Evolutionary Mechanisms of Deep Coal Rock Chemical Structures Under Various Pre-Fracturing Fluids
Abstract
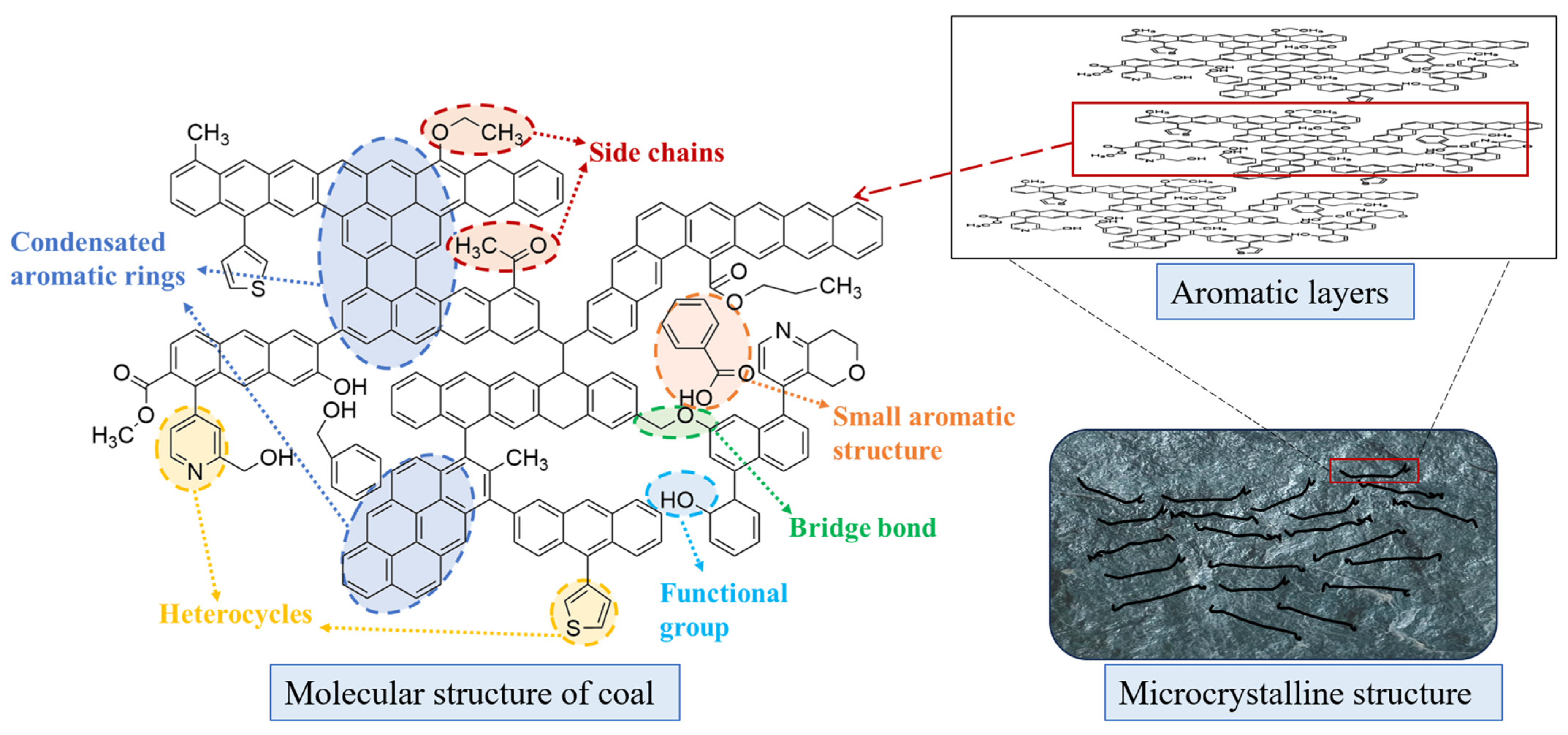
1. Introduction
2. Materials and Methods
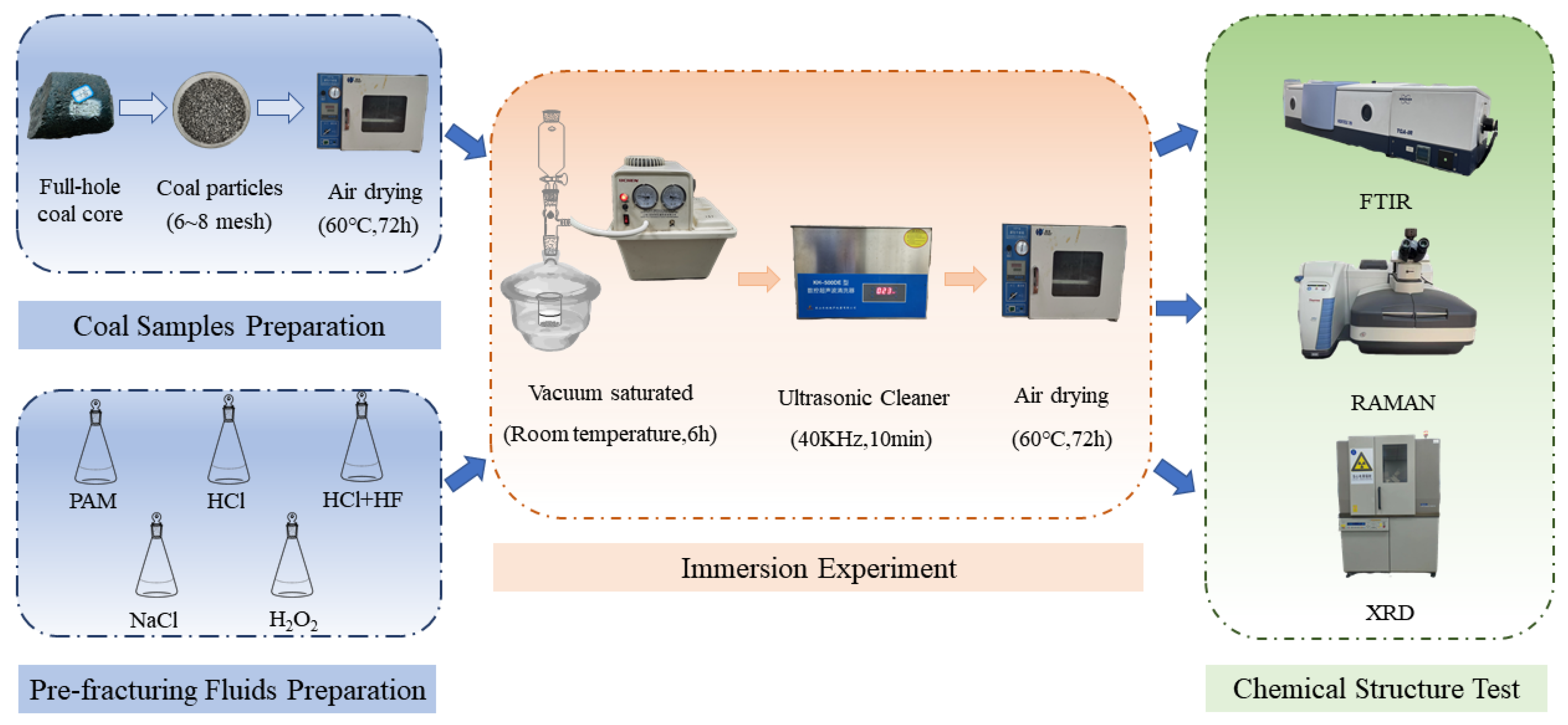
2.1. Coal Sample and Fluid Type
2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3. Raman Spectroscopy (RAMAN)
2.4. X-Ray Diffraction (XRD)
3. Results and Discussion
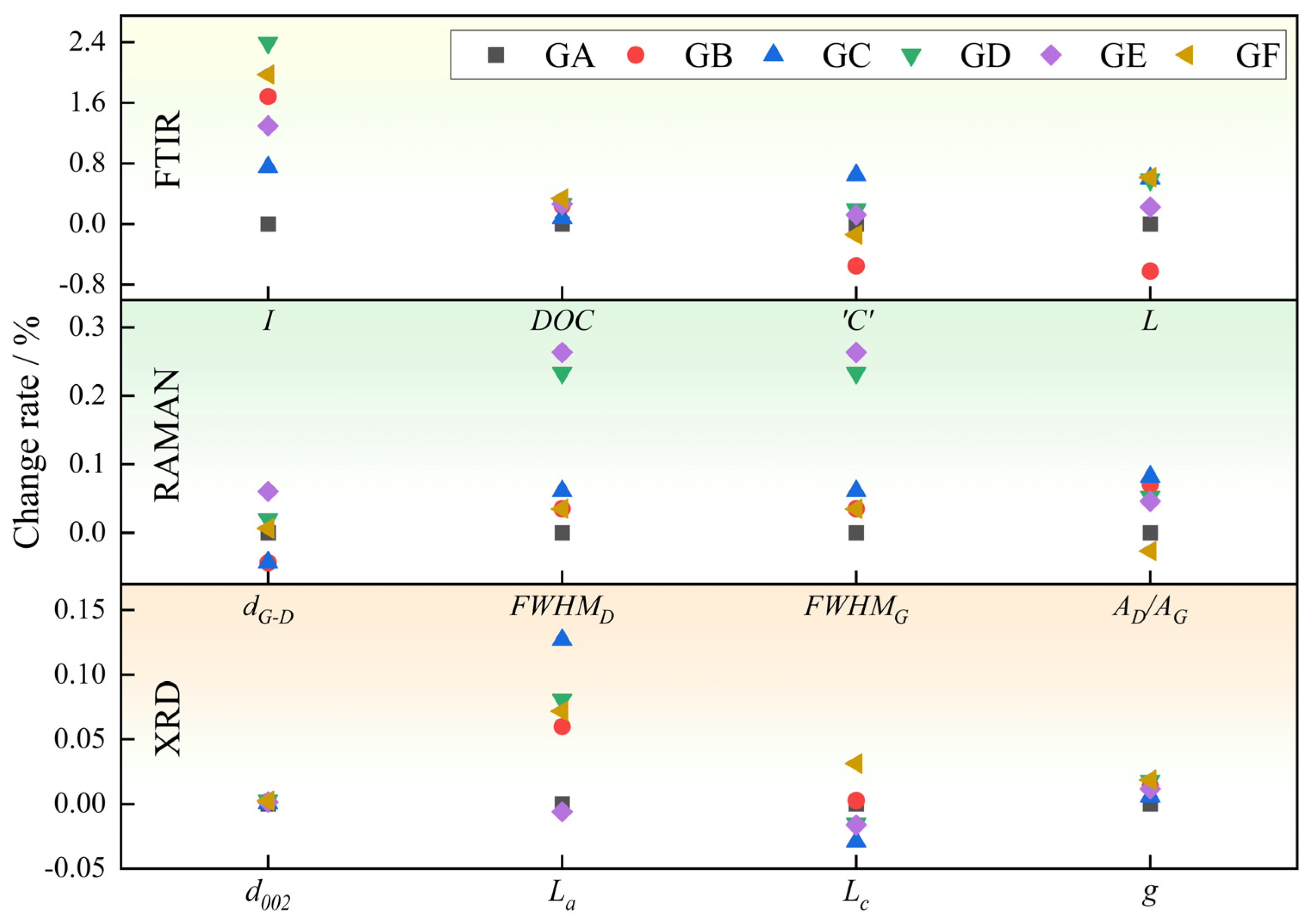
