Abstract
The conventional pyrolysis of tar-rich coals faces limitations in maximizing tar yield and optimizing tar composition, often resulting in inefficient resource utilization and elevated emissions of CO2. This study investigates a novel cryogenic pretreatment method using liquid nitrogen to enhance pyrolysis efficiency, aiming to improve tar yield and transform tar quality for sustainable coal utilization. Three tar-rich coals underwent cryogenic pretreatment at varying temperatures (0 to −90 °C) via liquid nitrogen, followed by pyrolysis. The product distribution (tar, gas) and quality were analyzed and compared to conventional pyrolysis and the Gray–King assay. The cryogenic pretreatment increased the tar yield by 25.8–44.6% compared to conventional methods, achieving a maximum yield of 7.8–16.0 wt% at −90 °C. The emissions of CO2 decreased by 12.7–27.4%, while CH4 and H2 proportions rose by 15.1–60.2%, enhancing gas energy content. The pretreatment reduced benzene compounds by 4.4–13.9 wt% and increased aromatic derivatives by 13.9–20.5 wt%, indicating a shift toward higher-value chemicals. The cryogenic approach demonstrates the dual benefits of boosting tar productivity while reducing carbon emissions, offering a promising path for cleaner and more efficient coal pyrolysis.
1. Introduction
Efficient and clean coal conversion is important for carbon reduction and energy security [1,2]. It reduces reliance on other energy sources, reduces pollutants and greenhouse gas emissions, promotes economic development, improves energy utilization efficiency, reduces costs, and enhances a country’s energy competitiveness [3,4].
Tar-rich coal refers to coal with a tar yield of 7–12 wt% under low-temperature carbonization, typically classified as medium- to low-rank coal, and is commonly found in lignite [5]. It benefits domestic oil and gas supply and improves the high-carbon properties of coal. Coal pyrolysis is an effective technology for converting tar-rich coal; it offers noteworthy benefits in terms of product versatility, ecological compatibility, and energy effectiveness. Nevertheless, it faces challenges like low yields, poor product quality, and equipment blockages [6,7,8,9].
Pretreatment techniques have been employed to modify the composition and characteristics of coal with the aim of enhancing the distribution and quality of pyrolysis products. Common pretreatment methods encompass physical, chemical, and thermal treatments. Physical pretreatment is preferred due to its operational simplicity and cost-effectiveness, leading to improved pyrolysis efficiency via increased coal surface area [10]. He et al. [11] studied the activity of inherent minerals (K, Na, Ca, Mg) in coal with an Fe catalyst. The results show that the Ca-Fe compound in raw coal promotes the formation of H2, and the Mg-Fe compound promotes the formation of liquid. However, it does not sufficiently remove minerals and certain organics. Chemical pretreatment effectively eliminates these substances and significantly enhances pyrolysis behavior such as tar yield [10,12]. Liang et al. [13] studied the effect of ionic liquid pretreatment on pyrolysis characteristics in a fixed-bed reactor. The results showed that the cross-linking bonds between coal macromolecular structures were destroyed after ionic liquid pretreatment, the distribution of pyrolysis products changed significantly, and the yield of the oil phase increased significantly. However, this approach is associated with secondary pollution risks and higher costs. Thermal pretreatment modifies the chemical structure of coal, leading to increased tar yield and an optimized pyrolysis environment, as exemplified in the hydrothermal treatment study by Bai et al. [14]. Nonetheless, the high thermal demand of this method results in considerable energy consumption. Other pretreatment strategies like biological treatment and mechanochemical techniques hold promise for enhancing coal permeability and pyrolysis characteristics. Despite their potential benefits, these methods still face challenges in terms of technological complexity and cost, necessitating further improvement.
Cryogenic treatment has demonstrated the potential to modify material characteristics and improve their properties, making it a relevant technique in aviation, aerospace, and materials science [15,16]. In the coal field, cryogenic technology is primarily utilized for the purpose of thermal fracturing and mining safety. This involves the injection of liquid nitrogen, which generates a thermal impact on the surface of the rocks, resulting in the efficient extraction of coal bed gas [17]. Additionally, cryogenic technology is also employed in the prevention and control of coal mine fires. Zhou et al. [18] proposed an instant liquid nitrogen injection firefighting method, and on-site testing verified its effectiveness. Within a brief period of 12 days, the coal mine was successfully unsealed, facilitating the swift and efficient management of the fire-affected area. Nonetheless, insufficient exploration exists in the field of coal conversion, particularly in the application of coal gasification. Currently, there is little research on cryogenic pretreatment for tar-rich coal before pyrolysis. However, according to our previous study [19], cryogenic pretreatment enhances the thermal diffusivity of tar-rich coal, thereby improving its internal heat transfer capacity during the pyrolysis process.
This study endeavors to elucidate the ramifications of cryogenic pretreatment on the production of coal tar. Furthermore, it endeavors to ascertain the fundamental mechanisms underpinning the genesis of coal tar products subjected to cryogenic pretreatment. Primarily, this investigation homes in on the meticulous analysis of the influence wielded by cryogenic temperatures on the coking propensities of coal, encompassing the intricate interplay of product distribution and composition.
The novelty of this study lies in the examination of an innovative pyrolysis technique that integrates cryogenic pretreatment to address challenges related to suboptimal product yields and poor quality encountered during coal pyrolysis. This study primarily compares the yields and distribution of key products obtained from tar-rich coal after cryogenic pretreatment. This research is significant as it introduces a pioneering and effective approach to the production of liquid fuels derived from coal.
2. Experimental Methodology
2.1. Coal Samples
The experimental coal samples were sourced from underground coal seams at the Xiaobaodang (XBD) coal mine, Yongshun (YS) coal mine, and Hecaogou (HCG) coal mine in China. Proximate analysis of the coal samples was carried out using a moisture analyzer and a muffle furnace, according to the international standard (ISO 1171/562:2010) [5]. The Vario EL III elemental analyzer was used to quantitatively analyze the C, H, and N elements in the coal samples, ICP-OES was used to quantitatively analyze the S element in coal, and the O element was determined by the difference method. Coal tar yield was determined by the Gray–King method, based on the international standard (ISO 502:2015) [5]. The relevant data can be found in Table 1.

Table 1.
Proximate analysis, ultimate analysis, and tar yield of coal samples.
Table 1 displays that the XBD sample possesses the highest fixed carbon content, measured at 53.05%. The volatile matter content in the samples varies slightly, ranging from 41.25% to 43.62%. According to ISO 11760 [5], all samples fall into the medium-rank coal category. This moderate range indicates a relatively similar degree of metamorphism among the three coal samples. The tar yield of the three coals ranges from 7.20 to 11.32 wt%, all within the 7–12 wt% range, indicating that they are tar-rich coals.
The air-dried coal samples underwent initial pulverization using a crusher, followed by sieving through a mesh to achieve particles smaller than 200 mesh (74 μm). Subsequently, the crushed samples were dried in a constant-temperature oven at 105 °C for 12 h. This process yielded fully dried coal samples with a moisture content of less than 0.5 wt%, which were then employed for the experimental procedures.
2.2. Pretreatment Method
The experimental setup included cryogenic pretreatment using liquid nitrogen, with real-time temperature monitoring facilitated by a T-type thermocouple. Initially, a substantial quantity of liquid nitrogen (0.4 L) was introduced into a Dewar flask. Following this, a 5 g coal sample was deposited into a porcelain boat, which was connected to the thermocouple, allowing for the real-time tracking of the coal particles’ surface temperature. The thermocouple was designed to measure temperatures within a range of −200 to 200 °C and featured a rapid response time of 0.01 s. For pretreatment, the coal samples, sealed in porcelain boats and placed in plastic bags, were placed in a Dewar flask and cooled for approximately 10 min to reach the lowest achievable temperature (around −178 °C). Subsequently, the coal samples were extracted from the liquid nitrogen and positioned at the far end of the tube furnace. Once the sample temperature, as measured by the thermocouple, rose to the target range (0 to −90 °C), the samples were promptly pushed into the center of the pyrolysis chamber.
2.3. Pyrolysis Experimental Procedure
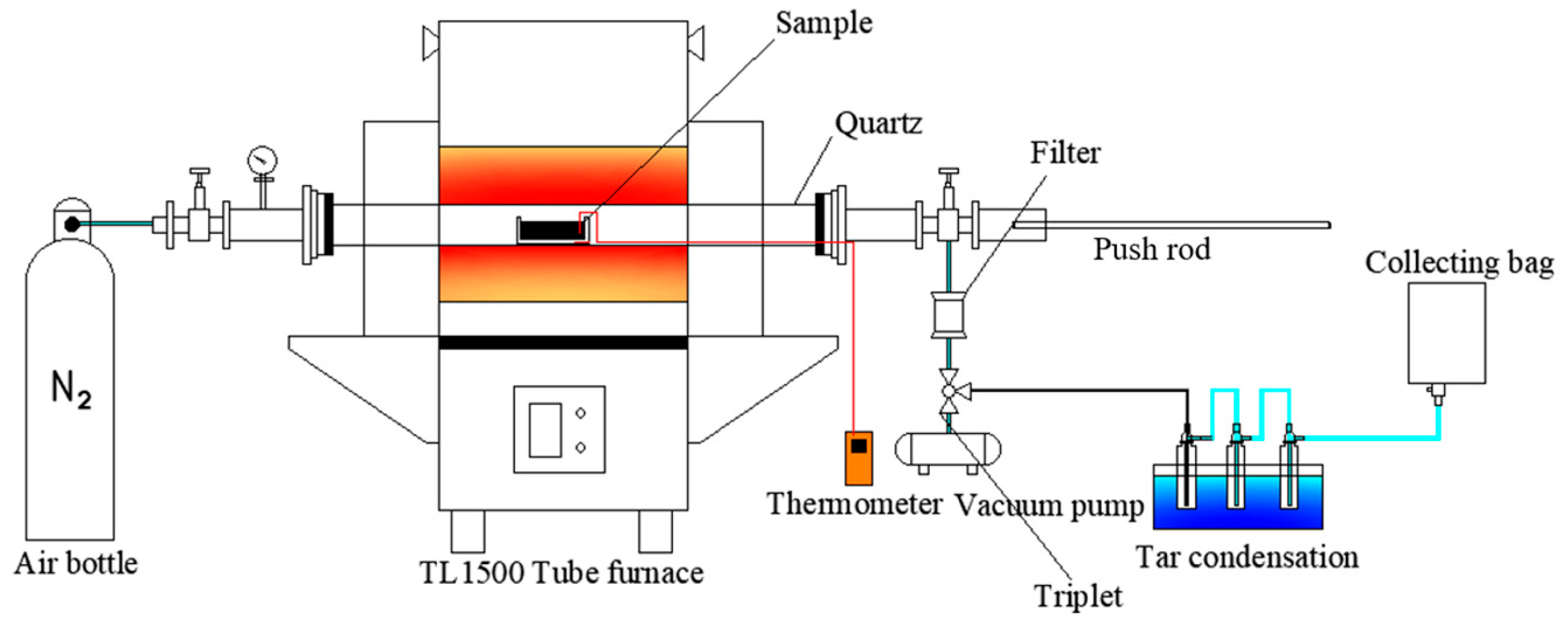
Figure 1 provides a detailed schematic of the coal sample pyrolysis experiment carried out using the TL1500 tube furnace (Bo Yuntong, Nanjing, China). A ceramic boat, containing 5 g of coal sample on a dry basis, was placed at the far-right end of the tube furnace. An ultrafine T-type thermocouple was centrally inserted into the coal sample and affixed to the ceramic boat, allowing for the monitoring of the heating rate during pyrolysis. The furnace’s temperature program was preset, and once it reached the specified range of 350 °C to 900 °C, nitrogen gas was introduced at a flow rate of 0.15 L per minute to purge the air, creating an inert atmosphere for the experiment. The coal sample was then quickly moved to the furnace’s central reaction zone for rapid pyrolysis.
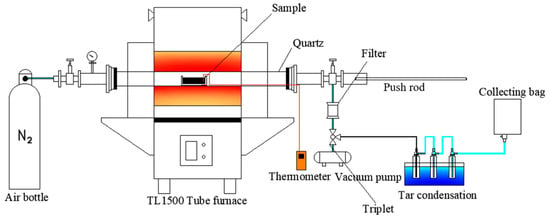
Figure 1.
The schematic of coal pyrolysis experiments.
A gas bag was utilized to collect the gaseous byproducts produced throughout the 30 min reaction period. After the reaction, the inflow of nitrogen gas and the gas bag were sealed. The resulting char was gathered, hermetically sealed, dried, and stored for subsequent analysis. Tar, an organic byproduct, was extracted from the condenser bottle and the inner walls of the tube furnace using isopropanol as a solvent. According to ASTM D5409 [13], isopropanol is commonly used as the optimal solvent for tar extraction due to its low toxicity and solubility. The mass of the uncollected tar is determined by the mass difference between the condensation bottle and the quartz tube before and after the experiment. The collected solution was filtered through a syringe filter with a 0.22-micrometre pore size to remove particulate impurities. The mass of the purified solution was measured and recorded. The prepared tar sample was then carefully stored in a refrigerated environment for future examination.
