The Methane Adsorption Ability of Lacustrine Shale and Its Controlling Factors: A Case Study of Shale from the Jurassic Lianggaoshan Formation in the Sichuan Basin
Abstract
:1. Introduction
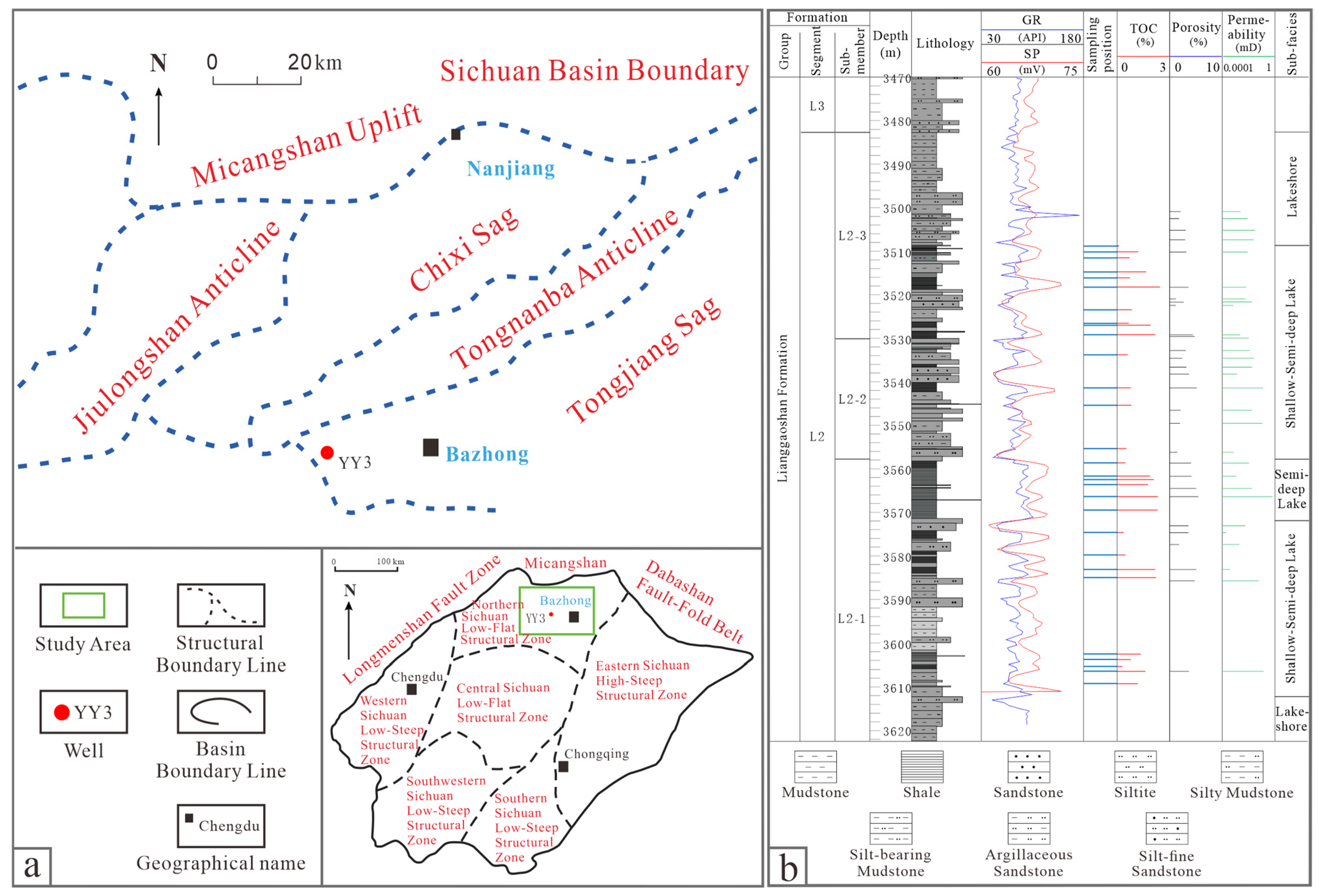
2. Geological Setting
3. Materials and Methods
3.1. Samples
3.2. TOC
3.3. Thermal Maturity and Type of Organic Matter
3.4. Mineral Composition
3.5. Bulk Porosity
3.6. SEM
3.7. High Methane Adsorption
4. Results
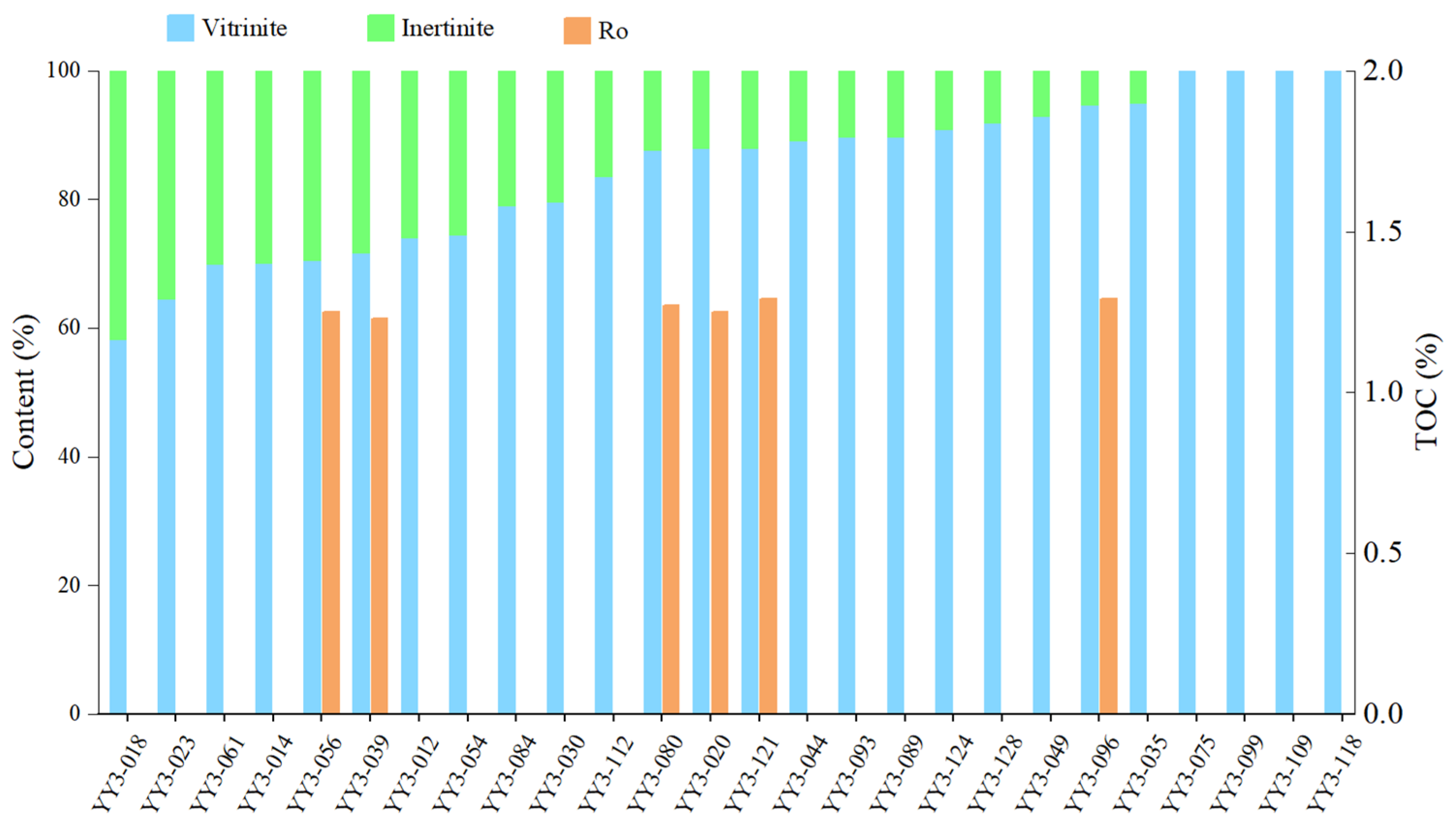
4.1. TOC Content and Organic Petrology
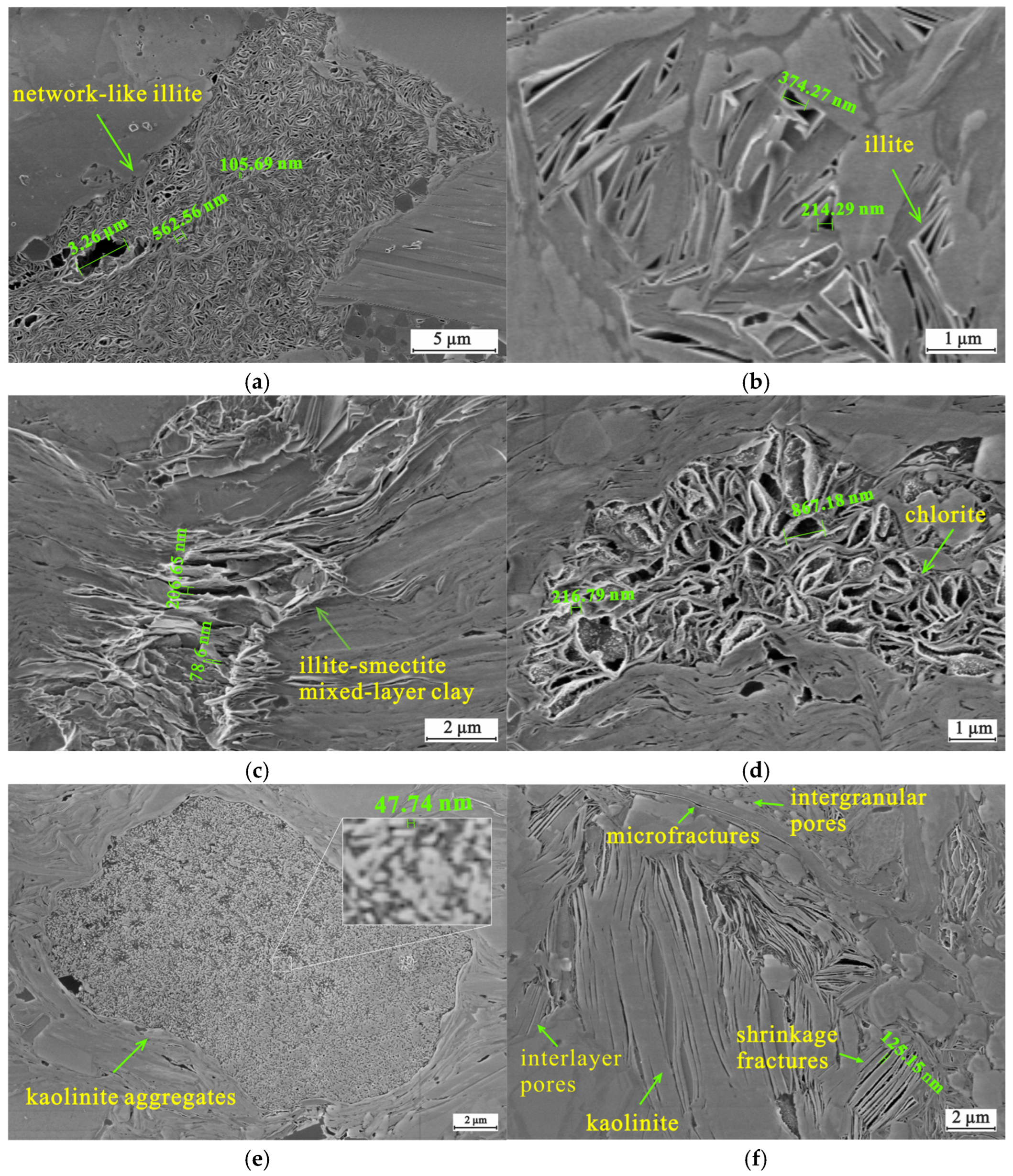
4.2. Inorganic Petrology
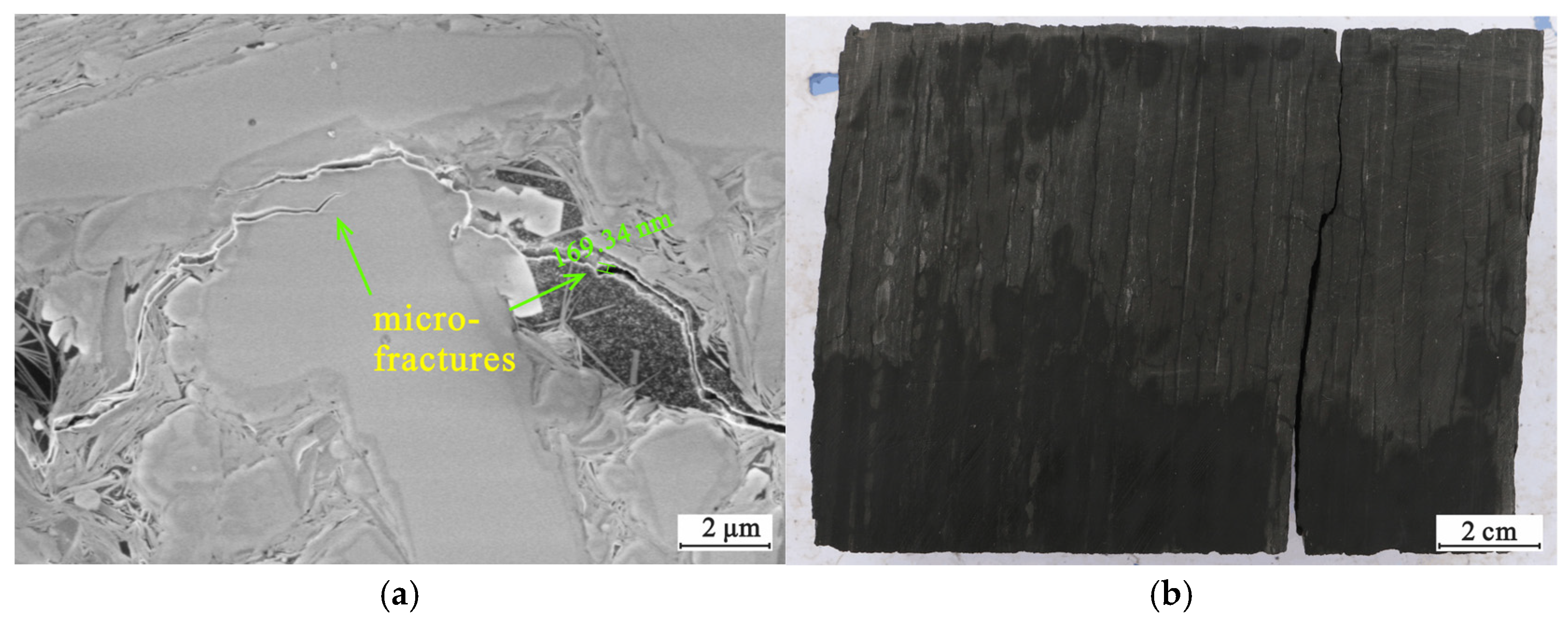
4.3. Reservoir Characteristics
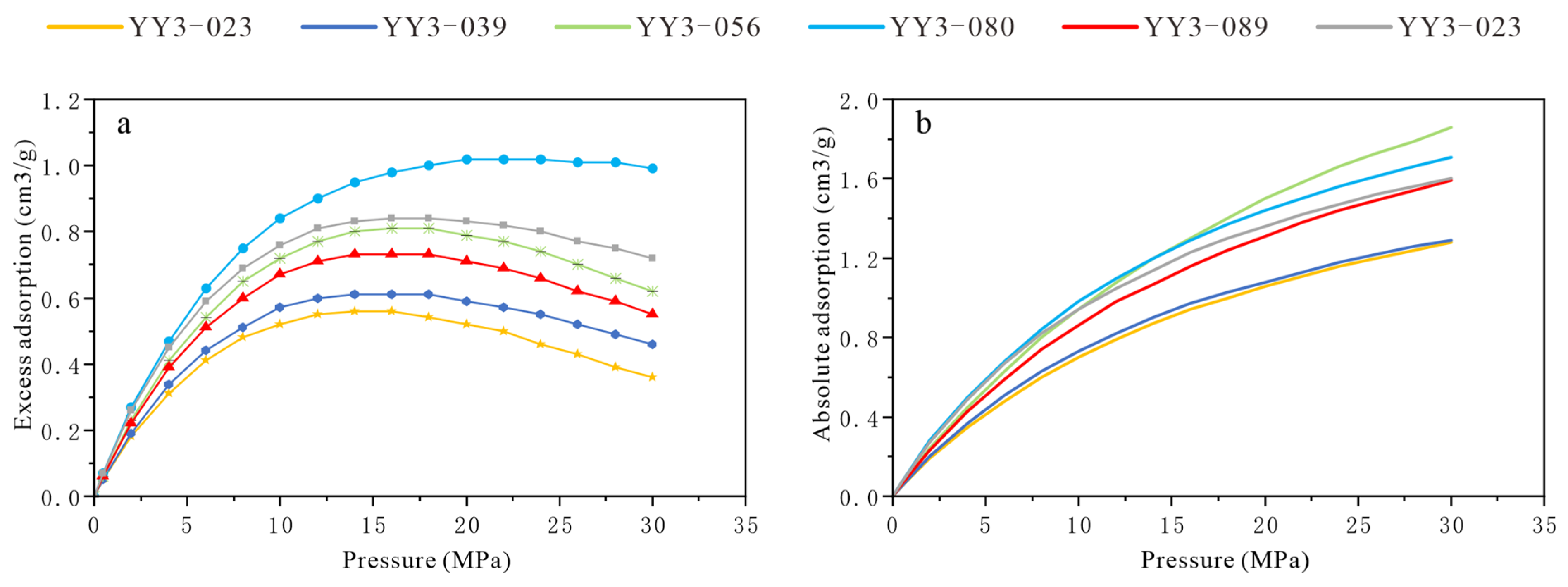
4.4. Methane Adsorption Ability
5. Discussion
5.1. Lacustrine and Marine Shale Pore Type Differences
5.2. The Effect of Inorganic Minerals on Adsorption Capacity
5.3. The Effect of TOC Content on Adsorption Capacity
5.4. Implications for Oil and Gas Exploration in Lianggaoshan Formation Shale
6. Conclusions
- (1)
- The lacustrine shale of the Lianggaoshan Formation has a moderate organic matter content, with organic types ranging from II to III, and maturation levels within the oil window. The mineral components are dominated by clay minerals, among which illite/smectite mixed layers have the highest proportion, followed by illite, chlorite and kaolinite.
- (2)
- The pore types in the lacustrine shale reservoir of the Lianggaoshan Formation mainly include clay mineral-related pores, feldspar and carbonate dissolution pores, organic matter pores, pyrite intercrystalline pores and micro-fractures. Among these, clay mineral-related pores are the most well developed.
- (3)
- The adsorption capacity of lacustrine shale from the Lianggaoshan Formation is primarily influenced by clay mineral content. A high clay mineral content significantly improves the pore structure of the reservoir, compensating for the limitations posed by moderate-to-low TOC in shale exploration and development. This highlights the exploration potential of shale deposits in semi-deep lacustrine environments.
- (4)
- Although lacustrine shale is less dependent on TOC, future research may still need to explore the synergistic or competitive effects between clay minerals and organic matter in the mixed system, and we urge the development of a comprehensive evaluation framework combining clay mineralogy, porosity, fracture density and geochemical data. The previously neglected lacustrine basins may occupy a dominant position in future oil and gas exploration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Chen, Z.; Liu, X.; Yin, X.; Shi, M.; Chen, Q.; Zhou, J. Key technologies for predicting sweet spots of Jurassic Lianggaoshan Formation continental shale oil and gas in the Fuxing area of the Sichuan Basin. Nat. Gas Ind. 2025, 45, 94–104. [Google Scholar]