3.1. Functional Group Evolution Characteristics
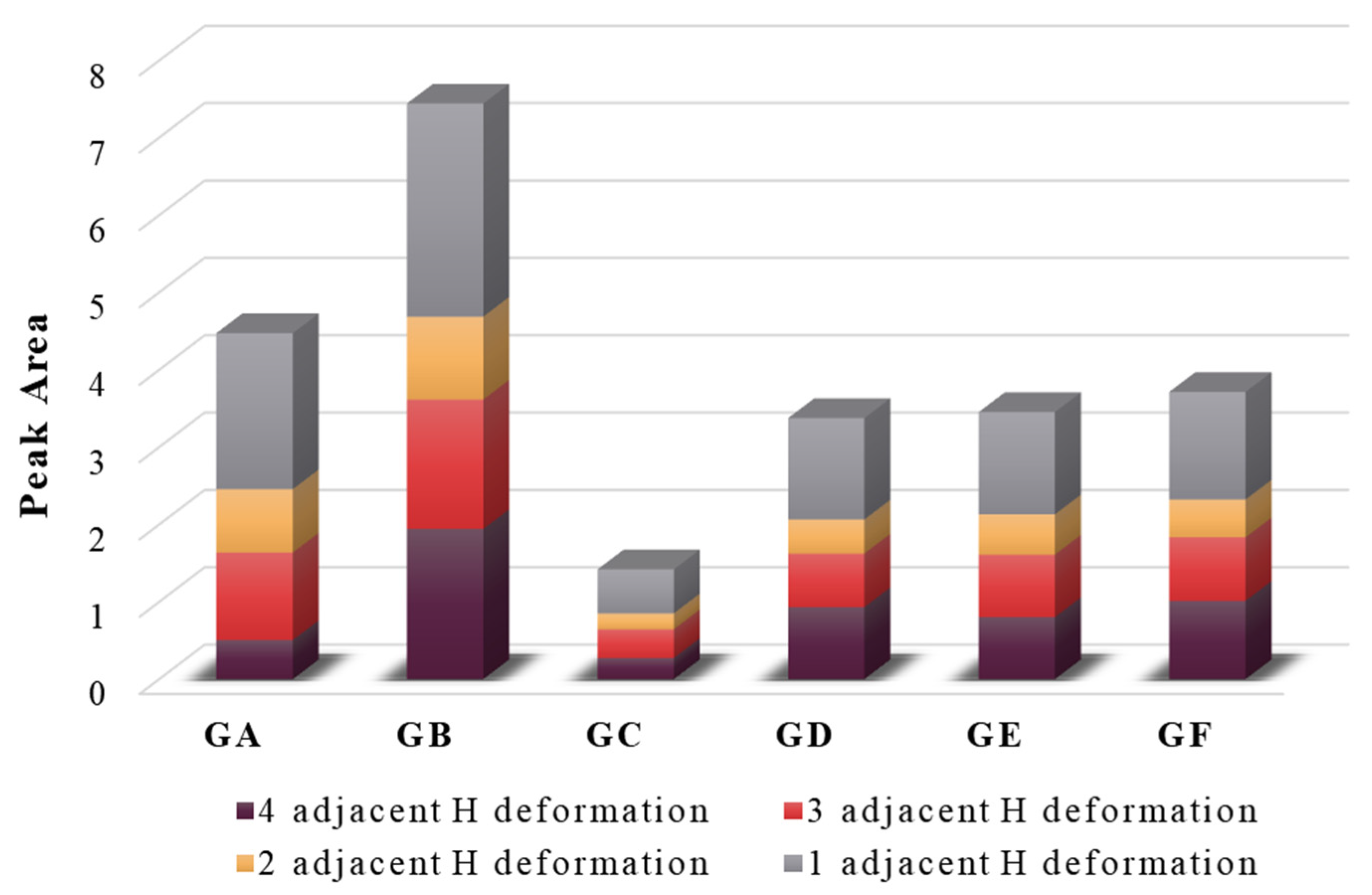
3.1.1. Aromatic Structure
- (1)
- The aromaticity index (I) is the ratio of aromatic functional groups to aliphatic functional groups in coal, representing the coal’s degree of aromaticity. It can be calculated as follows:where A represents the peak area at a specific wavenumber. The higher the value of I, the greater the degree of aromaticity [34].