The mass of the solid pyrolysis product was determined by weighing the char residue in the ceramic boat. The mass of the liquid product, or tar, was ascertained by measuring the difference in weight of the collection apparatus before and after the collection process. The mass of the gaseous products was calculated by subtracting the combined mass of the solid and liquid products from the total mass of the sample. To minimize potential experimental variations and ensure consistent results, the entire pyrolysis process was conducted three times under identical conditions. The average of these measurements was calculated to verify the reproducibility of the experiment.
The temperature control accuracy of the TL1500 tube furnace used in the experiment is ±1 °C, and the model of the gas flow control instrument is MT-52. During liquid nitrogen pretreatment, the immersion depth of the coal sample in the liquid nitrogen was 3 cm, and the sealing bag was made of polyethylene to ensure thermal insulation. The time interval for transferring the coal sample from pretreatment to the tube furnace during pyrolysis was 30 s, minimizing the impact of temperature rise on the experiment.
2.4. Analytical Methods
The yields of the different pyrolysis products were quantified using a solid-phase method. The solid product yield was measured by the mass of char, and the liquid product yield was determined by the weight of the collected tar. The yield of gaseous products was calculated using the method of difference.
To analyze the detailed composition of the gaseous products, a gas chromatograph (GC-2014, Shimadzu, Japan) was utilized. The instrument was equipped with a multi-column system (including HP-AS/L, DB-1, HaySep A/Q, and MolSieve 5A columns) for the efficient separation of polar and non-polar compounds, permanent gases, and light hydrocarbons. The column oven temperature followed a three-stage gradient program (initially at 60 °C, ramping up to 80 °C at 20 °C/min and then to 190 °C at 30 °C/min for a 4 min hold) to optimize the separation of components with different boiling points. The detectors included a Flame Ionization Detector (FID) set at 300 °C (detection limit for hydrocarbons: 0.01 ppm) and a Thermal Conductivity Detector (TCD) set at 250 °C. Calibration was performed using NIST-traceable standard gases (such as CH4 and CO2) to establish an external calibration curve (R2 > 0.999), with argon (Ar) as an internal standard to correct for injection errors (RSD < 2%). The reproducibility was validated through three parallel experiments, ensuring data reliability (deviation < 3%). The composition of the liquid products was examined using a gas chromatography–mass spectrometry (GC-MS) system (19091S-433, Agilent, CA, USA). The quantitative analysis of the liquid products was conducted by normalizing the areas under the respective peaks observed during the GC-MS procedure. This method provides high-resolution insights, revealing the transformation of benzene derivatives and polycyclic aromatic hydrocarbons (PAHs), directly supporting the research claims regarding the increased aromaticity of coal tar.
The carbon conversion rate (ηc) can reflect the energy utilization efficiency during the coal pyrolysis process and is calculated using Equation (1).
where m1 is the mass of raw coal, c1 is the carbon content of raw coal, m2 is the mass of char, and c2 is the carbon content of char.
ηc = 1 − (m2c2)/(m1c1)
By integrating elemental analysis with mass balance calculations, the carbon conversion rate metric (ηc) provided a direct link between the pretreatment conditions and energy utilization efficiency. This method revealed that cryogenic pretreatment enhanced the ηc by up to 4.7% compared to the Gray–King assay, a result aligned with this study’s goal of optimizing resource utilization.
3. Results and Discussion
3.1. Effect of Cryogenic Pretreatment on Heating Rate of Coal During Pyrolysis
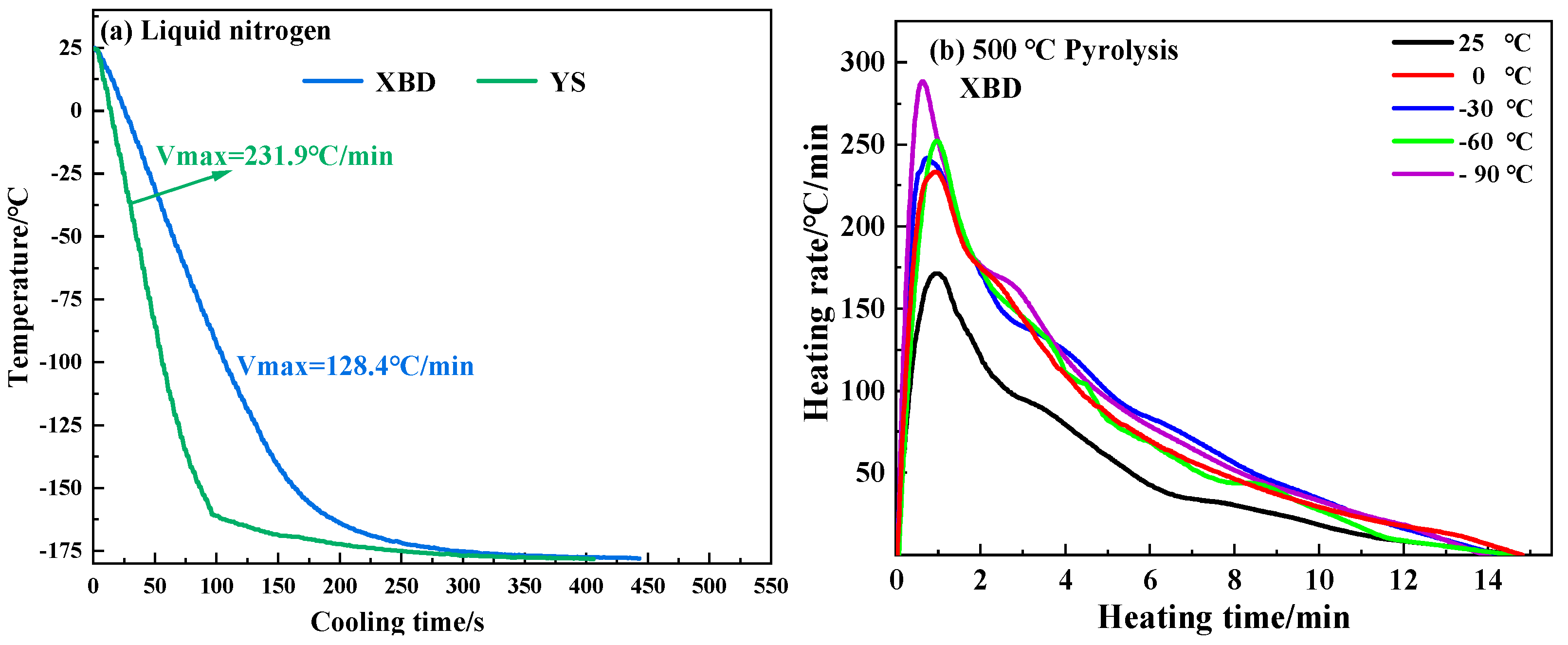
Figure 2 illustrates the cooling rates of coal samples in liquid nitrogen and the heating rates of cryogenic XBD coal samples during pyrolysis at 500 °C. Notably, the XBD and YS coal samples exhibit maximum cooling rates of −231.9 °C/min and −128.4 °C/min, respectively, upon immersion in liquid nitrogen. During the 500 °C pyrolysis, the initial peak heating rate of the coal samples is directly linked to the temperature of the cryogenic pretreatment. A decrease in the pretreatment temperature is associated with an increase in the heating rate. The highest heating rate recorded for the XBD coal was 288.5 °C/min, which occurred at a pretreatment temperature of −90 °C. This elevated heating rate is attributed to the larger temperature gradient between the coal sample and the thermal environment due to the lower pretreatment temperature. Furthermore, as indicated by a recent study [19], cryogenic pretreatment can enhance the thermal diffusivity of coal, thereby contributing to the increased heating rate.
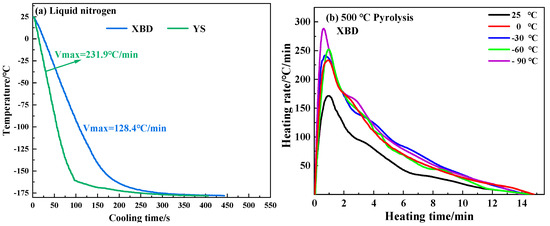
Figure 2.
Freezing rate of coal in liquid nitrogen and heating rate of XBD coal under pyrolysis environment of 500 °C.
3.2. Effect of Cryogenic Temperature on Product Yield
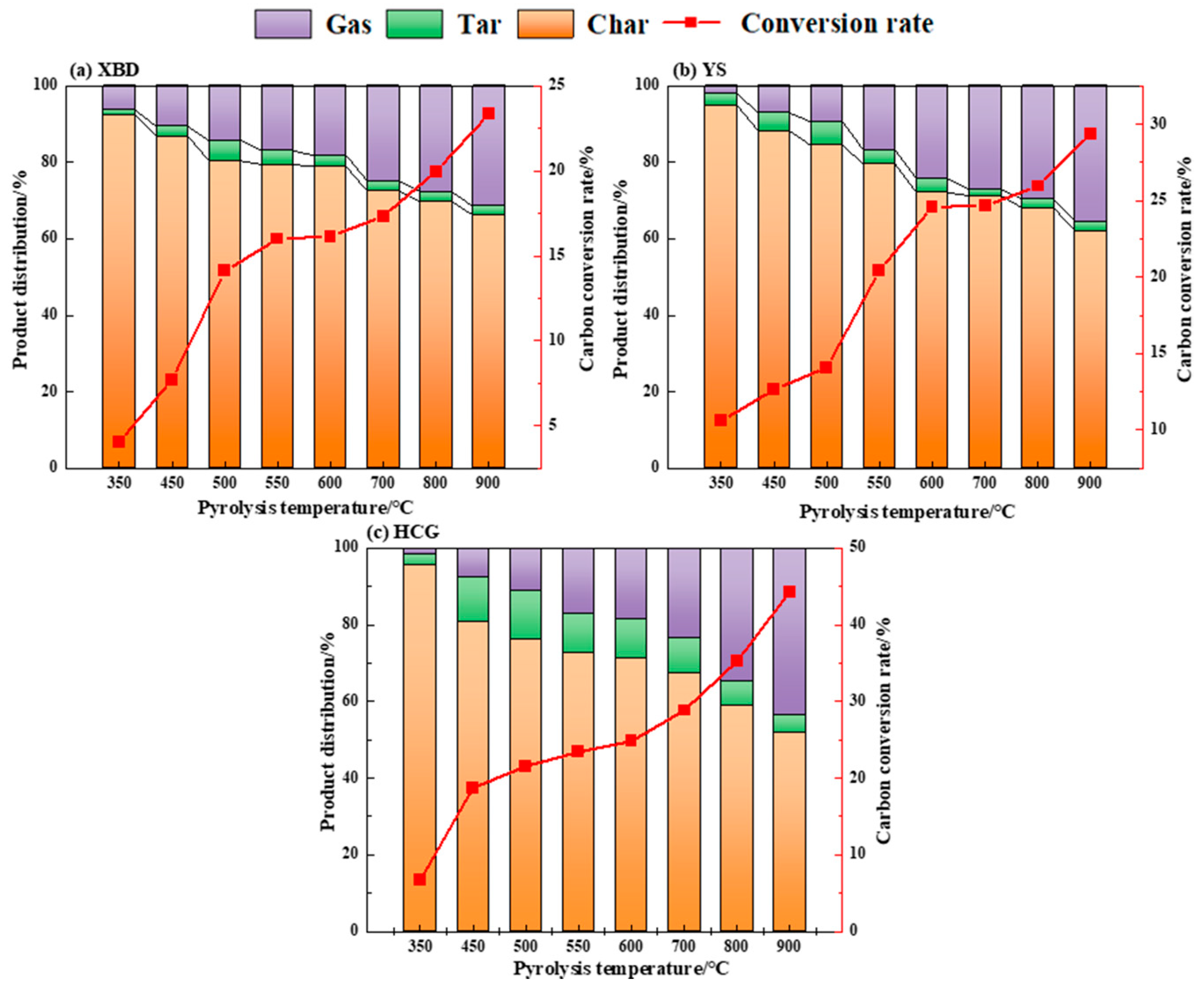
Figure 3 presents the product yield and carbon conversion rate of three coal samples under conventional pyrolysis with different temperatures. As shown, as the pyrolysis temperature escalates, the yield of gaseous products progressively increases, whereas the solid product yield decreases. The coal tar yield initially amplifies with temperature, hitting a maximum at 500 °C, before decreasing with further temperature elevation. The peak tar yields for XBD, YS, and HCG are recorded as 5.3 wt%, 6.2 wt%, and 12.5 wt%, respectively. This trend is attributed to the fact that a higher temperature encourages the primary thermal decomposition of the coal samples, stimulating the generation of volatiles. Beyond 500 °C, however, secondary thermal decomposition intensifies, leading the primary volatiles to further convert into gases and char. This enhances the release of gaseous products while constraining the formation of liquid products [20].
