type hexabromotellurate material. J. Mol. Struct. 2021, 1235, 130227. [Google Scholar] [CrossRef]
- Garcia-Rates, M.; Neese, F. Effect of the Solute Cavity on the Solvation Energy and its Derivatives within the Framework of the Gaussian Charge Scheme. J. Comput. Chem. 2020, 41, 922–939. [Google Scholar] [CrossRef]
- Maciel, G.S.; Garcia, E. Charges derived from electrostatic potentials: Exploring dependence on theory and geometry optimization levels for dipole moments. Chem. Phys. Lett. 2005, 409, 29–33. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1338. [Google Scholar] [CrossRef]
- Müller, S.; Nevolianis, T.; Garcia-Ratés, M.; Riplinger, C.; Leonhard, K.; Smirnova, I. Predicting solvation free energies for neutral molecules in any solvent with openCOSMO-RS. Fluid Ph. Equilibria 2025, 589, 114250. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6540442 (accessed on 1 March 2025).
- Zaruba, S.; Vishnikin, A.B.; Andruch, V. A novel vortex-assisted liquid–liquid microextraction approach using auxiliary solvent: Determination of iodide in mineral water samples. Talanta 2016, 149, 110–116. [Google Scholar] [CrossRef]
- Hedjazi, I.M.; Vishnikin, A.B.; Okovytyy, S.I.; Miekh, Y.V.; Bazel, Y.R. Use of dye aggregation phenomenon for spectrophotometric and SIA-LAV determination of bismuth (III) as a specific ion association complex between tetraiodobismuthate and Astra Phloxine. J. Mol. Struct. 2022, 1251, 132015. [Google Scholar] [CrossRef]
- Rasooly, R. PhloxineB, a versatile bacterial stain. FEMS Immunol. Med. Microbiol. 2007, 49, 261–265. [Google Scholar] [CrossRef]
- Bazeľ, Y.; Tóth, J.; Fizer, M.; Sidey, V.; Balogh, I. Estimation of ground and excitedstate dipole moments of three symmetric carbocyanine dyes via the analysis of luminescence properties. J. Mol. Liq. 2021, 337, 116476. [Google Scholar] [CrossRef]
- Kakalejčíková, S.; Bazel, Y.; Fizer, M. Extraction-less green spectrofluorimetric method for determination of mercury using an Astra Phloxine fluorophore: Comprehensive experimental and theoretical studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 310, 123946. [Google Scholar] [CrossRef]
- Shkumbatiuk, R.; Bazel, Y.; Andruch, V.; Török, M. Investigation of 2-[(E)-2-(4-diethylaminophenyl)-1-ethenyl]-1,3,3-trimethyl-3H-indolium as a new highly sensitive reagent for the spectrophotometric determination of nitrophenols. Anal. Bioanal. Chem. 2005, 382, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Slivka, M.; Korol, N.; Fizer, M.; Baumer, V.; Lendel, V. Thiazolo[3,2-b][1,2,4]triazol-7-ium salts: Synthesis, properties and structural studies. Heterocycl. Commun. 2018, 24, 197–203. [Google Scholar] [CrossRef]
- Fizer, M.; Slivka, M.; Baumer, V.; Slivka, M.; Fizer, O. Alkylation of 2-oxo(thioxo)-thieno[2,3-d]pyrimidine-4-ones: Experimental and theoretical study. J. Mol. Struct. 2019, 1198, 126858. [Google Scholar] [CrossRef]
- Yankovych, H.; Bodnár, G.; Elsaesser, M.S.; Fizer, M.; Storozhuk, L.; Kolev, H.; Melnyk, I.; Václavíková, M. Carbon composites for rapid and effective photodegradation of 4-halogenophenols: Characterization, removal performance, and computational studies. J. Photochem. Photobiol. A Chem. 2023, 441, 114753. [Google Scholar] [CrossRef]