- (2)
- The degree of aromatic condensation polymerization (DOC) is the ratio of aromatic structure to C=C bonds, which can be calculated as follows:
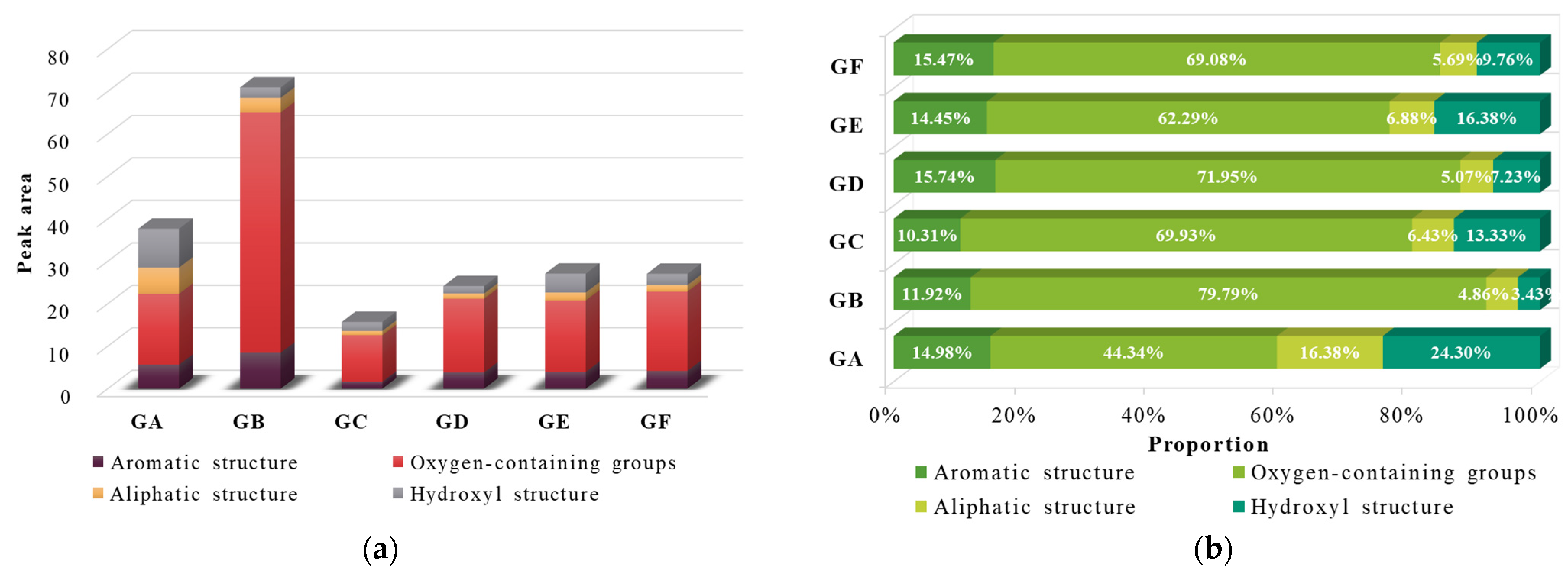
3.1.2. Oxygen-Containing Functional Groups
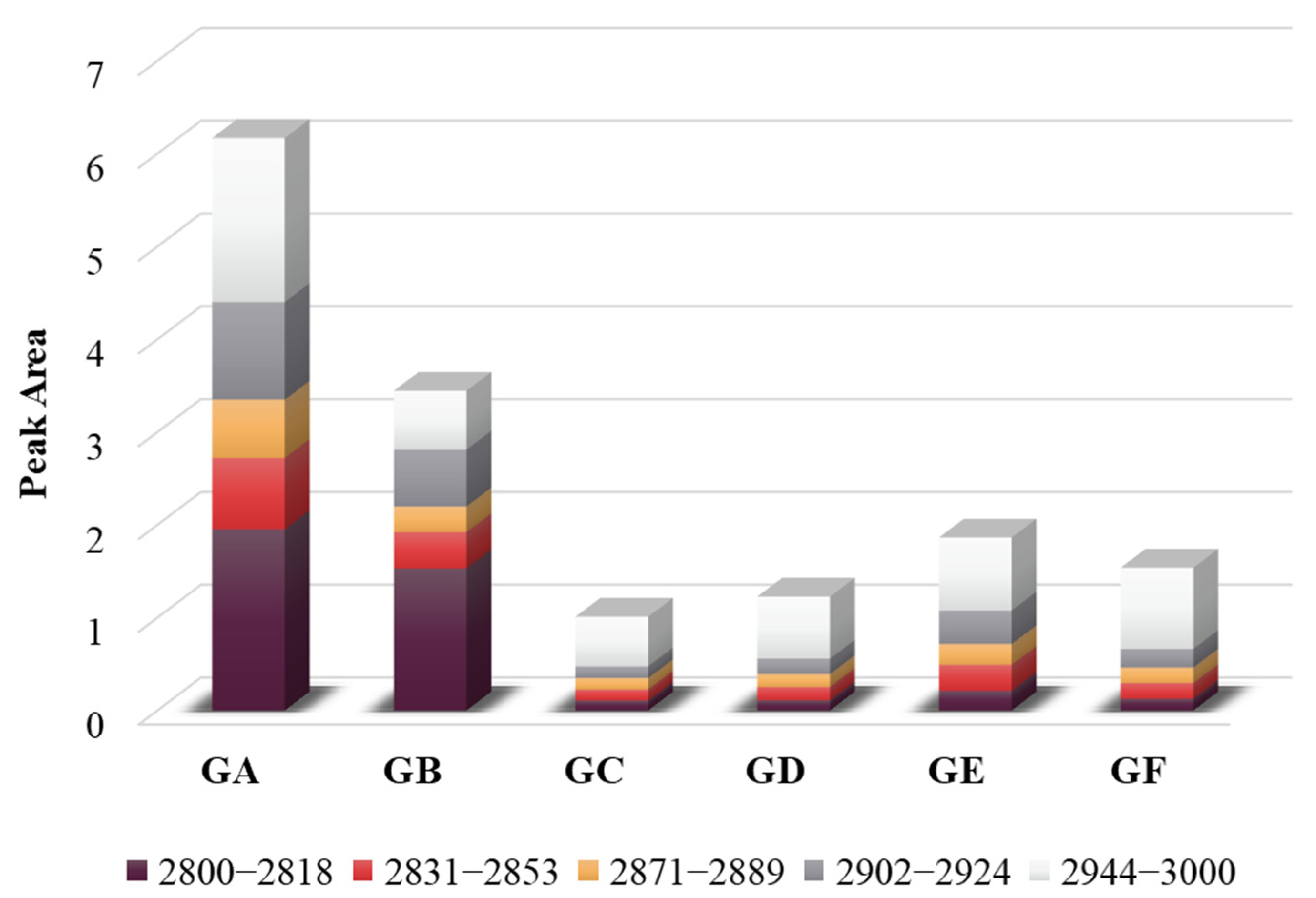
3.1.3. Aliphatic Structures
3.1.4. Hydroxyl Structure
3.2. Macromolecular Structure Evolution Characteristics
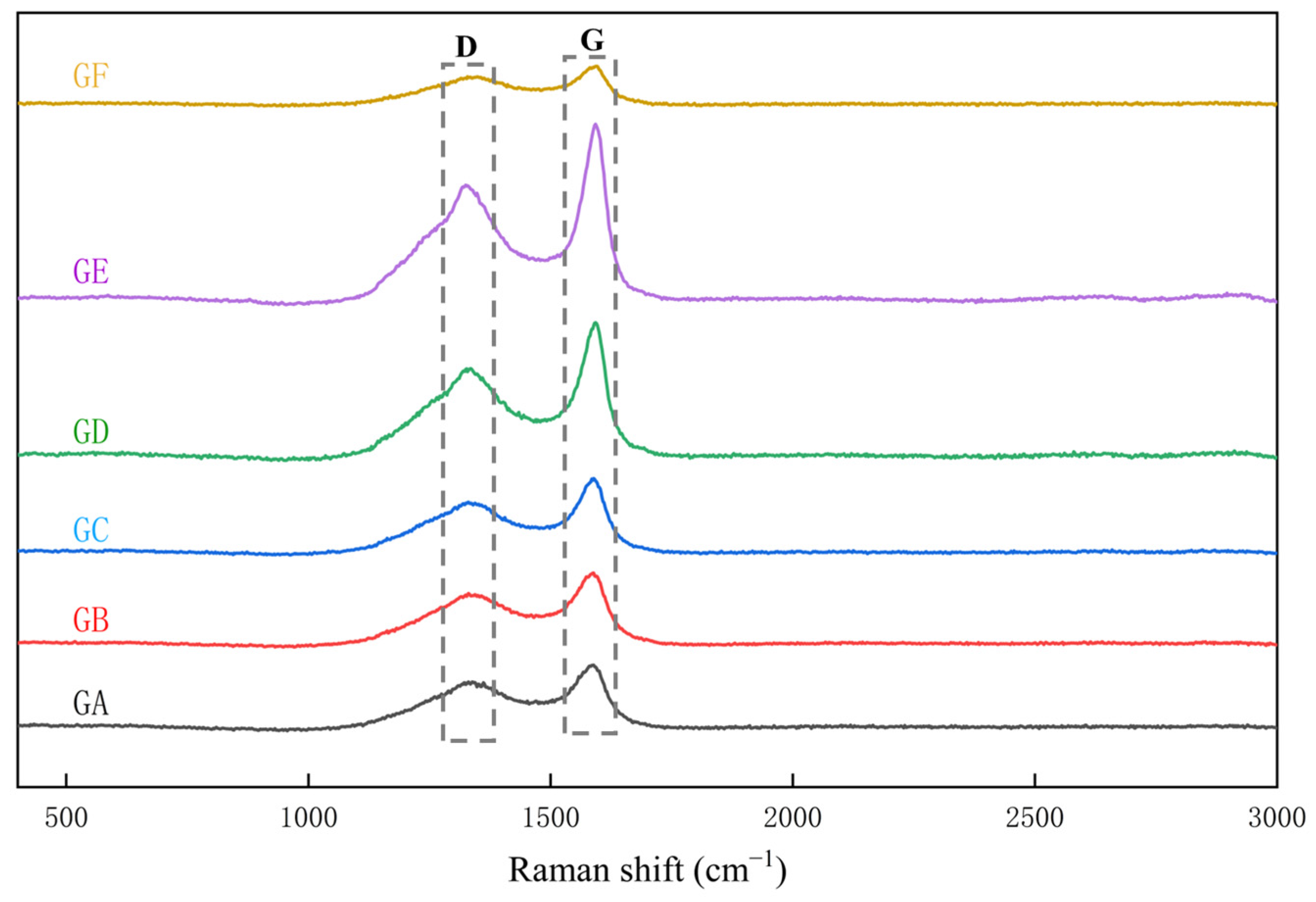
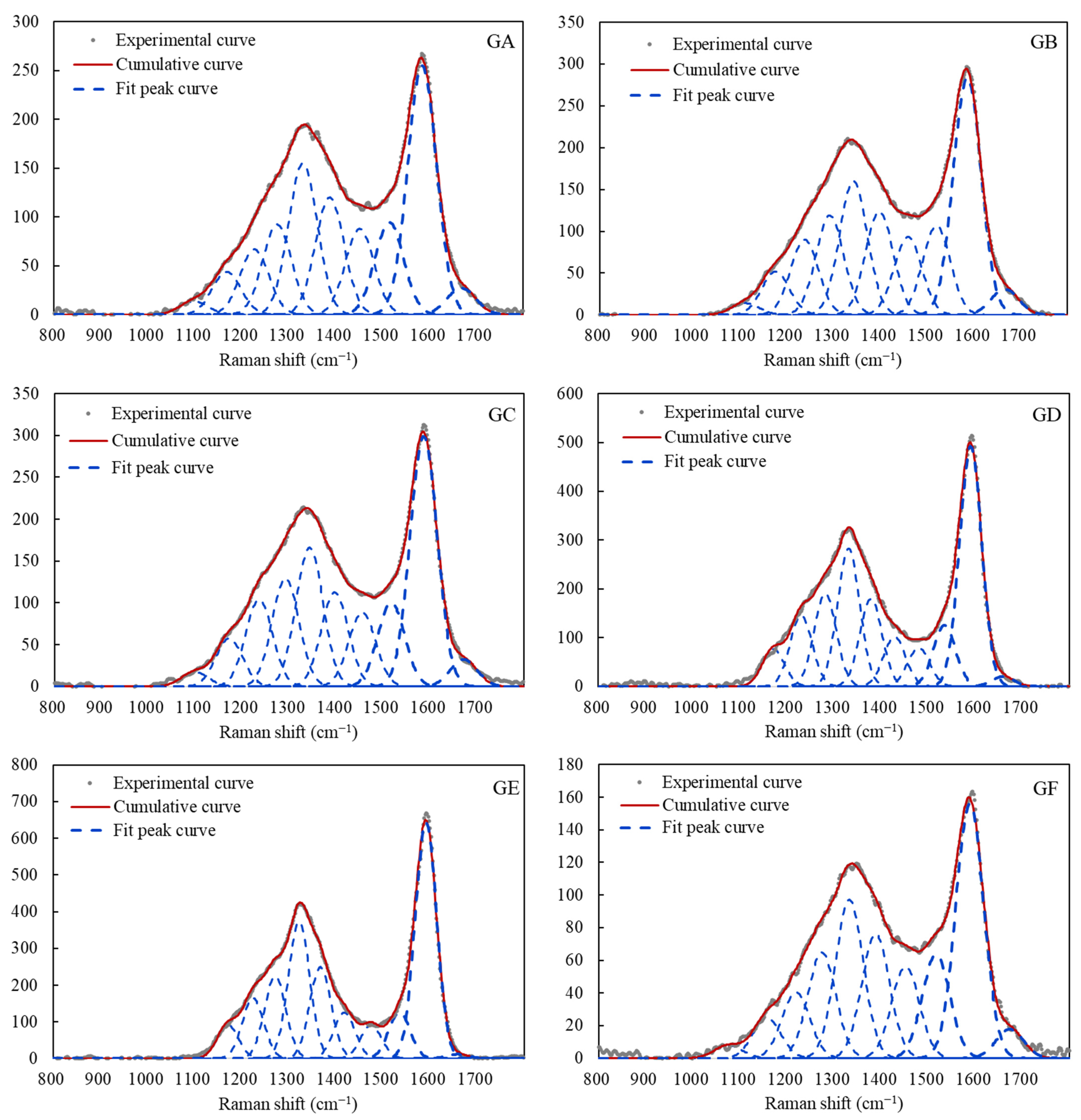
3.2.1. Peak Position
3.2.2. Full Width at Half Maximum (FWHM)
3.2.3. Peak Position Difference (dG–D)
3.2.4. Peak Area Ratio AD/AG
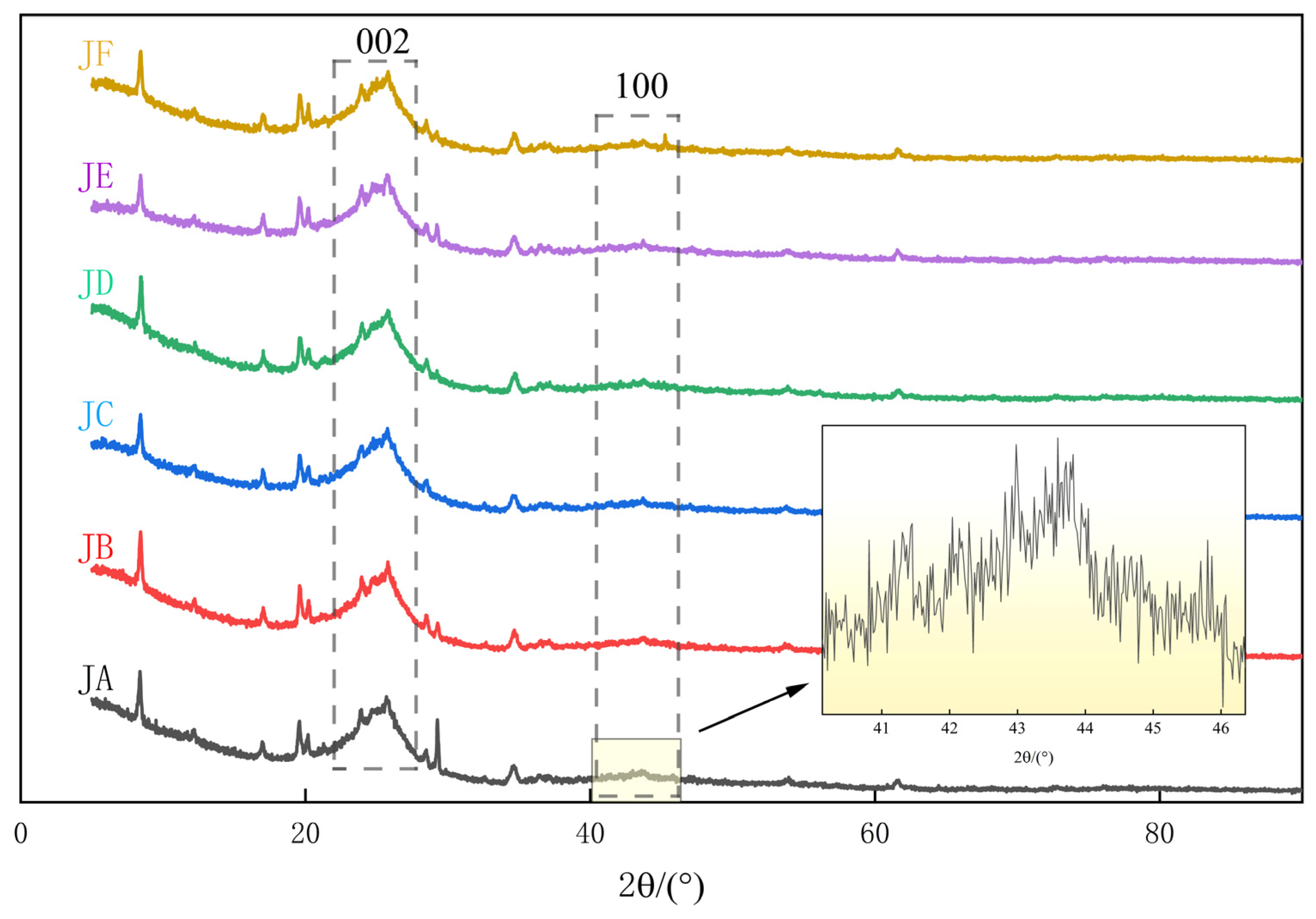
3.3. Microcrystalline Structure Evolution Characteristics
3.3.1. Interlayer Distance d002
3.3.2. Crystal Diameter La
3.3.3. Crystal Height Lc
3.3.4. Effective Stacking Layer Number Nave
3.3.5. Graphitization Degree g
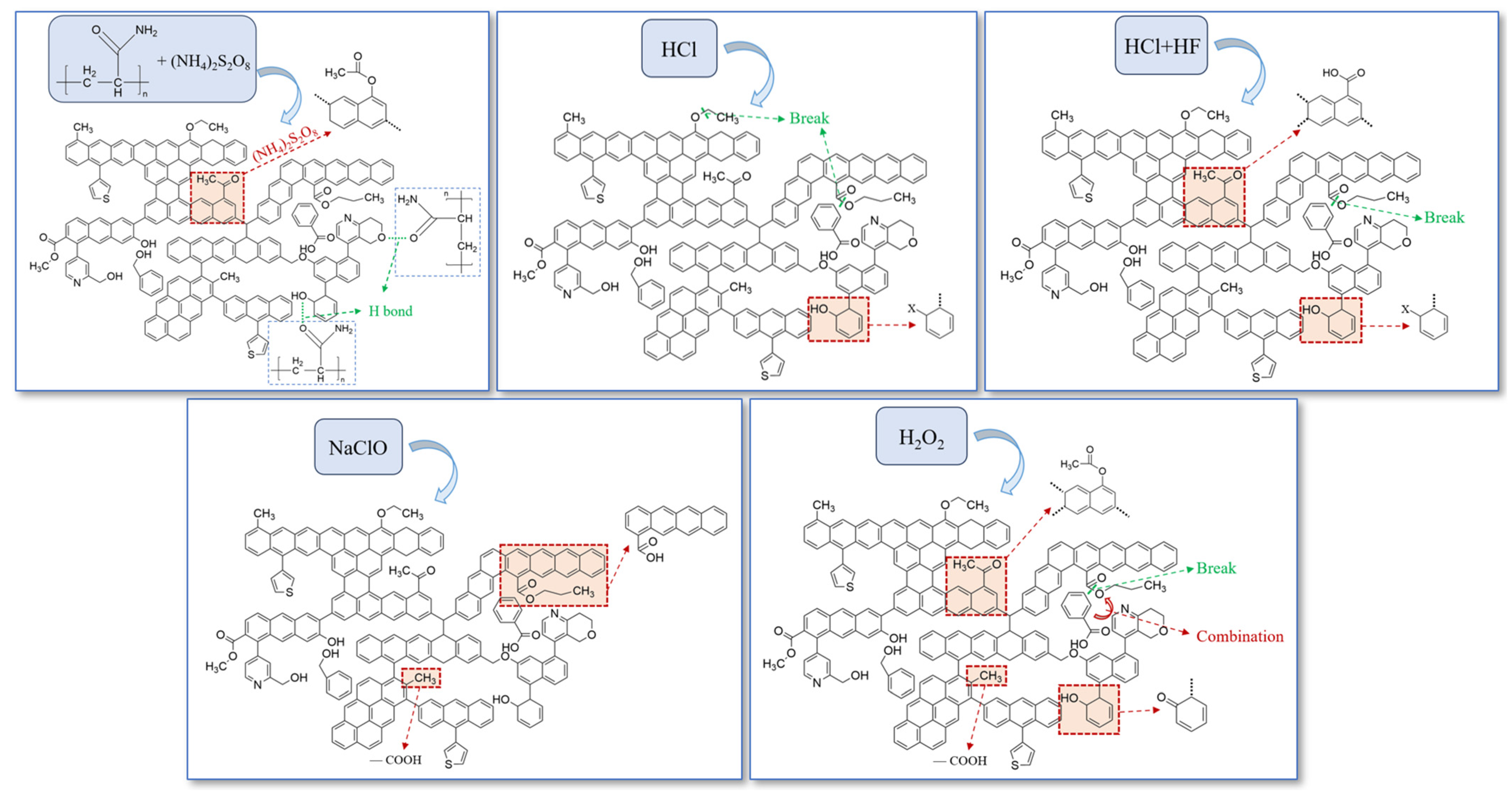
3.4. Mechanisms of Chemical Structure Evolution
4. Conclusions
- (1)
- Slick water, acid solutions and oxidizer solutions exert the same effect on the proportion of oxygen-containing functional groups (increase), aliphatic structures (decrease) and hydroxyl structures (decrease). After being treated by the five kinds of pre-fracturing fluids, I increased and DOC decreased. L increased after being treated by PAM, but decreased after being treated by acids and oxidizers.
- (2)
- Acids have the greatest effect on decreasing the content of functional groups; NaClO and HCl + HF have the most significant influence on the ordering of coal macromolecular structure, while H2O2 and HCl + HF exert the greatest influence on the graphitization degree of coal.
- (3)
- HCl + HF has a more pronounced effect on increasing the degree of aromaticity and graphitization of microcrystals than HCl.
- (4)
- NaClO has the most significant effect on the ordering of macromolecular structure, while H2O2 has the most significant effect on the graphitization degree of microcrystalline structure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Li, S.; Qin, Y.; Tang, D.; Shen, J.; Wang, J.; Chen, S. A comprehensive review of deep coalbed methane and recent developments in China. Int. J. Coal Geol. 2023, 279, 104369. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; He, H.; He, X.; Zhao, Z. Coal-rock gas: Concept, connotation and classification criteria. Pet. Explor. Dev. 2024, 51, 783–795. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 2. Adsorption rate modeling. Fuel 1999, 78, 1345–1362. [Google Scholar] [CrossRef]