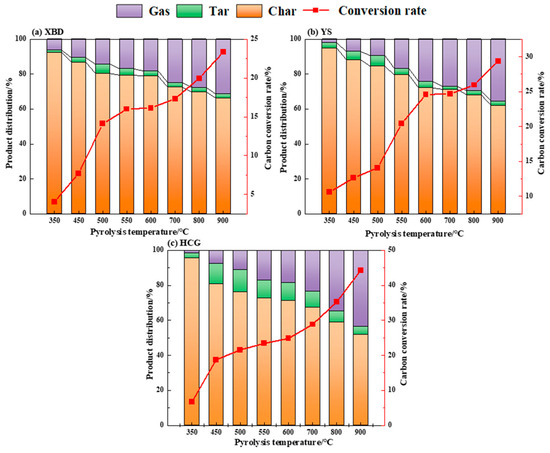
Figure 3.
Yield and carbon conversion of conventional pyrolysis products of coals.
The carbon conversion rate of coal directly correlates with the pyrolysis temperature. The maximum carbon conversion rates for the samples are observed at 900 °C, at 23.3 wt%, 29.3 wt%, and 44.3 wt%, respectively. The experimental results support the conclusion that higher temperatures expedite the progress of pyrolysis reactions, thereby boosting the carbon conversion rate. At a pyrolysis temperature of 500 °C, the tar yield of the three coals peaked, and therefore, the pyrolysis experiments for cryogenically pretreated coals were conducted at 500 °C.
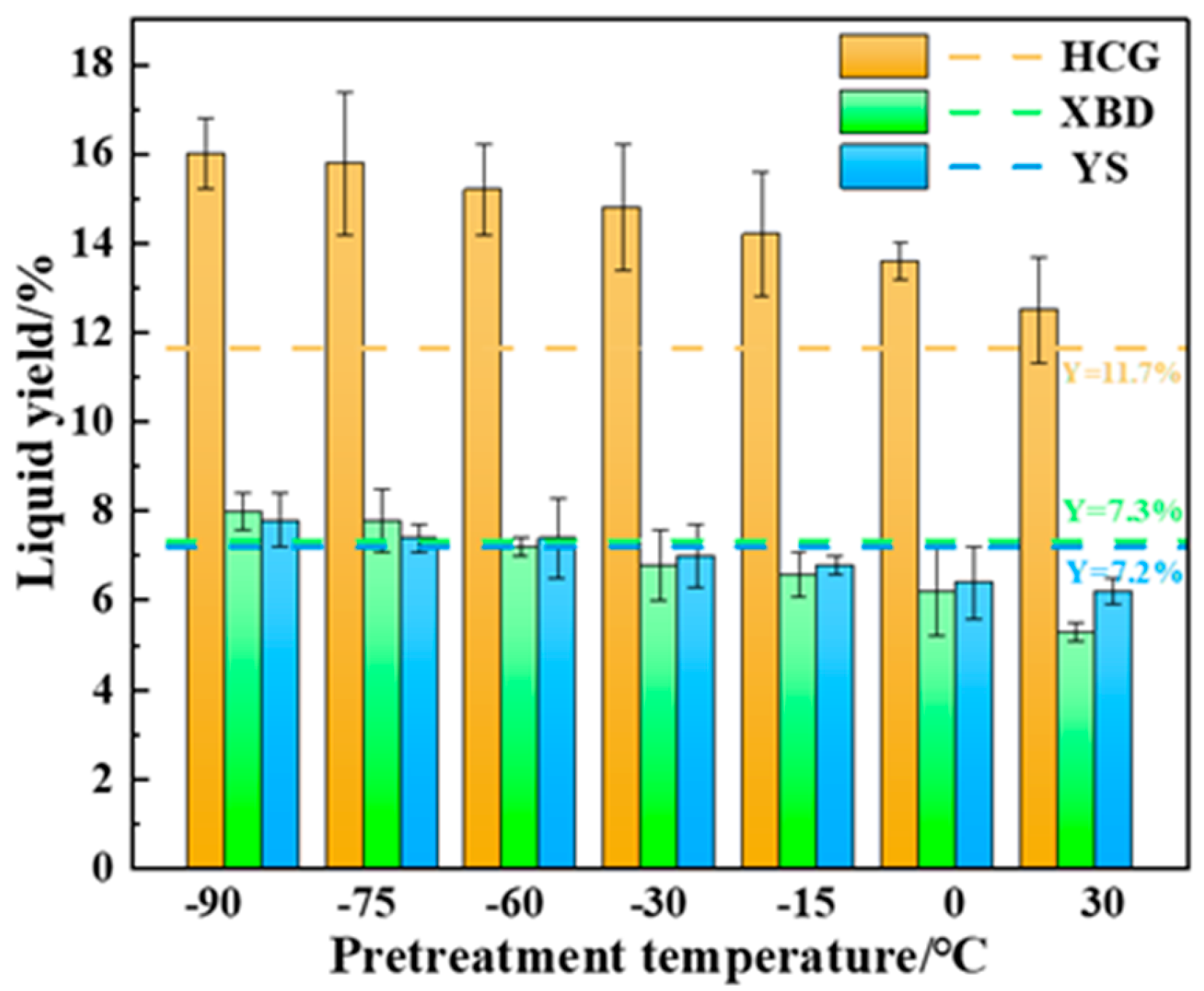
Figure 4 presents a comparison of the tar yields from coal subjected to cryogenic pretreatment (−90~0 °C), conventional pyrolysis, and Gray–King low-temperature carbonization. The tar yield under the Gray–King method is represented by Y. As seen, lower freezing temperatures result in higher tar production, with the maximum tar yield (7.8–16.0 wt%) achieved at −90 °C pretreatment. This exceeds the tar yield of the Gray–King method and conventional pyrolysis by 8.3–41.3% and 25.8 to 44.6%, respectively. This could be a result of a higher heating rate under cryogenic pretreatment. Cryogenic pretreatment enhances the heating rate of coal, as seen in Figure 2, and thereby increases the tar yield [21].
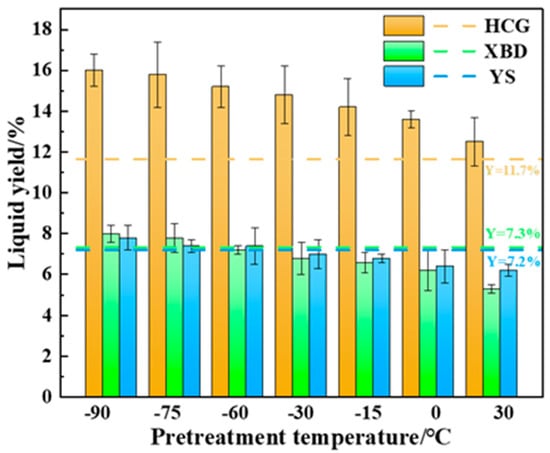
Figure 4.
Effect of cryogenic pretreatment on the tar yield of coals.
It is evident that the tar yield significantly increases with decreasing pretreatment temperature, reaching a maximum of 7.8–16.0 wt% at −90 °C, which is 25.8–44.6% higher than traditional pyrolysis, with the simultaneous optimization of the carbon conversion rate. This indicates that cryogenic pretreatment enhances thermal diffusivity, suppressing secondary cracking and maximizing tar production.
3.3. Effect of Cryogenic Temperature on Gas Product
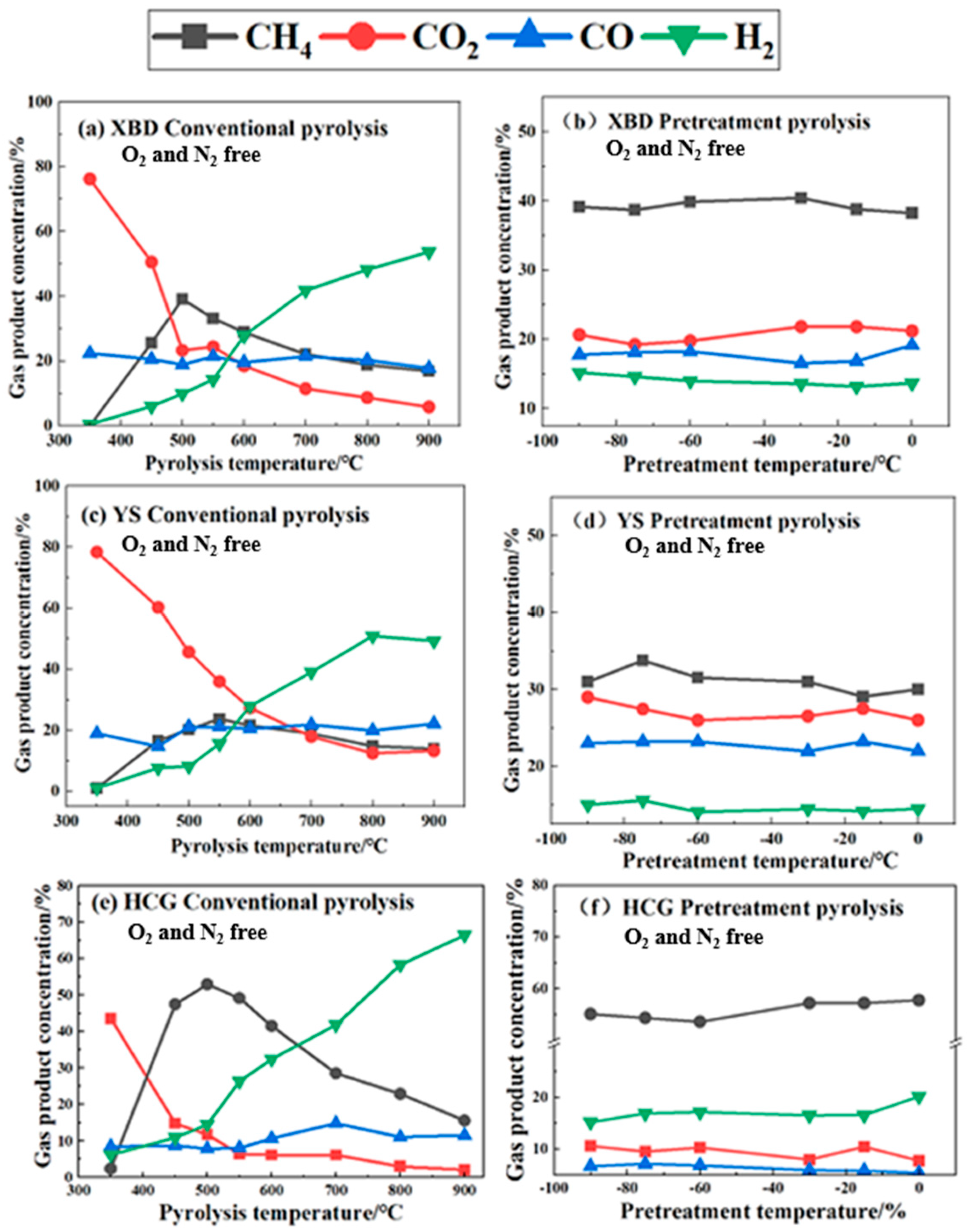
Figure 5 depicts the gas composition of the product resulting from both conventional pyrolysis and cryogenic pretreatment. As shown in Figure 5a,c,e, during conventional pyrolysis, the CO2 content in coal undergoes a substantial decrease of 43.5–65.2 vol% as the pyrolysis temperature rises, whereas the H2 content increases by 28.5–59.8 vol%. The reduction in the CO2 content is ascribed to the intensified gas-phase reaction between coal and CO2 at higher temperatures. Conversely, the rise in the H2 content is attributed to the stimulation of primary and secondary pyrolysis reactions at elevated temperatures, encompassing tar cracking, small-molecule decomposition, and secondary gas-phase reactions, all of which contribute to the release of H2.
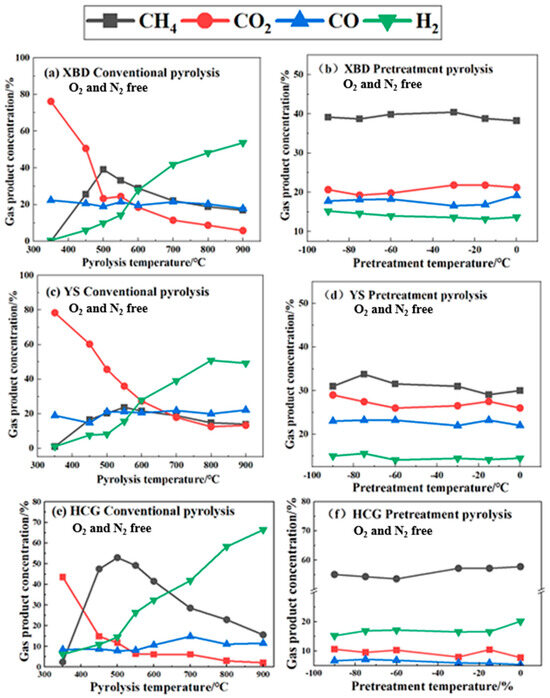
Figure 5.