- Luo, Q.; Goodarzi, F.; Zhong, N.; Qiu, N.; Wang, X.; Suchý, V.; Khan, I.; Zheng, X.; Liu, B.; Ardakani, O.H.; et al. Dispersed organic matter from pre-Devonian marine shales: A review on its composition, origin, evolution, and potential for hydrocarbon prospecting. Earth-Sci. Rev. 2025, 261, 105027. [Google Scholar]
- Li, P.; Liu, Z.-B.; Bi, H.; Jiang, T.; Bian, R.-K.; Wang, P.-W.; Shang, X.-Y. Differences in and factors controlling organic matter enrichment in the Ziliujing Formation shale in the Sichuan Basin. Pet. Sci. 2024, 21, 77–86. [Google Scholar] [CrossRef]
- Teng, J.; Liu, B.; Mastalerz, M.; Schieber, J. Origin of Organic Matter and Organic Pores in the Overmature Ordovician-Silurian Wufeng-Longmaxi Shale of the Sichuan Basin, China. Int. J. Coal Geol. 2022, 253, 103970. [Google Scholar]
- Abelly, E.N.; Yang, F.; Ngata, M.R.; Mwakipunda, G.C.; Shanghvi, E.R. A Field Study of Pore-Network Systems on the Tight Shale Gas Formation through Adsorption-Desorption Technique and Mercury Intrusion Capillary Porosimeter: Percolation Theory and Simulations. Energy 2024, 302, 131771. [Google Scholar]
- Ukaomah, C.F.; Sun, M.; Pan, Z.; Ostadhassan, M.; Liu, B.; Meng, Q.; Aminu, M.D.; Fischer, M. Experimental and Molecular Investigation of Water Adsorption Controls in Marine and Lacustrine Shale Reservoirs. J. Hydrol. 2023, 621, 129672. [Google Scholar]
- Li, X.; Jiang, Z.; Wang, S.; Wu, F.; Miao, Y.; Wang, X.; Wang, H.; Liu, X. Differences of Marine and Transitional Shales in the Case of Dominant Pore Types and Exploration Strategies, in China. J. Nat. Gas Sci. Eng. 2022, 103, 104628. [Google Scholar]
- Ali, A.M.; Kwaya, M.Y.; Mijinyawa, A.; Aminu, A.A.; Usman, Z.M. Molecular Dynamics and Energy Distribution of Methane Gas Adsorption in Shales. J. Nat. Gas Geosci. 2023, 8, 1–15. [Google Scholar]
- Yang, S.; Zhao, C.; Ji, B.; He, Y. Adsorption Isotherm Calculation and Mechanism of High Pressure and High Temperature Shale Gases. Fuel 2023, 331, 125854. [Google Scholar]
- Mudoi, M.P.; Sharma, P.; Khichi, A.S. A Review of Gas Adsorption on Shale and the Influencing Factors of CH4 and CO2 Adsorption. J. Pet. Sci. Eng. 2022, 217, 110897. [Google Scholar]