- Thomas, L.; Tang, H.; Kalyon, D.M.; Aktas, S.; Arthur, J.D.; Blotevogel, J.; Carey, J.W.; Filshill, A.; Fu, P.; Hsuan, G.; et al. Toward better hydraulic fracturing fluids and their application in energy production: A review of sustainable technologies and reduction of potential environmental impacts. J. Pet. Sci. Eng. 2019, 173, 793–803. [Google Scholar] [CrossRef]
- Guo, H.; Yu, Y.; Wang, Y.; Wang, K.; Yuan, L.; Xu, C.; Ren, B. Experimental study on the desorption law and diffusion kinetic characteristics of gas in raw coal and tectonic coal. Energy 2024, 289, 129924. [Google Scholar] [CrossRef]
- Kiani, A.; Sakurovs, R.; Grigore, M.; Sokolova, A. Gas sorption capacity, gas sorption rates and nanoporosity in coals. Int. J. Coal Geol. 2018, 200, 77–86. [Google Scholar] [CrossRef]
- Crosdale, P.J.; Beamish, B.B.; Valix, M. Coalbed methane sorption related to coal composition. Int. J. Coal Geol. 1998, 35, 147–158. [Google Scholar] [CrossRef]
- Sun, G.; Ni, G.; Fu, Z.; Yin, X.; Wang, G.; Wang, Z. Comparative Study of Organic and Inorganic Acids on the Mechanism of Physical Damage to Coal Mechanics. Energy Fuels 2023, 37, 19526–19537. [Google Scholar] [CrossRef]
- Haenel, M.W. Recent progress in coal structure research. Fuel 1992, 71, 1211–1223. [Google Scholar] [CrossRef]
- Li, X.P.; Zeng, Q. Development and Progress of Spectral Analysis in Coal Structure Research. Spectrosc. Spectr. Anal. 2022, 42, 350–357. [Google Scholar]
- Balucan, R.D.; Turner, L.G.; Steel, K.M. X-ray μCT investigations of the effects of cleat demineralization by HCl acidizing on coal permeability. J. Nat. Gas Sci. Eng. 2018, 55, 206–218. [Google Scholar] [CrossRef]
- Rui, G.; Shaojie, Z.; Junting, S.; Chengwei, L.; Feng, T.; Zhizhong, J.; Changwei, W.; Shouqing, P.; Zhiyuan, X. Effects of Different Concentrations of Weak Acid Fracturing Fluid on the Microstructure of Coal. Nat. Resour. Res. 2024, 33, 2215–2236. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Yang, Y.; Zhou, Y.; Kong, B.; Li, J.; Si, L. Effect of acid treatment on the characteristics and structures of high-sulfur bituminous coal. Fuel 2016, 184, 418–429. [Google Scholar] [CrossRef]
- Balucan, R.D.; Turner, L.G.; Steel, K.M. Acid-induced mineral alteration and its influence on the permeability and compressibility of coal. J. Nat. Gas Sci. Eng. 2016, 33, 973–987. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, R.; Ramakrishna, S. Comparative experimental study on the effects of organic and inorganic acids on coal dissolution. J. Mol. Liq. 2021, 339, 116730. [Google Scholar] [CrossRef]