Gas composition of coals under conventional pyrolysis and cryogenic pretreatment.
The CH4 content in the XBD, YS, and HCG coals initially rises with temperature, reaching its peak at 600 °C (40.3 vol%), 550 °C (23.7 vol%), and 500 °C (52.9 vol%), respectively, before declining. The initial ascent is fueled by the primary pyrolysis, which fosters CH4 production. The subsequent decrease may be attributed to the reaction of CH4 with CO2 generated during pyrolysis at high temperatures.
As shown in Figure 5d–f, after cryogenic pretreatment, the gas composition of the three coal samples exhibits relative stability, suggesting that the pretreatment temperature exerts a negligible influence on the resultant gases from coal pyrolysis. Contrasting with conventional pyrolysis, there is a noted decline in the CO2 and CO concentrations by 12.7 to 27.4%, alongside an elevation in the H2 and CH4 levels by 15.1 to 60.2%. The results indicate that the cryogenic pretreatment dampens the formation of both CO2 and CO during the pyrolysis of coal, while it encourages the liberation of CH4 and H2.
The results indicate that after pretreatment, the concentrations of CO2 and CO decrease by 12.7–27.4%, while the proportions of CH4 and H2 increase by 15.1–60.2%. This study further confirms that the direct impact of the pretreatment temperature on the gas composition is limited; however, it indirectly regulates gas release pathways by altering pyrolysis kinetics.
3.4. Effect of Cryogenic Temperature on Liquid Product
Table 2 illustrates the composition of the liquid products resulting from both conventional pyrolysis and cryogenic pretreatment. Due to the complexity of tar components, we have categorized them into six groups: benzene, phenol, PAHs, aromatic, alkanes, and others. The benzene group encompasses monocyclic aromatic hydrocarbons, including benzene, toluene, ethylbenzene, and xylenes (BTEXs), as well as poly-methyl-substituted benzenes. The PAH group includes polycyclic aromatic hydrocarbons, classified based on the number of benzene rings, including five types: naphthalene, phenanthrene, pyrene, benzo[a]pyrene, and benzo[ghi]perylene. The aromatic group includes all other benzene-ring-containing substances (contains elements other than C and H) not classified in the benzene and PAH groups. The alkane group represents aliphatic chains and cyclic compounds that do not contain benzene rings. The other group encompasses all unidentified compounds with a total concentration of below 0.01%. The specific components of tar are presented in Appendix A and Appendix B, indicating that the content of BTEX compounds in the aromatic fraction is extremely low.

Table 2.
Tar composition under cryogenic pretreatment and conventional pyrolysis.
As shown, as the temperature of conventional pyrolysis increases, the concentration of PAHs in the liquid products from the XBD, YS, and HCG coals experiences a substantial rise, with increases of 123%, 135%, and 326%, respectively. Simultaneously, the proportions of phenols and aliphatic hydrocarbons undergo significant decreases, with reductions spanning from 70 to 97% for phenols and 79 to 100% for aliphatic hydrocarbons. This shift in composition is attributed to the thermal cleavage of covalent bonds within aliphatic and phenolic compounds at higher pyrolysis temperatures. The free radical fragments generated during this process predominantly undergo condensation reactions to form PAHs with larger molecular weights [5,22].
The concentration of benzene-related compounds exhibits a positive correlation with the pyrolysis temperature. For instance, in the tar derived from the XBD coal, the benzene content escalates from 5.2 wt% at 500 °C to 15.4 wt% at 900 °C, while in the tar of the YS coal, it ascends from 14.5 wt% to 22.9 wt%. These findings suggest that an increase in the pyrolysis temperature promotes the formation of benzene and polycyclic aromatic hydrocarbons while concurrently inhibiting the generation of phenols and aliphatic compounds.
In contrast to conventional pyrolysis, cryogenic pretreatment markedly suppresses the formation of benzene compounds but significantly enhances the production of aromatic derivatives. After cryogenic pretreatment, the benzene content in the tar of the coal experiences a substantial decline, with a reduction of roughly 4.4 to 13.9 wt%. Concurrently, the content of aromatic derivatives sees a relative increase, with an increment of approximately 13.9 to 20.5 wt%. Nevertheless, the cryogenic pretreatment temperature appears to have a negligible effect on the phenols, polycyclic aromatic hydrocarbons, and aliphatic hydrocarbons in the pyrolysis tar composition, as there is no discernible pattern in the fluctuation of their contents with temperature changes.
The content of benzene derivatives decreases by 4.4–13.9 wt% after pretreatment, while aromatic derivatives increase by 13.9–20.5 wt%. Combined with GC-MS data, it is inferred that low-temperature rapid heat transfer suppresses the pathway for free radical condensation that produces polycyclic aromatic hydrocarbons (PAHs), thereby promoting the stabilization of aromatic rings.
3.5. Effect of Cryogenic Temperature on Solid Product
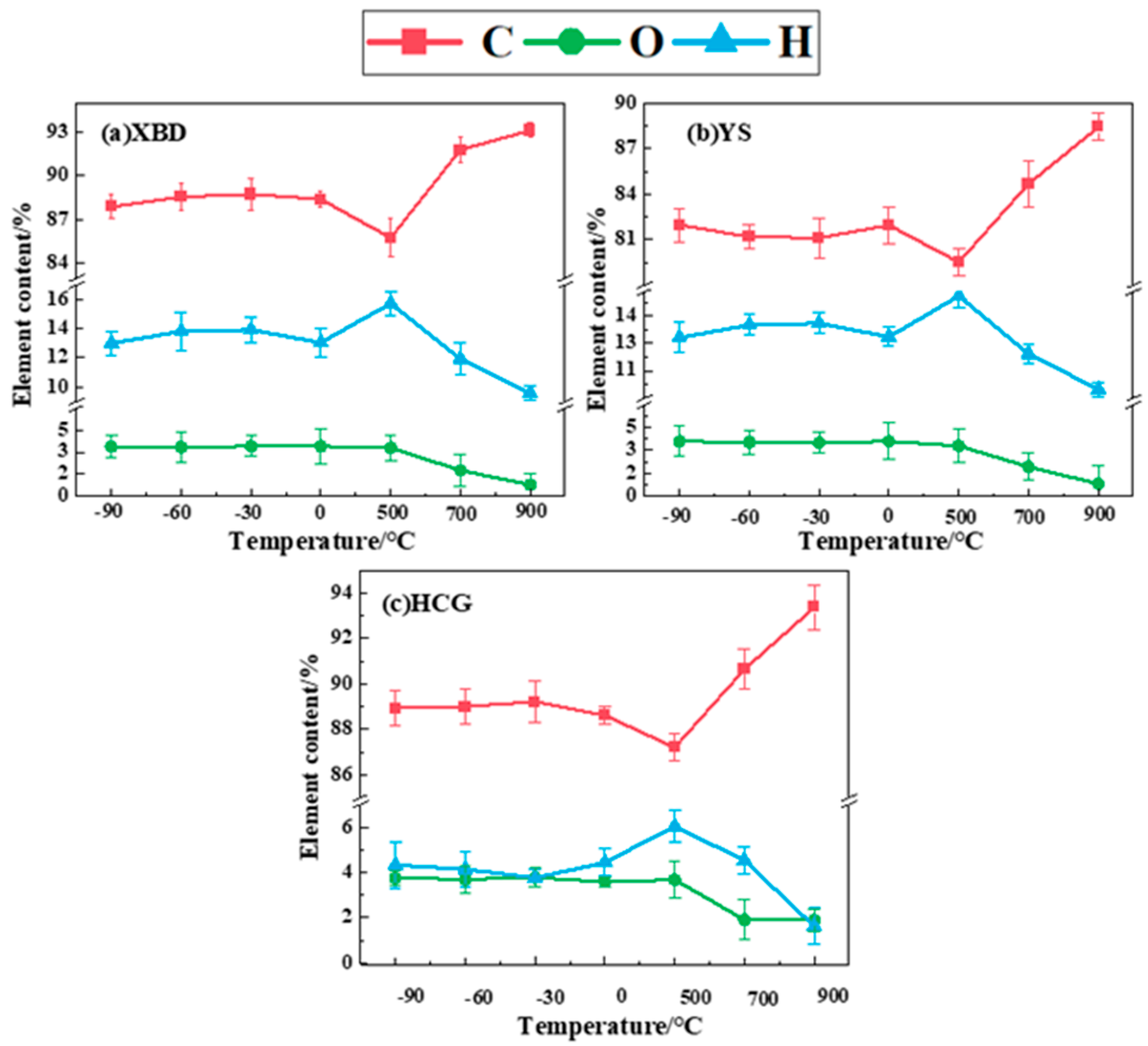
Figure 6 presents the elemental compositions of solid pyrolysis products under both conventional pyrolysis and cryogenic pretreatment. The data suggest that as the pyrolysis temperature rises from 500 °C to 900 °C, the carbon content in the char products from the three coal samples also increases, demonstrating respective growths of 7.37%, 8.91%, and 6.18%. This trend is attributed to the thermal decomposition of volatile components, predominantly those rich in H and O, as the temperature rises. These components are expelled as gases, leaving behind a solid residue that undergoes aromatization, thus leading to a higher proportion of carbon-rich constituents in the char.
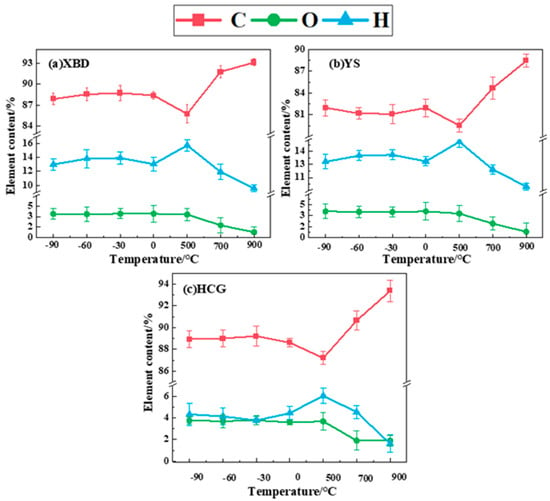
Figure 6.
Char composition under cryogenic pretreatment and conventional pyrolysis.
The ratios of H and O in the char products conversely decrease with the increase in pyrolysis temperature. This is primarily due to the evaporation of the coal’s inherent moisture and the elimination of chemical structures containing hydroxyl groups, consequently resulting in a diminished presence of H and O in the products. Moreover, the high temperature induces the breakdown of chemical bonds within the coal, causing the decomposition of functional groups like carboxyl, hydroxyl, and ether bonds. As these elements are released as gases such as H2, CH4, CO, and CO2, the content of H and O further diminishes.
At identical pyrolysis temperatures, the carbon (C) content in the char products of coal samples under cryogenic pretreatment pyrolysis exhibits a slight increase compared to that under conventional pyrolysis. Specifically, the C content in the XBD char rises by 2.65–2.99%, while the C content in the YS char increases by 1.61–2.45%, and the C content in the HCG char increases by 1.42–2.02%. This suggests that cryogenic pretreatment can yield char with a higher calorific value and superior combustion characteristics. Concurrently, the oxygen (O) content in the char decreases, with a reduction of 2.31–3.16% for the XBD char, 1.80–2.74% for the YS char, and 1.59–2.27% for the HCG char.
The cryogenic pretreatment enhances char properties through structural and chemical modifications. Rapid cooling induces micro-fractures in the coal matrix, promoting the decomposition of oxygen-containing groups (-OH, C=O) via decarboxylation and dehydration, thereby reducing the oxygen content (XBD: 2.31–3.16%; YS: 1.80–2.74%; HCG: 1.59–2.27%). Concurrently, carbon enrichment arises from aromatic condensation and partial graphitization, increasing the carbon content by up to 2.99%. These changes diminish hydrophilic interactions and oxidative exothermicity, effectively mitigating spontaneous combustion risks during storage. The optimized porosity and carbon structure further elevate the calorific value and combustion efficiency. By adjusting pretreatment temperatures (−90 °C to 0 °C), the char composition can be tailored for specific applications, such as high-energy fuels or stable adsorbents.
After pretreatment, the carbon content of the char increases by 1.42–2.99%, while the oxygen content decreases by 1.59–3.16%, indicating an enhancement in deoxygenation and aromatization reactions during pyrolysis. The elemental analysis confirms that cryogenic pretreatment optimizes the combustion properties and storage safety of the char by reducing the loss of H/O in the volatiles.