- Gerlach, T.; Müller, S.; González de Castilla, A.; Smirnova, I. An Open Source COSMO-RS Implementation and Parameterization Supporting the Efficient Implementation of Multiple Segment Descriptors. Fluid Ph. Equilibria 2022, 560, 113472. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y.; Mariychuk, R. Benchmark of different charges for prediction of the partitioning coefficient through the hydrophilic/lipophilic index. J. Mol. Model. 2018, 24, 141. [Google Scholar] [CrossRef]
- Starek, M.; Plenis, A.; Zagrobelna, M.; Dąbrowska, M. Assessment of Lipophilicity Descriptors of Selected NSAIDs Obtained at Different TLC Stationary Phases. Pharmaceutics 2021, 13, 440. [Google Scholar] [CrossRef]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y. Predicting the end point potential break values: A case of potentiometric titration of lipophilic anions with cetylpyridinium chloride. Microchem. J. 2021, 160, 105758. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]


| System | Linear Range (mg L−1) | LOD (mg L−1) | R2 | RSD (%) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|
| Supramolecular polymer material based on cucurbit[8]uril and 1,3,5-tris[4-(pyridin-4-yl) phenyl] benzene derivative with aggregation-induced emission | 0–36.7 | 0.58 | 0.9951 | 1.2–1.5 | 98.0–102.6 | [22] |
| Photoactivatable carbon dots as a label-free fluorescent probe | 0–34.4 | 10.50 | 0.9900 | — | 93.8–98.2 | [23] |
| Multifunctional B/N-carbon quantum dots | 0–6.9 | 0.41 | 0.9890 | 0.9–1.0 | 99.5–100.7 | [24] |
| Chemosensor based on 1,4-bis((9H-fluoren-9-ylidene)methyl)benzene | 0.05–0.2 | 0.07 | 0.9787 | — | — | [25] |
| Naphthaldehyde-based aggregation induced emission enhancement active “turn-off” fluorescent sensor | 0–0.6 | 0.56 | 0.9875 | — | 99.3–100.8 | [26] |
| Metal–organic frame material encapsulated Rhodamine 6G | 0.2–22.9 | 1.10 | 0.9983 | 1.8–2.7 | 98.2–101.9 | [27] |
| The VALLME-FLD method using AF | 0.92–11.45 µg L−1 | 0.40 µg L−1 | 0.9930 | 1.2–2.3 | 97.3–101.1 | This work |
| PA Added, µg L−1 | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|
| PA Determined, µg L−1 | RSD, % | Recovery, % | PA Determined, µg L−1 | RSD, % | Recovery, % | |
| 0.0 * | N.d. | – | – | – | – | – |
| 3.7 * | 3.5 ± 0.1 | 2.5 | 94.6 | 3.8 ± 0.2 | 4.2 | 102.7 |
| 9.2 * | 8.9 ± 0.4 | 3.7 | 96.7 | 9.6 ± 0.3 | 2.6 | 104.3 |
| 0.0 ** | N.d. | – | – | – | – | – |
| 3.7 ** | 3.6 ± 0.1 | 2.3 | 97.3 | – | – | – |
| 9.2 ** | 9.3 ± 0.1 | 1.2 | 101.1 | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakalejčíková, S.; Harenčár, D.; Bazeľ, Y.; Fizer, M. Fluorescence-Based Detection of Picric Acid Using Vortex-Assisted Liquid–Liquid Microextraction: An Innovative Analytical Approach. Processes 2025, 13, 1051. https://doi.org/10.3390/pr13041051
Kakalejčíková S, Harenčár D, Bazeľ Y, Fizer M. Fluorescence-Based Detection of Picric Acid Using Vortex-Assisted Liquid–Liquid Microextraction: An Innovative Analytical Approach. Processes. 2025; 13(4):1051. https://doi.org/10.3390/pr13041051
Chicago/Turabian StyleKakalejčíková, Sofia, Dominik Harenčár, Yaroslav Bazeľ, and Maksym Fizer. 2025. "Fluorescence-Based Detection of Picric Acid Using Vortex-Assisted Liquid–Liquid Microextraction: An Innovative Analytical Approach" Processes 13, no. 4: 1051. https://doi.org/10.3390/pr13041051
APA StyleKakalejčíková, S., Harenčár, D., Bazeľ, Y., & Fizer, M. (2025). Fluorescence-Based Detection of Picric Acid Using Vortex-Assisted Liquid–Liquid Microextraction: An Innovative Analytical Approach. Processes, 13(4), 1051. https://doi.org/10.3390/pr13041051