- Larsen, J.W.; Pan, C.S.; Shawver, S. Effect of demineralization on the macromolecular structure of coals. Energy Fuels 1989, 3, 557–561. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Yang, W.; Lu, J.; Lu, Y.; Liu, T.T.; Shi, S. Experimental study on erosion mechanism and pore structure evolution of bituminous and anthracite coal under matrix acidification and its significance to coalbed methane recovery. Energy 2023, 283, 128485. [Google Scholar] [CrossRef]
- Jing, Z.; Balucan, R.D.; Underschultz, J.R.; Steel, K.M. Oxidant stimulation for enhancing coal seam permeability: Swelling and solubilisation behaviour of unconfined coal particles in oxidants. Fuel 2018, 221, 320–328. [Google Scholar] [CrossRef]
- Liu, J.; Kang, Y.; Chen, Q.; Jiang, L.; Wang, J.; Gates, I.; You, L.; Sun, L.; Liu, D.; Sun, J. Understanding the interactions of NaClO oxidant with coal for intensified hydraulic fracturing effectiveness. Gas Sci. Eng. 2023, 117, 205082. [Google Scholar] [CrossRef]
- Jing, Z.; Rodrigues, S.; Strounina, E.; Li, M.; Wood, B.; Underschultz, J.R.; Esterle, J.S.; Steel, K.M. Use of FTIR, XPS, NMR to characterize oxidative effects of NaClO on coal molecular structures. Int. J. Coal Geol. 2019, 201, 1–13. [Google Scholar] [CrossRef]
- Mayo, F.R.; Kirshen, N.A. Oxidations of coal by aqueous sodium hypochlorite. Fuel 1979, 58, 698–704. [Google Scholar] [CrossRef]
- Jing, Z.; Mahoney, S.A.; Rodrigues, S.; Balucan, R.D.; Underschultz, J.; Esterle, J.S.; Rufford, T.E.; Steel, K.M. A preliminary study of oxidant stimulation for enhancing coal seam permeability: Effects of sodium hypochlorite oxidation on subbituminous and bituminous Australian coals. Int. J. Coal Geol. 2018, 200, 36–44. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, A.; Zhang, X. Effect of the oxidation coal of H2O2 on the properties of coal-based polyaniline material. Mater. Rep. 2010, 24, 41–44. [Google Scholar]
- Jing, Z.; Pan, S.; Wang, X.; Yuan, M.; Jim, U.; Karen, S. Oxidant stimulation to enhance coal seam permeability mechanism and application. J. China Coal Soc. 2022, 47, 3975–3989. [Google Scholar]
- GB/T 5751-2009; Chinese Classification of Coals. Standardization Administration of China: Beijing, China, 2009.
- Isah, U.A.; Rashid, M.I.; Lee, S.; Kiman, S.; Iyodo, H.M. Correlations of coal rank with the derived Fourier Transform Infra-Red (FTIR) spectroscopy structural parameters: A review. Infrared Phys. Technol. 2024, 141, 105456. [Google Scholar] [CrossRef]