4. Conclusions
This study significantly enhances the product efficiency and environmental benefits of coal pyrolysis through liquid nitrogen cryogenic pretreatment. Firstly, the cryogenic pretreatment promotes the release of CH4 and H2 (increases of 15.1–60.2%) while inhibiting the generation of CO2 and CO (reductions of 12.7–27.4%), optimizing the gas energy value. Secondly, the tar yield improves by 25.8–44.6% compared to conventional pyrolysis, with a decrease in the benzene compound content by 4.4–13.9 wt% and an increase in high-value aromatic derivatives by 13.9–20.5 wt%, indicating that cryogenic pretreatment can selectively regulate tar quality. Additionally, the carbon content of the residual char increases by 2.99%, and the oxygen content decreases by 3.16%, enhancing the combustion performance and reducing the risk of spontaneous ignition during storage and transportation. These findings provide a new pathway for clean and efficient coal pyrolysis technology, balancing resource utilization with carbon reduction goals.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by L.C., J.C. and Y.L. The experimental work was done by L.C., J.C., X.W., X.Y. and Z.Y. in collaboration. The first draft of this manuscript was written by L.C. and T.X. and all authors commented on previous versions of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Basic Research Program of Shaanxi, grant number 2022JM-232. The APC was funded by Xi’an University of Science and Technology.
Data Availability Statement
Data are contained within this article.
Acknowledgments
This work was supported by the Natural Science Basic Research Program of Shaanxi (2022JM-232). In addition, the authors also acknowledge the experimental platform provided by Xi’an University of Science and Technology.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
BTEX components in tar.
Table A1.
BTEX components in tar.
| T/°C C/wt% | Conventional Pyrolysis Temperature | Cryogenic Pretreatment Temperature | |||||
|---|---|---|---|---|---|---|---|
| 500 | 700 | 900 | 0 | −30 | −60 | −90 | |
| XBD | |||||||
| Benzene | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Toluene | 0 | 0 | 0 | 0 | 0 | 0 | 0.16 |
| Ethylbenzene | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xylenes | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| YS | |||||||
| Benzene | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Toluene | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ethylbenzene | 0 | 0 | 0 | 0 | 0.75 | 0 | 0 |
| Xylenes | 0 | 0 | 0 | 0.26 | 0.14 | 0 | 0 |
| HCG | |||||||
| Benzene | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Toluene | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ethylbenzene | 0 | 0 | 0 | 0 | 0.26 | 0 | 0 |
| Xylenes | 0 | 0 | 0 | 0 | 0.80 | 0.70 | 0.46 |
Appendix B

Table A2.
Tar composition of XBD sample.
Table A2.
Tar composition of XBD sample.
| XBD/wt% | Pyrolysis | Cryogenic |
|---|---|---|
| 500 °C | −90 °C | |
| Benzenes | ||
| Propylbenzene | 1.51 | 0 |
| 1-Ethyl-2-meth | 2.57 | 0 |
| Toluene | 0 | 0.19 |
| Tribenzocyclo | 0.61 | 0 |
| 1-Methyl-4-[(4-propylphenyl)ethynyl]benzene | 0.47 | 0 |
| Pentamethylbenzene | 0 | 0.30 |
| Phenols | ||
| Phenol | 1.37 | 0 |
| 2-Methylphenol | 1.48 | 0 |
| 3-Methylphenol | 4.02 | 0 |
| 2-Ethylphenol | 0.27 | 0 |
| 2,4-Dimethylphenol | 2.02 | 0 |
| 3-Ethylphenol | 2.32 | 0 |
| 2,3-Dimethylphenol | 0.23 | 0 |
| 2-Ethyl-4-methylphenol | 1.55 | 0 |
| 2,4,5-Trimethylphenol | 0.39 | 0 |
| 2,3,5-Trimethylphenol | 0.35 | 0.21 |
| 1-Naphthol | 1.34 | 1.04 |
| 2-Naphthol | 1.33 | 0.33 |
| p-Cresol | 0 | 1.12 |
| 4-Ethyl-2-methylphenol | 0 | 1.32 |
| 3,4-Dimethylphenol | 0 | 0.6 |
| 2-Methyl-1-naphthol | 1.76 | 0 |
| 6,7-Dimethyl-1-naphthol | 0.64 | 0 |
| Phenol, 3,4-dimethyl | 0 | 0.46 |
| 3,5-Diethylphenol | 0 | 0.31 |
| Phenol, 4-ethyl-2-methyl | 0 | 1.02 |
| 4-Methylthio-2,6-dimethylphenol | 0 | 0.13 |
| p-(Benzaldehyde amine)phenol | 0 | 0.32 |
| Phenol, 2-[[(4-methylphenyl)imino]methyl] | 0 | 0.32 |
| 4-Ethylphenol | 0 | 2.15 |
| PAHs | ||
| Naphthalenes | ||
| Naphthalene | 1.35 | 0 |
| 2-Methylnaphthalene | 0.82 | 0.46 |
| 1-Methylnaphthalene | 0.97 | 0 |
| 1,6-Dimethylnaphthalene | 1.73 | 0 |
| 2,3-Dimethylnaphthalene | 1.39 | 0 |
| 1,3-Dimethylnaphthalene | 0.65 | 0 |
| 1,6,7-Trimethylnaphthalene | 1.33 | 4.37 |
| 2,3,6-Trimethylnaphthalene | 1.15 | 0 |
| 1,4,5-Trimethylnaphthalene | 1.39 | 0 |
| Naphthalene,1,2,3,4-tetramethyl | 0 | 1.09 |
| Naphthalene,7-butyl-1-hexyl- | 0 | 0.14 |
| 1,4,5,8-Tetramethylnaphthalene | 0 | 0.19 |
| 1,5-Dimethylnaphthalene | 0 | 1.31 |
| 1,2-Dimethylnaphthalene | 0 | 1.92 |
| Phenanthrenes | ||
| Fluorene | 1.53 | 0 |
| 2-Hydroxyfluorene | 0.93 | 0 |
| 9-Methylfluorene | 0.7 | 0 |
| 4-Methylphenanthrene | 0.67 | 0 |
| 2-Methylphenanthrene | 1.03 | 0 |
| 1-Methylanthracene | 1.22 | 0 |
| 1-Methylphenanthrene | 0.41 | 0 |
| 4,5-Dimethylphenanthrene | 0.72 | 0 |
| 3,6-Dimethylphenanthrene | 0.51 | 0 |
| 2,3,5-Trimethylphenanthrene | 1.46 | 1.71 |
| Retene | 4.44 | 0 |
| Anthracene | 0 | 0.25 |
| 9-Methoxy-anthracene | 0 | 0.17 |
| Di-p-tolylethyne | 0 | 0.64 |
| Phenanthrene, 3,6-dimethyl | 0 | 0.53 |
| 1-Phenyl-naphthalene | 0 | 0.62 |
| 1H-Indene, 2-phenyl | 0 | 0.45 |
| 1,1,4,5,6-Pentamethyl-2,3-dihydro-1H-indene | 0 | 0.52 |
| 2-Methylanthracene | 0 | 2.49 |
| 2-Isopropyl-10-methylphenanthrene | 0 | 1.02 |
| 9-Methyl-9H-fluorene | 0 | 1.55 |
| 1,9-Dimethyl-9H-fluorene | 0 | 0.37 |
| 9H-Fluorene, 9-methylene | 0 | 2.43 |
| 7H-Benzophenanthrene | 0 | 0.41 |
| 2-Phenyl-1H-indene | 0 | 1.34 |
| 1,4-Dimethyl-7-(1-methylethyl)-azulene | 0 | 0.24 |
| Acenaphthene | 0 | 0.25 |
| Pyrenes | ||
| Pyrene | 1.68 | 1.34 |
| 1-Methylpyrene | 1.82 | 0 |
| 11H-Benzo[a]fluorene | 0 | 1.85 |
| Benzo[a]pyrenes | ||
| Benzo[j]fluoranthene | 1.87 | 0 |
| Benzo[e]pyrene | 1.18 | 0 |
| Perylene | 1.20 | 0 |
| Indeno[1,2,3-cd]anthracene | 0.34 | 0 |
| Aromatics | ||
| Oxygen-containing aromatics | ||
| 1-Ethyl-4-methoxybenzene | 0.23 | 0 |
| 3-Methyl-1,2-dihydroxybenzene | 0.44 | 0 |
| 1,1′-(Dioxydiethyl)dibenzene | 0.28 | 0 |
| 2,5-Dimethyl-1,4-dihydroxybenzene | 0.24 | 0 |
| 2,3-Dihydro-3,3,5,6-tetramethyl-1H-indenone | 0.52 | 0 |
| E-15-Heptadecenal | 0.17 | 0 |
| 7-Hexyltridecan-1-ol | 0.26 | 0 |
| n-Nonadecan-1-ol | 0.17 | 0 |
| 2-Methyl-1-hexadecanol | 0.38 | 0 |
| 3,7,11-Trimethyldodecanol | 0.2 | 0 |
| 1-Phenyl-1,3-cyclohexadien-5-ol | 0.41 | 0 |
| 9H-Fluoren-9-one, hydrazone | 0.39 | 0 |
| 1-Tricosanol | 1.33 | 0 |
| 2,5-Furandione, dihydro-3-octadecyl | 0.38 | 0 |
| 3,19:14,15-Diepoxy-20-ketopregnane, 3,11,18-triacetoxy | 0.37 | 0 |
| 4-Methyldibenzofuran | 0.83 | 0 |
| 3-Phenylfuran | 0 | 1.63 |
| cis-2,5-Dimethyltetrahydrofuran | 0 | 0.09 |
| Naphtho[2,1-b]furan | 0 | 0.37 |
| 2,3-Dihydroinden-5-ol | 0 | 0.12 |
| Tetratriacontyn-4-ethylbenzoate | 0 | 0.26 |
| 4-(1-Phenyl-2-propenyloxy)benzaldehyde | 0 | 0.11 |
| Naphthalenemethanol | 0 | 0.83 |
| Phenyl borate phthalate | 0 | 0.28 |
| 1,8-Naphthyridine, 2,4,7-trimethyl ester | 0 | 0.22 |
| 2-Butoxyethanol | 0 | 19.43 |
| Nitrogen-containing aromatics | ||
| 1H-Phenanthro[9,10-c]pyrazole | 0.41 | 0 |
| 9H-Fluoren-9-one, hydrazone | 0.39 | 0 |
| 2-(1-Methylethyl)phenol methylcarbamate | 0.16 | 0 |
| 5,6-Dimethyl-1H-benzimidazole | 0 | 0.19 |
| 5,7-Dimethylpyrimido[a]indole | 0 | 0.26 |
| 2-(1-Methylcyclopropyl)aniline | 0 | 0.46 |
| 2-Phenyl-4,5-dihydro-1H-imidazole | 0 | 0.07 |
| 1H-Benzimidazole, 5,6-dimethyl- | 0 | 0.38 |
| 8-Methyl-5H-pyrido[4,3-b]indole | 0 | 0.12 |
| 1-Benzyl-2-methyl-1H-imidazole | 0 | 0.13 |
| 1,8-Naphthyridine, 2,4,7-trimethyl ester | 0 | 2.50 |
| p-Aminotoluene | 0 | 0.10 |
| Sulfur-containing aromatics | ||
| 3-Methylbenzothiophene | 0 | 0.14 |
| 4-Methylthio-2,6-dimethylphenol | 0 | 0.08 |
| Halogen-containing aromatics | ||
| 4-Isopropylphenol-4-chlorobutyrate | 0 | 0.09 |
| Naphthylmethyl ester | 0 | 0.24 |
| Alkanes | ||
| Tetradecane | 0.49 | 0 |
| Heptadecane | 0.81 | 0 |
| Octadecane | 0.72 | 0 |
| Nonadecane | 1.05 | 0.65 |
| Eicosane | 0.96 | 0 |
| 9-Hexylheptadecane | 1.95 | 0 |
| Tricosane | 1.60 | 0 |
| 3-Ethyl-5-(2-ethylbutyl)-octadecane | 2.16 | 0 |
| Benzo[b]naphtho[2,3-h]bicyclo[2.2.2]octane | 0.56 | 0 |
| Pentacosane | 1.59 | 0 |
| 3-Ethyl-5-(2-ethylbutyl)-octadecane | 1.53 | 0 |
| 28-Nor-17-(H)-hopane | 0.31 | 0 |
| 7-Hexyltridecan-1-ol | 0.39 | 0 |
| n-Nonadecan-1-ol | 0.26 | 0 |
| 2-Methyl-1-hexadecanol | 0.57 | 0 |
| 1-Tricosanol | 1.95 | 0 |
| 1-Bromotriacontane | 0.29 | 0 |
| 3,7,11-Trimethyldodecanol | 0.31 | 0 |
| 2,5,5,8a-Tetramethyl-4-methylene-4a,5,6,7,8,8a-hexahydro-4H-chromene | 0.30 | 0 |
| 2,4,5,5,8a-Pentamethyl-6,7,8,8a-tetrahydro-5H-chromene | 0.68 | 0 |
| Di-tert-dodecyl disulfide | 0.85 | 0 |
| 2,7,10-Trimethyldodecane | 0 | 0.60 |
| Hexadecane | 0 | 0.42 |
| 1,2-Dibromopropane | 0 | 0.06 |
| 1,7-Dimethyl-4-(1-methylethyl)cyclodecane | 0 | 0.25 |
| Heptadecane | 0 | 0.47 |
| 1,2-Dibromopropane | 0 | 0.06 |
| Hexane, 3,3-dimethyl- | 0 | 0.03 |
| 1-Phenyl-1-phenoxyethane | 0 | 0.03 |
| 2,2,3,3-Tetramethyl-butane | 0 | 0.27 |
| 2-Ethyl-2-phenyl-1,3-benzodioxole | 0 | 0.05 |
| Hexadecane, 1-iodo | 0 | 1.40 |
| 4-Methyl-2-pentanol | 0 | 1.07 |
| Allyl isobutyrate | 0 | 0.05 |
| 2,3,4-Trimethylpentyl tetradecanoate | 0 | 0.07 |
| 2-Butoxyethanol | 0 | 11.66 |
| 4-Heptanol, 2,6-dimethyl-, acetate | 0 | 0.24 |
| 2,3-Dimethyl-2-cyclopenten-1-one | 0 | 0.06 |
| 1,2,3,4-Butanetetrol, tetraacetate | 0 | 0.10 |
| 1-Hexene, 5,5-dimethyl | 0 | 0.10 |
| 2-Propylamine, N,N′-methanetetramine | 0 | 0.12 |
| Others | 16.35 | 16.58 |

Table A3.