- Liu, B.; Mohammadi, M.-R.; Ma, Z.; Bai, L.; Wang, L.; Wen, Z.; Liu, Y.; Morta, H.B.; Hemmati-Sarapardeh, A.; Ostadhassan, M. Experimental Investigation and Intelligent Modeling of Pore Structure Changes in Type III Kerogen-Rich Shale Artificially Matured by Hydrous and Anhydrous Pyrolysis. Energy 2023, 282, 128799. [Google Scholar]
- Aruah, B.; Sakhaee-Pour, A.; Hatzignatiou, D.G. Alteration of Pore Structure and Effects on Critical Properties of Shale Formations Undergone Cryogenic Thermal Shock. Geoenergy Sci. Eng. 2024, 240, 213002. [Google Scholar] [CrossRef]
- Mishra, V.K.; Mendhe, V.A.; Kamble, A.D.; Pandey, S.; Singh, V.P.; Shukla, P. Significance of Organo-Mineralogical Constituents on Pore Distribution, Fractals and Gas Sorption Mechanism of Permian Shale Beds in Korba Sub-Basin of the Son-Mahanadi Valley, India. Geoenergy Sci. Eng. 2023, 231, 212334. [Google Scholar]
- Hazarika, S.; Boruah, A.; Kumar, H. Study of Pore Structure of Shale Formation for CO2 Storage. Mater. Today Proc. 2024, 99, 138–144. [Google Scholar]
- Ren, Y.-J.; Wei, B.; Ji, B.-X.; Pu, W.-F.; Wang, D.-L.; Tang, J.-Y.; Lu, J. Pore-Scale Probing CO2 Huff-n-Puff in Extracting Shale Oil from Different Types of Pores Using Online T1–T2 Nuclear Magnetic Resonance Spectroscopy. Pet. Sci. 2024, 21, 4119–4129. [Google Scholar]
- Zhang, N.; Wang, X.; Wang, S.; Wang, R.; Wu, J.; Li, Z.; Song, Y. Multifractal characteristics on pore structure of Longmaxi shale using nuclear magnetic resonance (NMR). Geoenergy Sci. Eng. 2024, 241, 213176. [Google Scholar]
- Zheng, H.; Yang, F.; Guo, Q.; Liu, K. Upscaling characterizing pore connectivity, morphology and orientation of shale from nano-scale to micro-scale. Mar. Pet. Geol. 2025, 172, 107213. [Google Scholar]
- Jiang, W.; Lin, M.; Luo, C.; Chen, Z.; Cao, G.; Ji, L.; Dou, W.; Zhong, K.; Hao, F. Optimal selection of inversion method for gas-adsorption pore characterization of shales in Wufeng and Longmaxi Formation, Sichuan Basin. Chem. Eng. J. 2024, 485, 149889. [Google Scholar]
- Taghavinejad, A.; Rabbani, A.; Falcone, G.; Shang, J.; Arif, M.; Zhang, Y. Pore Network Modelling of CO2-Shale Interaction for Carbon Storage: Swelling Effect and Fracture Permeability. Int. J. Greenh. Gas Control 2025, 141, 104294. [Google Scholar]
- Hui, G.; Chen, Z.; Wang, Y.; Zhang, D.; Gu, F. An Integrated Machine Learning-Based Approach to Identifying Controlling Factors of Unconventional Shale Productivity. Energy 2023, 266, 126512. [Google Scholar]