- Ibarra, J.; Muñoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- Li, X.; Zeng, F.; Wang, W.; Dong, K.; Cheng, L. FTIR characterization of structural evolution in low-middle rank coals. J. China Coal Soc. 2015, 40, 2900–2908. [Google Scholar]
- Okolo, G.N.; Neomagus, H.W.J.P.; Everson, R.C.; Roberts, M.J.; Bunt, J.R.; Sakurovs, R.; Mathews, J.P. Chemical–structural properties of South African bituminous coals: Insights from wide angle XRD–carbon fraction analysis, ATR–FTIR, solid state 13C NMR, and HRTEM techniques. Fuel 2015, 158, 779–792. [Google Scholar] [CrossRef]
- Hao, P.; Meng, Y.; Zeng, F.; Yan, T.; Xu, G. Quantitative Study of Chemical Structures of Different Rank Coals Based on Infrared Spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 787–792. [Google Scholar]
- Liang, H.; Wang, C.; Zeng, F.; Li, M.; Xiang, J. Effect of demineralization on lignite structure from Yinmin coalfield by FT-IR investigation. J. Fuel Chem. Technol. 2014, 42, 129–137. [Google Scholar]
- Fan, S.; Li, Y.; Song, D.; Zhang, X. Macromolecular structure evolution mechanism of tectonically deformed coal under different deformation mechanisms. Coal Sci. Technol. 2019, 47, 239–246. [Google Scholar]
- Wang, Z. Microstructure Evolution and Mechanical Properties of Deep Coal Under the Combined Action of Temperature. Ph.D. Thesis, Chongqing University, Chongqing, China, 2022. [Google Scholar]
- Yan, J.; Lei, Z.; Li, Z.-K.; Wang, Z.; Ren, S.; Kang, S.; Wang, X.; Shui, H. Molecular structure characterization of low-medium rank coals via XRD, solid state 13C NMR and FTIR spectroscopy. Fuel 2020, 268, 117038. [Google Scholar] [CrossRef]
- Cheng, N.; Shi, M.; Hou, Q.; Pan, J.; Han, Y. Application of Raman spectroscopy in characterization of coal macromolecular structure. J. China Coal Soc. 2023, 48, 1311–1324. [Google Scholar]
- Xu, J.; Tang, H.; Su, S.; Liu, J.; Xu, K.; Qian, K.; Wang, Y.; Zhou, Y.; Hu, S.; Zhang, A.; et al. A study of the relationships between coal structures and combustion characteristics: The insights from micro-Raman spectroscopy based on 32 kinds of Chinese coals. Appl. Energy 2018, 212, 46–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z. Raman spectroscopic study of chemical structure and thermal maturity of vitrinite from a suite of Australia coals. Fuel 2019, 241, 188–198. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.-I.; Li, C.-Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, H.; Jiang, D.; Huang, F.; Su, S.; Bai, H.-J. Raman spectroscopy of coal component of Late Permian coals from Southern China. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 767–770. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.H.; Guo, Y.; Gao, W.; Lu, X. FTIR and simultaneous TG/MS/FTIR study of Late Permian coals from Southern China. J. Anal. Appl. Pyrolysis 2013, 100, 75–80. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, Y.; Li, W.; Hao, C.; Hu, B.; Jiang, J. Microcrystalline Characterization and Morphological Structure of Tectonic Anthracite Using XRD, Liquid Nitrogen Adsorption, Mercury Porosimetry, and Micro-CT. Energy Fuels 2019, 33, 10844–10851. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Li, X.; Zeng, F.; Wang, W.; Kui, D. XRD characterization of structural evolution in low-middle rank coals. J. Fuel Chem. Technol. 2016, 44, 777–783. [Google Scholar]
- Cheng, J.; Zhu, Y.; Gao, L.; Zhao, X. Evolution of coke microcrystalline structure during calcination process of coal-based needle coke. J. Fuel Chem. Technol. 2020, 48, 1071–1078. [Google Scholar]






| Industrial Analysis (wt.%) | Elemental Analysis (wt.%, daf) | |||||||
|---|---|---|---|---|---|---|---|---|
| Moisture, Mad | Ash yield, Aad | Volatile matter yield, Vdaf | Fixed carbon, FCdaf | C | H | O | N | S |
| 1.16 | 11.14 | 6.96 | 93.04 | 89.23 | 3.36 | 4.39 | 0.95 | 2.08 |
| Quartz | Calcite | Ammonium mica | non-crystal | Clay minerals | Clay Mineral Content (%) | |||
| (%) | (%) | (%) | (%) | (%) | K | C | I | I/S |
| 0.7 | 0.4 | 13.4 | 75.3 | 10.2 | 26.9 | 13.4 | 32.7 | 27.0 |
| Coal Sample Number | Pre-Fracturing Fluid System |
|---|---|
| GA | None |
| GB | 0.2%PAM + 2%KCl + 0.04%(NH4)2S2O8 |
| GC | 15%HCl + 2%KCl + 0.5%EDTA |
| GD | 12%HCl + 3%HF + 2%KCl + 0.5%EDTA |
| GE | 10%NaClO + 2%KCl |
| GF | 10%H2O2 + 2%KCl |
| Functional Group Category | Wavelength Range/cm−1 | Absorption Peak Form and Corresponding Structure |
|---|---|---|
| Aromatic structures | 730~750 | 4 Adjacent H deformation |
| 750~810 | 3 Adjacent H deformation | |
| 810~850 | 2 Adjacent H deformation | |
| 850~900 | 1 Adjacent H deformation | |
| Oxygen-containing structures | 1001~1021 | Ash, stretching vibration of Si-O group |
| 1072~1077 | C-O stretching vibration in alkyl ether | |
| 1127~1135 | C-O stretching vibration in aryl ether | |
| 1175~1327 | Aromatic C-O stretching vibration | |
| 1373~1378 | Symmetric bending vibration of CH3 | |
| 1426~1431 | Antisymmetric deformation vibration of CH2 | |
| 1574~1600 | C=C stretching vibrations in conjugated or aromatic rings | |
| 1680~1725 | Aromatic C=O stretching vibration | |