Tar composition of YS sample.
Table A3.
Tar composition of YS sample.
| YS/wt% | Pyrolysis | Cryogenic |
|---|---|---|
| 500 °C | −90 °C | |
| Benzenes | ||
| 2-Phenyl-1H-indene | 0 | 0.31 |
| 1-Methyl-4-[4-propylphenylethynyl]benzene | 0 | 0.04 |
| Pentamethylbenzene | 0 | 0.05 |
| p-Tolylethyne | 0.52 | 0.05 |
| Propylbenzene | 4.82 | 0 |
| 1-Ethyl-2-methylbenzene | 7.9 | 0 |
| 1,2,3-Trimethylbenzene | 0.65 | 0 |
| 2,2′-Dimethylbiphenyl | 0 | 0.02 |
| 3,3′,4,4′-Tetramethyl-1,1′-biphenyl | 0 | 0 |
| 1,2-Diethyl-3,4-dimethylbenzene | 0 | 0.03 |
| 1,1′-(1,3-Butadiyne-1,4-diyl)diferrocene-benzene | 0.57 | 0 |
| Phenols | ||
| Phenol | 0 | 0.32 |
| Phenol | 2.97 | 0 |
| p-Cresol | 0 | 2.37 |
| 2-Methylphenol | 1.74 | 0.47 |
| 3-Methylphenol | 5.73 | 0 |
| 2,3-Dimethylphenol | 0.47 | 0 |
| 2,4-Dimethylphenol | 2.07 | 0 |
| 3,4-Dimethylphenol | 0 | 1.49 |
| 3,4,5-Trimethylphenol | 0 | 0.10 |
| 2,3,5-Trimethylphenol | 0 | 0.10 |
| 2-Ethylphenol | 0.26 | 0 |
| 3-Ethylphenol | 3.17 | 1.14 |
| 3,5-Dimethylphenol | 0 | 0.10 |
| 3,5-Diethylphenol | 0 | 0 |
| 2,3,5-Trimethylphenol | 0.41 | 0 |
| Catechol | 0 | 0.15 |
| 2,2′-Methylenebis[6-(1,1-dimethylethyl)-4-methyl-phenol | 0 | 0.18 |
| 4-Ethyl-2-methylphenol | 0 | 0.34 |
| 1-Naphthol | 1.06 | 0 |
| 2-Naphthol | 1.20 | 0 |
| 2-Methyl-1-naphthol | 2.80 | 0 |
| 4-Methyl-1,2-benzenediol | 0 | 0.24 |
| 3-Methyl-1,2-benzenediol | 0 | 0.12 |
| 4-Ethyl-1,3-benzenediol | 0 | 0.11 |
| 4-(1-Phenylethyl)-phenol | 0 | 0.11 |
| 3-Ethyl-5-methylphenol | 0.96 | 0 |
| 2-Ethyl-4-methylphenol | 0 | 0.80 |
| p-Cresol, TMS derivative | 0 | 0.28 |
| Orcinol (also known as: Orcinol) | 0 | 0.12 |
| Resorcinol | 0 | 0.44 |
| PAHs | ||
| Naphthalenes | ||
| 2-Methylnaphthalene | 2.74 | 1.09 |
| 1,2-Dimethylnaphthalene | 0 | 0.17 |
| 1,3-Dimethylnaphthalene | 1.80 | 0.67 |
| 1,5-Dimethylnaphthalene | 0 | 0.41 |
| Azulene, 1,4-dimethyl-7-(1-methylethyl)- | 0 | 0.09 |
| 1,6-Dimethylnaphthalene | 0.95 | 0.63 |
| 1,4,6-Trimethylnaphthalene | 0 | 0.78 |
| 2,3,6-Trimethylnaphthalene | 1.12 | 0.48 |
| 1,6,7-Trimethylnaphthalene | 0.93 | 1.74 |
| Pyrene | 1.38 | 0 |
| 2,7-Dimethylnaphthalene | 1.58 | 0 |
| 1-Ethylnaphthalene | 0.51 | 0 |
| 1,4,5,8-Tetramethylnaphthalene | 1.64 | 0 |
| 1,4-Dimethylnaphthalene | 0 | 0.22 |
| 2-Vinylnaphthalene | 0 | 0 |
| Biphenyl | 0.70 | 0.36 |
| 7-Butyl-1-hexyl-naphthalene | 0 | 0.66 |
| 3,6,8-Trimethyl-1,2-dihydronaphthalene | 0 | 0.35 |
| 1-(1,1-Dimethylethyl)-naphthalene | 0 | 0.16 |
| 1,6-Dimethyl-4-(1-methylethyl)-naphthalene | 0 | 0.13 |
| 2-(1-Methylethyl)-naphthalene | 0 | 0.11 |
| phenanthrenes | ||
| 1-Phenylnaphthalene | 0 | 0.13 |
| Fluorene | 1.44 | 0.37 |
| 9-Methyl-9H-fluorene | 0.88 | 1.17 |
| 1,4-Dimethyl-6-phenylnaphthalene | 0 | 0.11 |
| Acenaphthene | 0 | 0.12 |
| 2,3,5-Trimethylphenanthrene | 0.75 | 0 |
| 1-Azafluorenone | 0 | 0.19 |
| 3-Methylphenanthrene | 0 | 0 |
| 3,6-Dimethylphenanthrene | 0 | 0.30 |
| 9,9-Dimethyl-9H-fluorene | 0 | 0.28 |
| 1,1-Dimethylindene | 0 | 0.08 |
| 8-Isopropyl-1,3-dimethylphenanthrene | 0 | 0.57 |
| 4,5-Dimethylphenanthrene | 0.58 | 0.21 |
| 2-Methylphenanthrene | 0 | 2.14 |
| 1-Ethyl-2-methylphenanthrene | 0 | 0.59 |
| 2,3-Dimethylphenanthrene | 0 | 0.13 |
| 9-Methylene-9H-fluorene | 0 | 1.10 |
| Anthracene | 0.69 | 0 |
| 4-Methylphenanthrene | 0.63 | 0 |
| 1-Methylanthracene | 0.88 | 0 |
| 1a,9b-Dihydro-1H-cyclopropa[l]phenanthrene | 1.09 | 0 |
| Pyrenes | ||
| Pyrene | 0.41 | 1.00 |
| 11H-Benzo[b]fluorene | 0 | 1.87 |
| Benzo[b]naphtho[2,3-d]furan | 0 | 0.2 |
| Benzo[a]fluorene | 0 | 0.29 |
| 1,9-Dimethylpyrene | 0 | 0.74 |
| Triphenylene | 0.34 | 0 |
| Pyranthrene | 0 | 2.05 |
| 4,12-Dimethylbenzo[a]anthracene | 0 | 0.14 |
| 1-Methylpyrene | 0.85 | 0 |
| Naphthophenanthrene | 0 | 0.96 |
| Benzo[a]pyrenes | ||
| Perylene | 1.34 | 0.37 |
| 8H-Indeno[2,1-b]phenanthrene | 0 | 0.23 |
| Benzo[ghi]perylenes | ||
| Benzo[ghi]perylene | 0 | 0 |
| Benzo[ghi]perylene | 0 | 0.16 |
| Aromatics | ||
| Oxygen-containing aromatics | ||
| 1,3-Dihydro-2H-indenone | 0 | 0.09 |
| 2,3,4,6,8,9,10,11-Octahydro-6-oxo-1H-pyrido[3,2-a]quinazoline | 0 | 0.09 |
| Cyclohexanecarboxylic acid, (1H-tetrazol-5-yl)amide | 0 | 0.53 |
| Cyclopentanone, O-methoxime | 0 | 0.30 |
| 11-Oxodibenzo[b,e]oxepin-11(7H)-one | 0 | 0.08 |
| Phenanthro[1,9-cd]pyrazol-6(2H)-one | 0 | 0.15 |
| 4-Fluoro-2-(trifluoromethyl)benzoic acid, 2-nitro-5-fluorophenyl ester | 0 | 0.08 |
| 9,9′-Bis(9H-fluorene), 9,9′-dihydroxy- | 0 | 0.18 |
| 1-Acetamido-bicyclo [3.2.0]heptan-2-one | 0 | 0.14 |
| 2-(2,4,6-Cycloheptatrienyl)-1,3-indenone | 0 | 0.12 |
| 1-(1-Methylcyclohexyl)acetone | 0 | 0.19 |
| Trimesic acid, propyl tridec-2-ynyl ester | 0 | 0.08 |
| o-Isopropylphenol methylcarbamate | 0 | 0.08 |
| 5-(p-Phenoxyphenyl)pentanal | 0 | 0.08 |
| 4-Ethyl-3-methyl-3-phenylpyrrolidine-2,5-dione, TMS (isomer 2) | 0 | 0.08 |
| 1H-Benzimidazole, 5,6-dimethyl- | 0 | 0.08 |
| 3,3-Diethylpyrrolidine-2,4-dione | 0 | 0.08 |
| 3-Phenylfuran | 0 | 1.1 |
| Naphtho[2,1-b]furan | 0 | 0.14 |
| Opianic acid | 0 | 0.11 |
| Flavone | 0 | 0.12 |
| 2-Benzylpyridine | 0 | 0.22 |
| 3-Acetyl-2,5,6-trimethylhydroquinone | 0 | 0.09 |
| 7-Methyl-2-naphthol | 0 | 0.83 |
| Benzo(b)naphtho(1,2-d)furan | 0 | 0.19 |
| [[4-(1,1-Dimethylethyl)phenoxy]methyl]-oxirane | 0 | 0.32 |
| 2-Methyl-1-naphthol | 0 | 0.83 |
| 9H-Fluorenol | 0 | 1.33 |
| 6-(4-Cyanophenyl)-2-naphthyl hexanoate | 0 | 1.16 |
| 1-Naphthol | 0 | 0.39 |
| 2,3-Dihydro-1H-inden-5-ol | 0 | 0.35 |
| 9H-Fluorenol | 0 | 0.28 |
| 9,10-Dihydro-9,10-dimethyl-9,10-ethanophenanthrene-11,12-dicarboxylic acid, dimethyl ester | 0 | 0.25 |
| 4,5-Dimethoxy-2-hydroxyacetic acid phenyl ester | 0 | 0.22 |
| 2-Phenanthrol | 0 | 0.22 |
| Benzo(a)pyrene-7-ol | 0 | 0.21 |
| 1-(4-Hydroxyphenyl)propane-1,2-diol (isomer 2) | 0 | 0.17 |
