Investigating the Effectiveness of a Simple Water-Purifying Gadget Using Moringa oleifera Seeds as the Active Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area and Sampling Points
2.2. Sampling Procedures
2.3. Moringa oleifera Seed Preparation
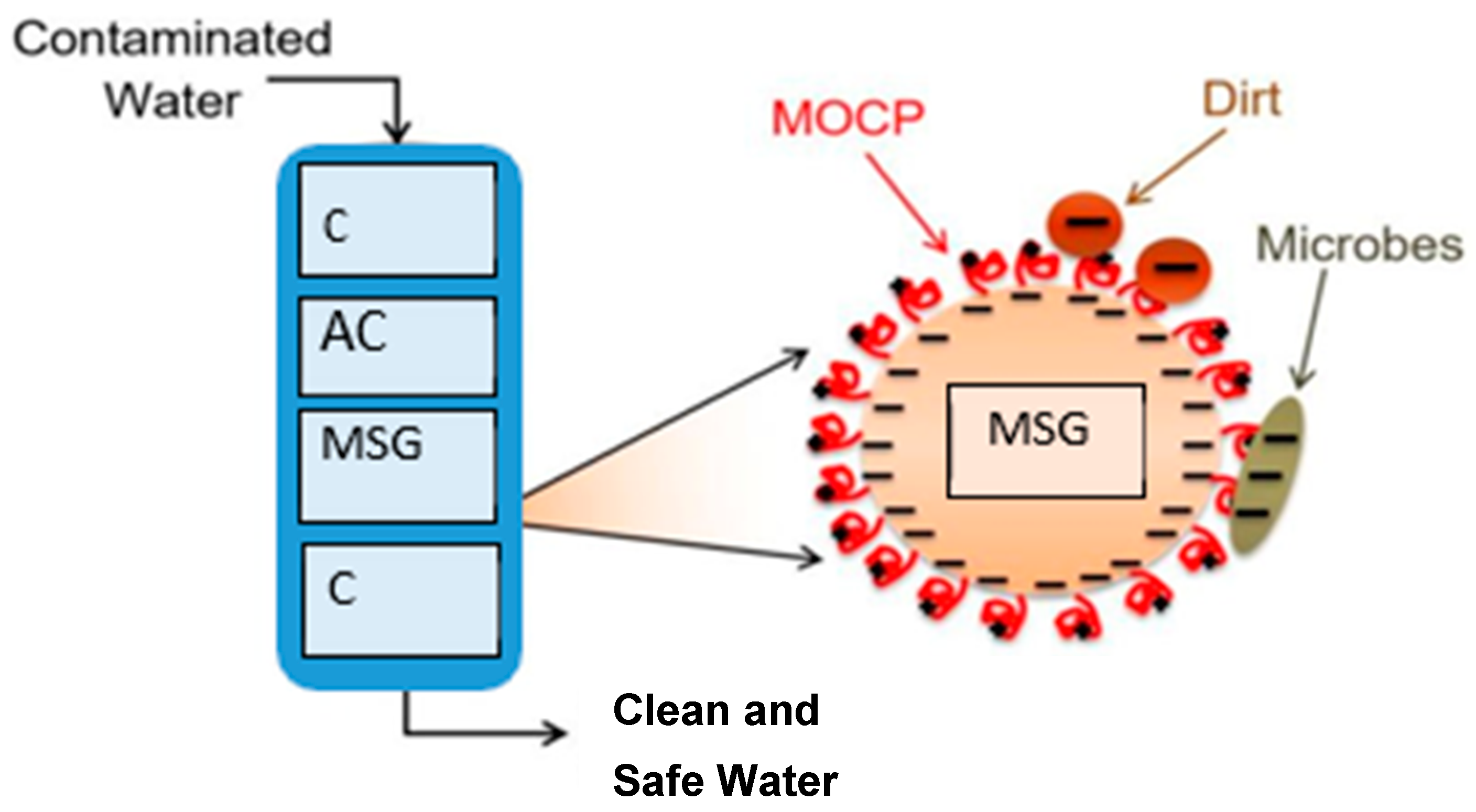
2.4. Prototype Water Filtering Device
2.5. Water Quality Analysis
2.6. Microbiological Analysis
2.7. Data Analysis
3. Results
3.1. Physicochemical Parameters
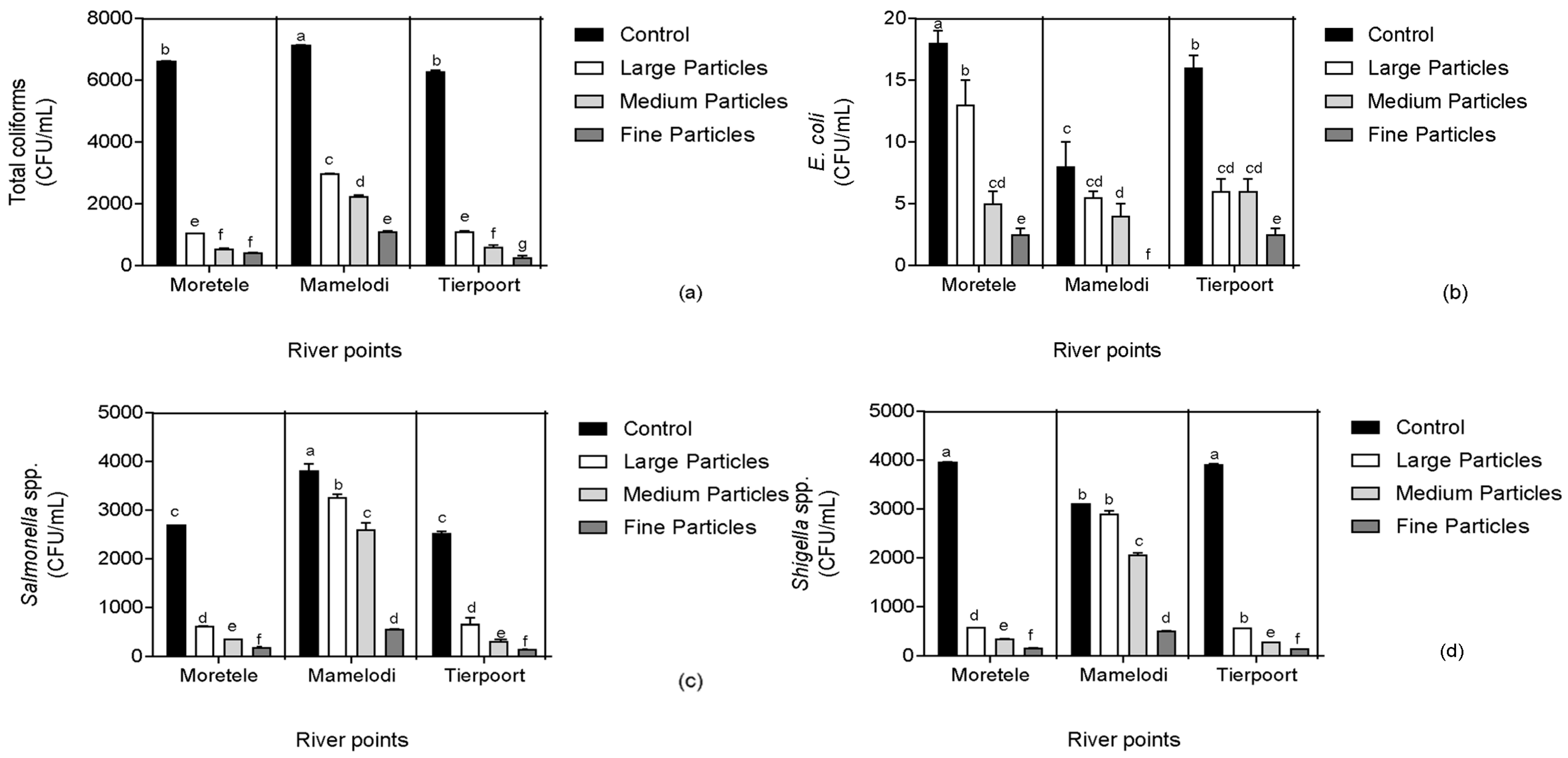
3.2. Microbiological Parameters
4. Discussion
4.1. Physicochemical Parameters
4.2. Microbiological Parameters
4.3. Implications and Future Directions
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okafor, C.O.; Ude, U.I.; Okoh, F.N.; Eromonsele, B.O. Safe Drinking Water: The Need and Challenges in Developing Countries. In Water Quality-New Perspectives; IntechOpen: London, UK, 2024. [Google Scholar]
- Stoll, D. Water Crisis in South Africa: Causes, Effects, And Solutions. Available online: https://earth.org/water-crisis-in-south-africa/ (accessed on 21 July 2024).