| 1726~1772 | C=O stretching vibration of ketone, aldehyde, and -COOH | |
| 1790~1800 | C=O stretching vibration of aryl ester | |
| Aliphatic structures | 2800~2818 | Methylene symmetric stretching vibration |
| 2831~2853 | Methyl symmetrical stretching vibration | |
| 2871~2889 | Stretching vibration of methylidene | |
| 2902~2924 | Antisymmetric stretching vibration of methylene | |
| 2944~3000 | Antisymmetric stretching vibration of methyl group | |
| Hydroxyl structure | 3000~3089 | Aromatic CH stretching vibration |
| 3109~3232 | Cyclic association hydroxy-hydrogen bond | |
| 3248~3348 | Hydroxy-ether-oxygen hydrogen bond | |
| 3369~3468 | Hydroxy self-associated hydrogen bond | |
| 3488~3549 | Hydroxy-π hydrogen bond | |
| 3558~3600 | Free hydroxyl group |
| Coal Sample Number | I | DOC | ‘C’ | L |
|---|---|---|---|---|
| GA | 0.91 | 2.02 | 0.22 | 0.59 |
| GB | 2.45 | 1.52 | 0.10 | 0.96 |
| GC | 1.60 | 1.86 | 0.36 | 0.24 |
| GD | 3.10 | 1.49 | 0.26 | 0.25 |
| GE | 2.10 | 1.49 | 0.25 | 0.46 |
| GF | 2.72 | 1.34 | 0.19 | 0.23 |
| Coal Sample Number | d002/nm | La/nm | Lc/nm | Nave | g |
|---|---|---|---|---|---|
| GA | 0.350705544 | 3.218234866 | 2.05262101 | 5.852833086 | 0.753533916 |
| GB | 0.350063984 | 3.411132285 | 2.058300763 | 5.879784437 | 0.763864996 |
| GC | 0.350430176 | 3.627404715 | 1.992973588 | 5.687220229 | 0.757968176 |
| GD | 0.349867739 | 3.477856057 | 2.021044311 | 5.77659522 | 0.767025131 |
| GE | 0.350159259 | 3.198774174 | 2.019204833 | 5.766532739 | 0.762330767 |
| GF | 0.349827912 | 3.449525198 | 2.116703564 | 6.050699473 | 0.76766647 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sun, Z.; Wang, Z.; Zhou, D.; Xu, J. Evolutionary Mechanisms of Deep Coal Rock Chemical Structures Under Various Pre-Fracturing Fluids. Processes 2025, 13, 1166. https://doi.org/10.3390/pr13041166
Wang X, Sun Z, Wang Z, Zhou D, Xu J. Evolutionary Mechanisms of Deep Coal Rock Chemical Structures Under Various Pre-Fracturing Fluids. Processes. 2025; 13(4):1166. https://doi.org/10.3390/pr13041166
Chicago/Turabian StyleWang, Xiaoxiang, Zirui Sun, Ziyuan Wang, Desheng Zhou, and Jinze Xu. 2025. "Evolutionary Mechanisms of Deep Coal Rock Chemical Structures Under Various Pre-Fracturing Fluids" Processes 13, no. 4: 1166. https://doi.org/10.3390/pr13041166
APA StyleWang, X., Sun, Z., Wang, Z., Zhou, D., & Xu, J. (2025). Evolutionary Mechanisms of Deep Coal Rock Chemical Structures Under Various Pre-Fracturing Fluids. Processes, 13(4), 1166. https://doi.org/10.3390/pr13041166