| 3-Amino-3-(4-methoxy-1-naphthyl)-propanoic acid | 0 | 0.17 |
| Glutaric acid, monoamide, N,N-bis(4-methylphenyl)-, nonyl ester | 0 | 0.16 |
| 4-Methyl-1-naphthyl methyl ether | 0 | 0.29 |
| 2-Methoxyfluorene | 0 | 0.13 |
| N-Phenylpropargyl-3-phenolamine | 0 | 0.13 |
| Tuberostemonine | 0 | 0.13 |
| 2-(1-Methylethyl)-phenol, methylcarbamate | 0 | 0.12 |
| 9H-Fluorenone | 0 | 0.12 |
| Catechol phenylboronate | 0 | 0.11 |
| Dibenzo-para-dioxin | 0 | 0.10 |
| 1-(1-Hydroxy-2-methylpropyl)-cyclopropanecarboxylic acid, 2,6-bis(1,1-dimethylethyl)-4-methylphenyl ester | 0 | 0.13 |
| 1-Vinylphenanthrol, 1,2-dihydro-1-methyl- | 0 | 0.09 |
| Phthalic acid, propyl-2-tridec-1-ynyl ester | 0 | 0.09 |
| 9H-Fluorenone | 0 | 0.08 |
| 1-Ethyl-4-methoxybenzene | 0.72 | 0 |
| 3,4-Dihydro-8-methyl-1(2H)-naphthalenone (one benzene ring containing oxygen, oxygen not on the benzene ring) | 0.43 | 0 |
| 1,1′-(Dioxydiethylidene)bis[3-ethylbenzene] (two benzene rings, oxygen not on the benzene rings) | 0.41 | 0 |
| 1-Methyl-2-indenone (1, O not on) | 0.41 | 0 |
| 1,2,3,4-Tetrahydro-5,6,7,8-tetramethylnaphthalene | 1.37 | 0 |
| 4-Methyldibenzofuran | 2.54 | 0 |
| 2,3-Diphenyl-2-cyclopropen-1-one (2, O not on) | 1.02 | 0 |
| N-Methyl-melochinone (1, O and N not on) | 0.45 | 0 |
| 1-Oxindole benzo[b]naphtho[e]bicyclo[2.2.2]octane (3, O not on) | 0.34 | 0 |
| Indeno[2,1-b]chromene | 0 | 0.13 |
| 1-Phenyl-1-pentyne (monocyclic containing oxygen, oxygen not on the ring) | 0.31 | 0 |
| Rhinitis tablet base | 0 | 0.09 |
| Nitrogen-containing aromatics | ||
| 5,7-Dimethylpyrano[3,4-a]indole | 0 | 0.38 |
| 1H-Phenanthro[9,10-d]imidazole-2-amine | 0 | 0.09 |
| 4-tert-Butylphthalonitrile | 0 | 0.08 |
| 3-Phenylpyridine | 0 | 0.15 |
| 10H-Benzothiophene-2-carbonitrile, trimethylsilyl derivative | 0 | 7.47 |
| 4-Pyridinamine, N-(1-naphthylmethyl)- | 0 | 0.23 |
| 5,6-Dimethyl-1H-benzimidazole | 0 | 0.21 |
| Benzylhydrazine | 0 | 0.33 |
| 2-(1-Methylcyclopropyl)aniline | 0 | 0.26 |
| 1,8-Naphthyridine, 2,4,7-trimethyl- | 0 | 0.09 |
| 4-Methylamino-5-amino-fluorene | 0 | 0.09 |
| Aniline, 4,4′-(1,2-ethylenediyl)bis- | 0 | 0.09 |
| 3-Methyl-3-phenyl(3H)benzo[c]pyrrole (three rings, two benzene rings, N on another ring) | 0.91 | 0 |
| 4-Propylthiazole | 0 | 0.38 |
| 1H-Phenanthro[9,10-c]pyrazole (four rings, three benzene rings, N on another ring) | 0.30 | 0 |
| Sulfur-containing aromatics | ||
| Acetoxy-2-norbornyl succinate | 0 | 0.13 |
| 4,9-Dimethylnaphtho[2,3-b]thiophene (three aromatic rings, two benzene rings, S on the thiophene ring) | 0.46 | 0 |
| Halogen-containing aromatics | ||
| 1-[4-(Benzyloxy)phenyl]-2-bromo-1-propanone | 0 | 0.08 |
| 1-(3-Chloro-4-methylphenylsulfonyl)-4-piperidinecarboxylic acid, isopropylamide | 0 | 0.19 |
| Alkanes | ||
| 2,5-Furandicarboxaldehyde | 0 | 0.18 |
| Hexadecane | 0 | 0.81 |
| Heptadecane | 0.40 | 0 |
| Pyrrolidine-2,4-dione | 0 | 0.77 |
| Nonadecane | 0.36 | 2.64 |
| Tetracosane | 0.55 | 1.67 |
| Octadecane | 0.32 | 2.24 |
| 9-Hexylheptadecane | 1.44 | 0 |
| Pyrene | 0.93 | 0 |
| 9-Hexylheptadecane | 0.63 | 0 |
| 1,7-Dimethyl-4-(1-methylethyl)cyclodecane | 0 | 0.43 |
| 4-Methyl-2-pentanol | 0 | 1.44 |
| 1-Propylcyclopentene | 0 | 0.10 |
| 1,1,4,5,6-Pentamethyl-2,3-dihydro-1H-indene | 0 | 0.31 |
| 9-Methyl-1-undecene | 0 | 0.58 |
| Cyclohexylmethyl nonyl ester sulfate | 0 | 0.17 |
| Heptacosane | 0 | 2.11 |
| 2,6,10,14-Tetramethylhexadecane | 0 | 1.82 |
| 9-Methylfluorene | 0 | 0.1 |
| 4-(Trimethylsilyl)pyrazole, 2TMS derivative | 0 | 0.25 |
| Heneicosane | 0 | 1.59 |
| 2,6,11-Trimethyldodecane | 0 | 0.6 |
| 1-Tetradecene | 0 | 0.57 |
| 5-(1-Adamantyl)salicylic acid, methyl ester | 0 | 0.5 |
| Benzyl acetate | 0 | 0.49 |
| 2,4,6-Trimethyloctane | 0 | 0.37 |
| Butyl decanyl ester sulfate | 0 | 0.33 |
| Anisole | 0 | 0.28 |
| 2-Octen-4-one | 0 | 0.25 |
| 3-Ethoxy-3,4-dimethyl-1-hexyne | 0 | 0.22 |
| 1-Methylchrysene | 0 | 0.21 |
| 1,3,5,6-Tetramethyladamantane | 0 | 0.25 |
| Homobicyclo[2.2.1]heptan-2-one ethyl acetate | 0 | 0.16 |
| Methyl diisopropoxyethoxy silane | 0 | 0.16 |
| 1-(Hexyloxy)-4-methylhexane | 0 | 0.15 |
| 1-Adamantanamine, N-tert-butyldimethylsilyl- | 0 | 0.12 |
| 1,3-Di-n-propyladamantane | 0 | 0.11 |
| Heptafluorobutyric acid, undecyl ester | 0 | 0.45 |
| 2-Chloroacetic acid, 1,4-cyclohexanediylbis(oxy-2,1-ethanediyl) ester | 0 | 0.29 |
| 2-Bromotetradecane | 0 | 0.26 |
| Bromochloronitromethane | 0 | 0.21 |
| Carbonic acid, allyl-pentafluorobenzyl ester | 0 | 0.12 |
| 2-Fluoropyridine | 0 | 0.26 |
| 2-(2-Benzyl-1,2-dihydroisoquinolin-3-yl)ethanol | 0 | 0.13 |
| 4-Ethyloctane | 0.16 | 0 |
| 1-Bromo-heptadecane | 0.1 | 0 |
| Acenaphthene | 0.27 | 0 |
| 2,6,10-Trimethyl-tetradecane | 0.26 | 0 |
| 3-Ethyl-5-(2-ethylbutyl)-octadecane | 0.73 | 0 |
| 2,3-Dihydro-5,6-dimethyl-1H-indene | 0.31 | 0 |
| 3-(2-Methyl-1-propenyl)-1H-indene | 0.47 | 0 |
| 1,7-Dimethyl-3-phenyltricyclo[4.1.0.0(2,7)]heptene | 0.41 | 0 |
| 17.α.,21.β.-28,30-Bisnorhopane | 0.22 | 0 |
| 2,3-Dimethyl-2-cyclopenten-1-one | 0.09 | 0 |
| 3,7,11-Trimethyl-dodecanol | 0.10 | 0 |
| 7-Hexyltridecan-1-ol | 0.22 | 0 |
| Methyl 5,8,11,14-eicosatetraynoate | 0.86 | 0 |
| Podophyllotoxin | 0.32 | 0 |
| 9-Hexadecenoic acid | 0.27 | 0 |
| (Z)-Eicosyl 9-hexadecenoate | 0.42 | 0 |
| n-Hexadecyl succinic anhydride | 0.34 | 0 |
| Tetrahydrofishtoxin 287b | 0.25 | 0 |
| 1-Tricosanol | 1.84 | 0 |
| 3,13,16,20-Tetraacetoxy-3-deoxy-3,16-dihydroxy-12-deoxypodophyllotoxin | 0.21 | 0 |
| tert-Hexadecyl mercaptan | 0.34 | 0 |
| 2-Amino-3-cyano-4,5,6,7-tetrahydro-5,7-dimethylthionaphthene | 0.26 | 0 |
| 2-Chloro-2-nitropropane | 0 | 0.10 |
| 2-Butyl-1,3-dioxane-5,5-dimethanol | 0 | 0.10 |
| 28-Nor-17α-hopane | 0 | 0.10 |
| 1-Methoxycyclododecanemethanol | 0 | 0.09 |
| Others | 16.73 | 19.62 |

Table A4.
Tar composition of HCG sample.
Table A4.
Tar composition of HCG sample.