- Liu, B.; Mohammadi, M.-R.; Ma, Z.; Bai, L.; Wang, L.; Xu, Y.; Hemmati-Sarapardeh, A.; Ostadhassan, M. Pore structure characterization of solvent extracted shale containing kerogen type III during artificial maturation: Experiments and tree-based machine learning modeling. Energy 2023, 283, 128885. [Google Scholar]
- Hui, S. Quantifying the Relative Contribution and Evolution of Pore Types to Shale Reservoir Space: Constraints from over-Mature Marine Shale in the Sichuan Basin, SW China. J. Asian Earth Sci. 2023, 249, 105625. [Google Scholar] [CrossRef]
- Dong, Z.; Tian, S.; Xue, H.; Lu, S.; Liu, B.; Erastova, V.; Chen, G.; Zhang, Y. A Novel Method for Automatic Quantification of Different Pore Types in Shale Based on SEM-EDS Calibration. Mar. Pet. Geol. 2025, 173, 107278. [Google Scholar]
- Zhang, W.; Zhou, S.; Yu, Z.; Liu, X.; Wang, S.; Miao, H.; Liu, D.; Tian, J.; Wang, H. Controls of Clay Mineral Transformation and Organic Matter on Pore Networks of the Paleogene Lacustrine Shale Oil System in the Yitong Basin, NE China. J. Asian Earth Sci. 2025, 280, 106469. [Google Scholar]
- Hou, Y.; He, S.; Yi, J.; Zhang, B.; Chen, X.; Wang, Y.; Zhang, J.; Cheng, C. Effect of pore structure on methane sorption capacity of shales. Pet. Explor. Dev. 2014, 41, 248–256. [Google Scholar]
- Borrok, D.M.; Yang, W.; Wei, M.; Mokhtari, M. Heterogeneity of the Mineralogy and Organic Content of the Tuscaloosa Marine Shale. Mar. Pet. Geol. 2019, 109, 717–731. [Google Scholar]
- Lohr, C.D.; Merrill, M.D. Comparison of Measured versus Modeled TOC in the Tuscaloosa Marine Shale of Southwestern Mississippi, U.S.A. Mar. Pet. Geol. 2024, 164, 106655. [Google Scholar] [CrossRef]
- Galoski, C.E.; Froehner, S.; Jiménez Martínez, A.E.; Aquino, C.D.; França, A.; Borillo, G.C. Insights into Organic Matter Source and Depositional Environments of Shales from the Ponta Grossa Formation in Parana Basin, Brazil. J. S. Am. Earth Sci. 2024, 143, 104995. [Google Scholar]
- Chalmers, G.R.L.; Bustin, R.M. The Organic Matter Distribution and Methane Capacity of the Lower Cretaceous Strata of Northeastern British Columbia, Canada. Int. J. Coal Geol. 2007, 70, 223–239. [Google Scholar]
- Gao, P.; Xiao, X.; Hu, D.; Lash, G.G.; Liu, R.; Cai, Y.; Wang, Z.; Zhang, B.; Yuan, T.; Liu, S. Effect of Silica Diagenesis on Porosity Evolution of Deep Gas Shale Reservoir of the Lower Paleozoic Wufeng-Longmaxi Formations, Sichuan Basin. Mar. Pet. Geol. 2022, 145, 105873. [Google Scholar]