| HCG/wt% | Pyrolysis | Cryogenic |
|---|---|---|
| 500 °C | −90 °C | |
| Benzenes | ||
| Propylbenzene | 0.35 | 0 |
| 1-Ethyl-2-methylbenzene | 1.45 | 0 |
| 1,2,3-Trimethylbenzene | 0.36 | 0 |
| p-Xylene | 0 | 0.46 |
| 1-Hexene, 2-(o-phenyldimethyl)-4-methyl (C14H20O derivative) | 0.33 | 0 |
| 1-Ethyl-4-methoxybenzene (C9H12O derivative) | 0.77 | 0 |
| Di(2-ethylhexyl) phthalate (C24H38O4 derivative) | 0.54 | 0 |
| Phenols | ||
| Phenol | 2.51 | 0 |
| 2-Methylphenol | 3.27 | 2.28 |
| p-Cresol | 5.74 | 5.97 |
| 2,6-Dimethylphenol | 0.88 | 0 |
| 2-Ethylphenol | 0.80 | 0 |
| 2,4-Dimethylphenol | 4.32 | 0 |
| 3-Ethylphenol | 3.12 | 0 |
| 2,3-Dimethylphenol | 0.38 | 0 |
| 2-(1-Methylpropyl)phenol | 0.16 | 0 |
| 3,4-Dimethylphenol | 0.77 | 1.30 |
| 2,3,5-Trimethylphenol | 1.41 | 0 |
| 3-(1-Methylpropyl)phenol | 0.80 | 0 |
| 2-Ethyl-4-methylphenol | 0.23 | 0 |
| 3-Ethyl-5-methylphenol | 1.72 | 0 |
| 3,5-Diethylphenol | 0.43 | 0 |
| β-Naphthol | 0.44 | 0 |
| 7-Methyl-2-naphthol | 0.70 | 0 |
| 4-Ethylphenol | 0 | 0.32 |
| 4-Ethyl-2-methylphenol | 0 | 1.54 |
| PAHs | ||
| Naphthalenes | ||
| 2,3-Dihydro-5-methylinden | 0.38 | 0 |
| 2-Methylnaphthalene | 0.92 | 2.12 |
| 1-Methylnaphthalene | 0.60 | 0 |
| 1,2,3,4-Tetrahydro-6,7-dimethylnaphthalene | 0.16 | 0 |
| 2,3-Dihydro-5-indanol | 0.62 | 0 |
| 1,6-Dimethylnaphthalene | 0.59 | 0 |
| 1,3-Dimethylnaphthalene | 2.74 | 0 |
| 2,6-Dimethylnaphthalene | 0.71 | 0 |
| Dimethylbiphenyl | 0.35 | 0 |
| 1,4-Dimethylnaphthalene | 0.21 | 0 |
| 1,4,5-Trimethylnaphthalene | 1.43 | 0 |
| 2,3,6-Trimethylnaphthalene | 1.72 | 0 |
| 1,6,7-Trimethylnaphthalene | 0.49 | 0 |
| 1,4,5,8-Tetramethylnaphthalene | 0.83 | 1.04 |
| 1,2-Dimethylnaphthalene | 0 | 1.83 |
| 1,4,6-Trimethylnaphthalene | 0 | 2.63 |
| 2-Phenyl-1H-indene; | 0 | 1.19 |
| 4-Ethylbiphenyl | 0 | 0 |
| (4-Methoxybenzyl)phenethylamine (bicyclic C16H19NO) | 0.25 | 0 |
| 2,3-Dihydro-5-indanol (bicyclic C9H10O) | 0.61 | 0 |
| Phenanthrenes | ||
| 1-Methylphenanthrene | 0.37 | 0 |
| 9-Methylanthracene | 0 | 1.46 |
| 4-Methylphenanthrene | 1.07 | 0 |
| 3-Methylphenanthrene | 0.25 | 0 |
| 2,3,5-Trimethylphenanthrene | 0.46 | 0 |
| 2-Methylanthracene | 0 | 0.42 |
| Benzo[b]fluorene | 0 | 0.32 |
| 9-Hydroxyflavone | 0.65 | 0 |
| 9,9-Dimethylflavone | 0.25 | 0 |
| 2,3,3-Trimethyl-3H-pyrrolo[3,2-H]quinoline (tricyclic C14H14N2) | 0.34 | 0 |
| Pyrenes | ||
| Pyrene | 0 | 0.41 |
| Benzo[a]anthracene | 0 | 0.34 |
| Benzo[c]phenanthrene | 0 | 0.27 |
| 9,10-Dihydro-11,12-diacetyl-9,10-oxanthracene | 0.68 | 0 |
| Benzo[a]pyrenes | ||
| Benzo[e]pyrene | 0 | 0.31 |
| Aromatics | ||
| Nitrogen-containing aromatics | ||
| Norsamide | 0 | 0.55 |
| Ethylpyrazine | 0.13 | 0 |
| 2-Methyl-5-phenylpyrrole | 0.21 | 1.02 |
| 9,10-Dihydroacid diethyl ester | 0.65 | 0.7 |
| 1H-Pyrrolo[3,2-H]quinoline, 2,3,6,8-tetramethyl- | 0 | 0.31 |
| Pyridinemethanol, α-(azidomethyl)-1-[(pyridylcarbonyl)oxy]- | 0 | 4.10 |
| Phenol, 2-[(ethylamino)methyl]-4-nitro | 1.07 | 2.08 |
| 1-[(pyridylcarbonyl)oxy]-2,5-pyrrolidine-dione | 1.52 | 2.51 |
| 2,4,6-(1H,3H,5H)-pyrimidinetrione, 5-acetyl- | 0.53 | 1.25 |
| Oxygen-containing aromatics | ||
| 9H-Fluorene | 0.75 | 1.58 |
| 2-Vinylfuran | 1.44 | |
| Sulfur-containing aromatics | ||
| 1,5-Dimethoxy-naphthalene | 0.24 | |
| 9,9-Dimethylfluorene | 0.53 | 0.97 |
| 2,4-Dihydroxy-3,6-dimethylbenzaldehyde | 0.41 | 1.26 |
| 1,5-Diacetyl-2,6-naphthalenediol | 1.5 | 2 |
| Aromatics containing halogen elements | ||
| m-Cresol, TMS derivative | 0.12 | 0.23 |
| 5-Chloro-3-ethyl-1H-pyrrole-derived pyridine | 0.42 | |
| Succinic acid, 2,4,6-trichlorobenzyl-2-naphthyl ester | 0.6 | 0.94 |
| 2-Chloro-4-(2-hydroxyphenyl)pyrimidine | 0.41 | 0.8 |
| Alkanes | ||
| Decane | 0.22 | 0 |
| Undecane | 0.45 | 0 |
| 1-Dodecene | 0.14 | 0 |
| Dodecane | 0.48 | 0 |
| Tridecane | 1.18 | 0 |
| 1-Tetradecene | 0.56 | 0 |
| Tetradecane | 1.32 | 0 |
| Pentadecane | 1.51 | 0 |
| Hexadecane | 1.43 | 12.75 |
| Heptadecane | 1.38 | 0 |
| 2,6,10,14-Tetramethylpentadecane | 0.59 | 0 |
| Octadecane | 1.57 | 0 |
| Nonadecane | 1.94 | 0 |
| Eicosane | 1.93 | 0 |
| Heneicosane | 1.78 | 0 |
| Docosane | 1.85 | 0 |
| (Z)-9-Docosene | 0.24 | 0 |
| Tricosane | 1.34 | 0 |
| Tetracosane | 2.13 | 0 |
| Pentacosane | 2 | 0 |
| 3-Ethyl-5-(2-ethylbutyl)octadecane | 3.76 | 0 |
| Heptacosane | 1.45 | 0 |
| Butenoic acid, undecyl ester | 0.42 | 0 |
| 1-Dodecanol, 3,7,11-trimethyl | 0.86 | 0 |
| 5,8-Diethyl-dodecane | 0.48 | 0 |
| 1-Pentadecene | 0.57 | 0 |
| E-15-Heptenal | 0.45 | 0 |
| E-14-Hexenal | 0.49 | 0 |
| 5,8,11,14-Eicosatetraenoic acid | 0.67 | 0 |
| n-Nonadecanol | 0.65 | 0 |
| E-15-Heptenal | 0.58 | 0 |
| 17-Pentacosene | 0.64 | 0 |
| 1-Tricosanol | 1.58 | 0 |
| 3,3-Dimethylhexane | 0 | 3.98 |
| 1-Iodododecane | 0 | 1.85 |
| 2,2,5-Trimethyl-3,4-hexanedione | 0 | 0.41 |
| 2,5-Dimethyl-3-hexanone | 0 | 0 |
| 2,4-Dimethyldecane | 0 | 0.65 |
| 1,1,3-Trimethylcyclopentane | 0 | 0.88 |
| 3,7-Dimethyldecane | 0 | 0 |
| 2,7-Dimethyloctane | 0 | 1.55 |
| 2,2-Dimethyl-3-hexene | 0 | 0.40 |
| Propylene | 0 | 0.36 |
| (E)-5-Methyl-4-decene | 0 | 0.68 |
| 2,6,11-Trimethyl-dodecane | 0 | 3.16 |
| 3,7-Dimethylundecane | 0 | 2.93 |
| Acetone | 0 | 0.14 |
| Propyne | 0 | 0.17 |
| (2S,6R,7S,8E)-(+)-2,7-Epoxy-4,8-giant blue alkenyl | 0 | 2.00 |
| 1,3-Dihydro-1-methyl-5-(1-methylethyl)-2H-imidazole-2-thione | 0 | 0.52 |
| Phenobarbital | 0 | 0.64 |
| Cyclohexylmethylhexyl sulfate ester | 0 | 0.31 |
| Cyclohexyl(2-methylcyclohexyl)propanedinitrile | 0 | 0.16 |
| 3,3,6-Trimethyl-1,5-heptadiene-4-one | 0 | 0.14 |
| Di(cyclohexylmethyl) sulfate ester | 0 | 0.21 |
| N-Allyloctanamide | 0 | 1.35 |
| 1-(4,6-Dihydroxy-2,3,5-trimethyl-7-benzofuran-yl)ethanone | 0 | 0.22 |
| 2-Ethylhexyl isobutyl sulfate ester | 0 | 2.58 |
| Isobutylpentyl sulfate ester | 0 | 0.16 |
| Others | 6.78 | 15.18 |
References
- Ma, Z.-H.; Li, S.; Dong, X.-Q.; Li, M.; Liu, G.-H.; Liu, Z.-Q.; Liu, F.-J.; Zong, Z.-M.; Cong, X.-S.; Wei, X.-Y. Recent Advances in Characterization Technology for Value-Added Utilization of Coal Tars. Fuel 2023, 334, 126637. [Google Scholar] [CrossRef]
- Chen, J.; Xu, T.; Wu, Y.; Ke, Y.; Li, Y. In-Situ SANS Study on Spatial Evolution of Coal Nanoporosity during Pyrolysis at Elevated Temperatures. Powder Technol. 2024, 445, 120123. [Google Scholar] [CrossRef]
- Gong, B.; Tian, C.; Wang, X.; Chen, X.; Zhang, J. Mineralogical Characteristics and Arsenic Release of High Arsenic Coals from Southwestern Guizhou, China during Pyrolysis Process. Processes 2023, 11, 2321. [Google Scholar] [CrossRef]
- Du, Z.; Li, W. The Catalytic Effect from Alkaline Elements on the Tar-Rich Coal Pyrolysis. Catalysts 2022, 12, 376. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, W.; Yang, P.; Zhang, J.; Gao, K.; Shang, J.; Yang, B.; Wu, Z. In-Situ Infrared and Kinetic Characteristics Analysis on Pyrolysis of Tar-Rich Coal and Macerals. Fuel 2023, 348, 128601. [Google Scholar] [CrossRef]
- Melikoglu, M. Clean Coal Technologies: A Global to Local Review for Turkey. Energy Strategy Rev. 2018, 22, 313–319. [Google Scholar] [CrossRef]
- Zhu, Z.; Cong, R.; Zhou, L.; Zheng, H.; Tu, Y.; Wu, Z. Effects of Chemical Properties and Inherent Mineral Matters on Pyrolysis Kinetics of Low-Rank Coals. Processes 2021, 9, 2111. [Google Scholar] [CrossRef]
- Mi, Q.; Li, B.; Li, Y.; Ma, Y.; Shi, R. Kinetic Analysis of Pyrolysis Reaction of Hydrogen-Containing Low Rank Coals Based on Thermogravimetric Method. Processes 2023, 11, 706. [Google Scholar] [CrossRef]
- Luo, L.; Liu, J.; Zhang, H.; Ma, J.; Wang, X.; Jiang, X. TG-MS-FTIR Study on Pyrolysis Behavior of Superfine Pulverized Coal. J. Anal. Appl. Pyrolysis 2017, 128, 64–74. [Google Scholar] [CrossRef]
- Qiang, L.; Bai, B.; Peng, Z.; Zhang, S.; Chang, H.; Sun, M.; Xu, L.; Ma, X. Research on the Relationship between the Structure and Pyrolysis Characteristics of Pretreated Shendong Coal. Fuel 2021, 305, 121515. [Google Scholar] [CrossRef]
- He, R.Z.; Liu, H.; Lu, Q.X.; Zhao, Y.; Wang, X.; Xie, X.; Deng, X.; Yuan, S. Effects of Si and Al elements in coal on Fe-catalyzed brown coal pyrolysis. Fuel 2022, 315, 123170. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Y.; Xu, T.; Bhattacharya, S. A Review on in-Situ Process Analytical Techniques for the Thermochemical Conversion of Coal and Biomass. Rev. Chem. Eng. 2023, 40, 435–455. [Google Scholar] [CrossRef]
- Liang, P.; Qin, X.Z.; Bai, G.M.; Wu, Z.; Sun, D.; Zhang, Y.; Jiao, T. Effects of ionic liquid pretreatment on pyrolysis characteristics of a high-sulfur bituminous coal. Fuel 2019, 258, 116134. [Google Scholar] [CrossRef]
- Bai, B.; Qiang, L.; Zhang, S.; Mu, H.; Ma, X. Influence of Coal Structure Change Caused by Different Pretreatment Methods on Shengli Lignite Pyrolysis. Fuel 2023, 332, 126089. [Google Scholar] [CrossRef]
- Nelson, I.; Naleway, S.E. Intrinsic and Extrinsic Control of Freeze Casting. J. Mater. Res. Technol. 2019, 8, 2372–2385. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a Path to Build Complex Composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef]
- Cai, C.; Li, G.; Huang, Z.; Tian, S.; Shen, Z.; Fu, X. Experiment of Coal Damage Due to Super-Cooling with Liquid Nitrogen. J. Nat. Gas Sci. Eng. 2015, 22, 42–48. [Google Scholar] [CrossRef]
- Fu-bao, Z.; Bo-bo, S.; Jian-wei, C.; Ling-jun, M. A New Approach to Control a Serious Mine Fire with Using Liquid Nitrogen as Extinguishing Media. Fire Technol. 2015, 51, 325–334. [Google Scholar] [CrossRef]
- Xu, T.; Lei, Y.; Chen, L.; Wu, Y. Thermal Properties and Grey Correlation Degree Analysis of Tar-Rich Coal under Cryogenic and Pyrolysis Condition. Fuel 2024, 371, 132170. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Kang, G.; Li, K.; Ma, J.; Xu, G. Secondary Reactions Suppression during Fuel Fast Pyrolysis in an Infrared Heating Apparatus for the Fixed Bed Pyrolysis Process with Internals. J. Anal. Appl. Pyrolysis 2021, 156, 105163. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The Multi-Scale Challenges of Biomass Fast Pyrolysis and Bio-Oil Upgrading: Review of the State of Art and Future Research Directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Song, Y.; Lei, S.; Li, J.; Yin, N.; Zhou, J.; Lan, X. In Situ FT-IR Analysis of Coke Formation Mechanism during Co-Pyrolysis of Low-Rank Coal and Direct Coal Liquefaction Residue. Renew. Energy 2021, 179, 2048–2062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).