- Ross, D.J.K.; Bustin, R.M. The Importance of Shale Composition and Pore Structure upon Gas Storage Potential of Shale Gas Reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar]
- Hu, C.; Tan, J.; Lyu, Q.; Zhang, Y. Evolution of Organic Pores in Permian Low Maturity Shales from the Dalong Formation in the Sichuan Basin: Insights from a Thermal Simulation Experiment. Gas Sci. Eng. 2024, 121, 205166. [Google Scholar]
- Wang, Q. Effects of Clay Mineral Characteristics on Shale Adsorbed Gas: Based on the Experimental Analysis of Shale Samples in Longmaxi Formation of Dingshan Area, Southeast Sichuan. J. Chongqing Univ. Sci. Technol. Sci. Ed. 2019, 21, 20–24. [Google Scholar]
- Heller, R.; Zoback, M. Adsorption of Methane and Carbon Dioxide on Gas Shale and Pure Mineral Samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar]
- Wang, A.; Zhong, D.; Wang, W.; Zhou, Z.; Tang, Z. Controls of Multi-stage Tectonic Movement on Sandstones with Different Provenances. J. Southwest Pet. Univ. (Sci. Technol. Ed.) 2022, 44, 27–40. [Google Scholar]
- Wei, X.; Huang, J.; Li, Y.; Wang, Q.; Liu, R.; Wen, Z. The main controlling factors of continental shale gas enrichment and high yield in the Da’anzhai section of Yuanba area. Geol. China 2014, 41, 970–981. [Google Scholar]
- Xi, Y.; Wen, Z.; Zhao, W.; Zhang, L.; Zhang, H.; Sun, L.; Wu, C.; Song, H. Study on geological characteristics and enrichment law of shale gas of Carboniferous Benxi Formation in eastern Ordos Basin. Nat. Gas Geosci. 2022, 33, 1936–1950. [Google Scholar]
- Xin, L.; Li, H.; Wang, X.; Zhang, Z.; Hou, S.; He, S. Comparative study of shale reservoir characteristics between the Longmaxi Formation and the Da’anzhai Member in the Sichuan Basin: Highlighting facies differences between marine and lacustrine depositional environments. Chin. J. Geol. 2024, 59, 1003–1017. [Google Scholar]
- Chen, L.; Dong, J.; Hu, Y.; Ji, Y.; Ren, G. Mechanism of Organic Matter Enrichment in the Wufeng Formation–Longmaxi Formation Shale in the Upper Yangtze Platform. J. Southwest Pet. Univ. Technol. Ed. 2024, 46, 66–79. [Google Scholar]
- Miao, F.; Peng, Z.; Wang, C.; Yue, Y.; Wang, Z. Gas-Bearing Capacity and Controlling Factors of Niutitang Formation Shale in Well XZD-1, Western Margin of Xuefeng Uplift. Earth Sci. 2019, 44, 3662. [Google Scholar] [CrossRef]
- Shabani, M.; Moallemi, S.A.; Krooss, B.M.; Amann-Hildenbrand, A.; Zamani-Pozveh, Z.; Ghalavand, H.; Littke, R. Methane Sorption and Storage Characteristics of Organic-Rich Carbonaceous Rocks, Lurestan Province, Southwest Iran. Int. J. Coal Geol. 2018, 186, 51–64. [Google Scholar]
- Feng, Y.; Xiao, X.-M.; Wang, E.-Z.; Gao, P.; Lu, C.-G.; Li, G. Gas Storage in Shale Pore System: A Review of the Mechanism, Control and Assessment. Pet. Sci. 2023, 20, 2605–2636. [Google Scholar]
- Ghasemzadeh, H.; Babaei, S.; Tesson, S.; Azamat, J.; Ostadhassan, M. From Excess to Absolute Adsorption Isotherm: The Effect of the Adsorbed Density. Chem. Eng. J. 2021, 425, 131495. [Google Scholar]
- Rugarabamu, J.R. Modelling of Physical and Chemical Properties of Activated Carbons Which Affect Methane Adsorption Mechanisms. Pet. Res. 2025, 10, 188–203. [Google Scholar]
- de Araujo, I.S.; Jagadisan, A.; Heidari, Z. Impacts of Kerogen Type and Thermal Maturity on Methane and Water Adsorption Isotherms: A Molecular Simulation Approach. Fuel 2023, 352, 128944. [Google Scholar]
- Wei, S.; He, S.; Pan, Z.; Zhai, G.; Dong, T.; Guo, X.; Yang, R.; Han, Y.; Yang, W. Characteristics and evolution of pyrobitumen-hosted pores of the overmature Lower Cambrian Shuijingtuo Shale in the south of Huangling anticline, Yichang area, China: Evidence from FE-SEM petrography. Mar. Pet. Geol. 2020, 116, 104303. [Google Scholar]
- Zheng, X.-W.; Schovsbo, N.-H.; Bian, L.-B.; Rudra, A.; Sanei, H. Organic Geochemical and Petrographic Characteristics of the Cambrian-Ordovician Organic-Rich Marine Shales in Scandinavia. Pet. Sci. 2023, 20, 2637–2647. [Google Scholar]
- Atwah, I.; Adeboye, O.O.; Zhang, J.; Wilcoxson, R.; Marcantonio, F. Linking Biomarkers with Elemental Geochemistry to Reveal Controls on Organic Richness in Devonian-Mississippian Mudrocks of Oklahoma. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 611, 111355. [Google Scholar]
- Teng, J.; Mastalerz, M.; Liu, B. Petrographic and Chemical Structure Characteristics of Amorphous Organic Matter in Marine Black Shales: Insights from Pennsylvanian and Devonian Black Shales in the Illinois Basin. Int. J. Coal Geol. 2021, 235, 103676. [Google Scholar]
- Wu, Z.; Grohmann, S.; Littke, R. Geochemistry and Petrology of Early Permian Lacustrine Shales in the Lodève Basin, Southern France: Depositional History, Organic Matter Accumulation and Thermal Maturity. Int. J. Coal Geol. 2024, 284, 104469. [Google Scholar]
- Wei, S.; Hu, M.; He, S.; Shu, Y.; Dong, T.; He, Q.; Yang, W.; Cai, Q. Effects of Quartz Precipitation on the Abundance and Preservation of Organic Matter Pores in Cambrian Marine Shale in South China. JMSE 2023, 11, 1267. [Google Scholar]
- Zhang, Y.; Zuo, S.; Li, Y.; Huang, Y.; Mo, Y. Analysis on Pore Evolution and Microscopic Mechanism of Red Clay during Loss Water and Shrinkage. Chin. J. Undergr. Space Eng. 2022, 18, 1882–1890. [Google Scholar]
- Zhou, J.; Fan, M.; Xian, X.; Yang, K.; Lu, Z. Correction calculation of shale gas absolute adsorption capacity and its influencing factors analysis. Coal Sci. Technol. 2022, 50, 154–162. [Google Scholar]
- Li, A.; Ding, W.; Zhou, X.; Cao, X.; Zhang, M.; Fu, F.; Chen, E. Investigation of the Methane Adsorption Characteristics of Marine Shale: A Case Study of Lower Cambrian Qiongzhusi Shale in Eastern Yunnan Province, South China. Energy Fuels 2017, 31, 2625–2635. [Google Scholar]
- Tan, J.; Weniger, P.; Krooss, B.; Merkel, A.; Horsfield, B.; Zhang, J.; Boreham, C.J.; Graas, G.; Tocher, B.A. Shale gas potential of the major marine shale formations in the upper yangtze platform, south China, part II: Methane sorption capacity. Fuel 2014, 129, 204–218. [Google Scholar]
- Rexer, T.F.; Mathia, E.J.; Aplin, A.C.; Thomas, K.M. High-Pressure Methane Adsorption and Characterization of Pores in Posidonia Shales and Isolated Kerogens. Energy Fuels 2014, 28, 2886–2901. [Google Scholar]






| Sample ID | TOC (%) | Mineral Composition (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Quartz | Feldspar | Calcite | Dolomite | Pyrite | Siderite | Clay Minerals | ||
| YY3-005 | 0.23 | 35.30 | 4.40 | 0.20 | 0.50 | 0.40 | 0.80 | 55.40 |
| YY3-010 | 0.10 | 50.20 | 25.30 | 3.40 | 0.60 | 0.10 | 0.20 | 19.40 |
| YY3-012 | 1.20 | 28.00 | 3.00 | 2.80 | 0.70 | 3.30 | 3.10 | 57.60 |
| YY3-014 | 0.71 | 33.70 | 22.40 | 0.20 | 0.00 | 0.30 | 0.30 | 42.20 |
| YY3-018 | 1.66 | 29.60 | 2.60 | 9.00 | 0.20 | 2.10 | 0.80 | 55.00 |
| YY3-020 | 0.71 | 33.50 | 4.70 | 0.90 | 1.00 | 1.80 | 1.00 | 56.20 |
| YY3-023 | 2.45 | 34.10 | 3.30 | 1.50 | 0.50 | 4.20 | 1.80 | 53.60 |
| YY3-030 | 0.83 | 42.80 | 10.20 | 1.80 | 0.10 | 0.60 | 1.00 | 42.60 |
| YY3-035 | 0.66 | 40.80 | 4.90 | 4.50 | 0.60 | 0.10 | 0.40 | 48.00 |
| YY3-036 | 1.92 | 31.90 | 3.70 | 0.40 | 0.10 | 1.10 | 0.90 | 60.90 |
| YY3-039 | 2.18 | 31.10 | 2.50 | 3.70 | 0.10 | 0.30 | 1.70 | 56.70 |
| YY3-046 | 0.59 | 35.50 | 5.90 | 0.10 | 1.50 | 0.20 | 0.80 | 55.00 |
| YY3-056 | 0.77 | 30.50 | 3.50 | 0.50 | 0.20 | 0.30 | 1.00 | 60.50 |
| YY3-061 | 0.81 | 40.00 | 17.90 | 0.50 | 0.60 | 0.20 | 1.00 | 39.00 |
| YY3-071 | 0.52 | 36.10 | 5.70 | 0.20 | 1.00 | 0.20 | 1.10 | 53.30 |
| YY3-075 | 0.46 | 32.60 | 4.20 | 0.20 | 0.10 | 0.20 | 0.50 | 59.30 |
| YY3-080 | 1.87 | 33.70 | 4.70 | 0.20 | 0.10 | 1.20 | 1.60 | 57.00 |
| YY3-081 | 2.09 | 35.30 | 4.90 | 8.00 | 0.40 | 2.60 | 1.50 | 46.30 |
| YY3-084 | 1.78 | 29.80 | 4.10 | 0.20 | 0.10 | 2.60 | 1.40 | 57.90 |
| YY3-089 | 2.33 | 36.40 | 4.90 | 0.40 | 0.10 | 2.00 | 1.20 | 51.20 |
| YY3-093 | 2.32 | 28.20 | 2.70 | 0.30 | 1.30 | 1.60 | 0.30 | 61.50 |
| YY3-099 | 0.36 | 34.30 | 7.20 | 1.00 | 0.10 | 0.10 | 0.60 | 54.50 |
| YY3-106 | 0.47 | 41.30 | 9.80 | 0.60 | 1.80 | 0.30 | 1.00 | 44.00 |
| YY3-112 | 2.18 | 27.90 | 3.10 | 9.70 | 0.20 | 2.30 | 0.90 | 54.60 |
| YY3-115 | 2.22 | 32.70 | 3.30 | 0.20 | 1.10 | 0.40 | 1.00 | 60.40 |
| YY3-118 | 1.35 | 40.80 | 4.50 | 7.80 | 0.10 | 0.70 | 0.50 | 44.60 |
| YY3-121 | 0.78 | 34.40 | 2.70 | 0.60 | 0.20 | 1.40 | 6.50 | 51.30 |
| YY3-123 | 0.29 | 60.90 | 6.80 | 9.90 | 0.20 | 0.50 | 0.70 | 20.50 |
| YY3-124 | 1.61 | 42.30 | 3.60 | 4.80 | 0.40 | 0.80 | 0.60 | 44.70 |
| YY3-128 | 1.19 | 48.40 | 3.70 | 2.40 | 0.30 | 0.60 | 0.50 | 41.50 |
| Sample ID | Depth (m) | Langmuir Volume (m3/t) | Langmuir Pressure (MPa) |
|---|---|---|---|
| YY3-023 | 3518.26 | 2.18 | 21.08 |
| YY3-039 | 3529.2 | 2.09 | 18.68 |
| YY3-056 | 3541.42 | 3.59 | 27.95 |
| YY3-080 | 3561.7 | 2.72 | 17.72 |
| YY3-089 | 3566.4 | 2.72 | 21.44 |
| YY3-112 | 3583.15 | 2.46 | 16.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, P.; Zhang, D.; Hu, M.; Yang, G.; Wei, S.; Zeng, F. The Methane Adsorption Ability of Lacustrine Shale and Its Controlling Factors: A Case Study of Shale from the Jurassic Lianggaoshan Formation in the Sichuan Basin. Processes 2025, 13, 1061. https://doi.org/10.3390/pr13041061
Fu P, Zhang D, Hu M, Yang G, Wei S, Zeng F. The Methane Adsorption Ability of Lacustrine Shale and Its Controlling Factors: A Case Study of Shale from the Jurassic Lianggaoshan Formation in the Sichuan Basin. Processes. 2025; 13(4):1061. https://doi.org/10.3390/pr13041061
Chicago/Turabian StyleFu, Pei, Dazhi Zhang, Mingyi Hu, Gang Yang, Sile Wei, and Fan Zeng. 2025. "The Methane Adsorption Ability of Lacustrine Shale and Its Controlling Factors: A Case Study of Shale from the Jurassic Lianggaoshan Formation in the Sichuan Basin" Processes 13, no. 4: 1061. https://doi.org/10.3390/pr13041061
APA StyleFu, P., Zhang, D., Hu, M., Yang, G., Wei, S., & Zeng, F. (2025). The Methane Adsorption Ability of Lacustrine Shale and Its Controlling Factors: A Case Study of Shale from the Jurassic Lianggaoshan Formation in the Sichuan Basin. Processes, 13(4), 1061. https://doi.org/10.3390/pr13041061