- Claassen, M. Scenarios for the South African water sector in 2025. Water SA 2013, 39, 143–150. [Google Scholar] [CrossRef][Green Version]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Programme, W.W.A.; UN-Water. Water in a Changing World. Available online: https://www.afro.who.int/health-topics/water#:~:text=663%20million%20people%20rely%20on,%2C%20dysentery%2C%20typhoid%20and%20polio (accessed on 21 July 2024).
- Bazaanah, P.; Mothapo, R.A. Sustainability of drinking water and sanitation delivery systems in rural communities of the Lepelle Nkumpi Local Municipality, South Africa. Environ. Dev. Sustain. 2024, 26, 14223–14255. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.R.; Guimarães, R.N.; Moser, P.B.; Santos, L.V.; de Paula, E.C.; Lebron, Y.A.; Silva, A.F.R.; Casella, G.S.; Amaral, M.C. Restrictions in water treatment by conventional processes (coagulation, flocculation, and sand-filtration) following scenarios of dam failure. J. Water Process Eng. 2023, 51, 103450. [Google Scholar] [CrossRef]
- Dzuvor, C.K.; Pan, S.; Amanze, C.; Amuzu, P.; Asakiya, C.; Kubi, F. Bioactive components from Moringa oleifera seeds: Production, functionalities and applications—A critical review. Crit. Rev. Biotechnol. 2022, 42, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Rifi, S.K.; Souabi, S.; El Fels, L.; Driouich, A.; Nassri, I.; Haddaji, C.; Hafidi, M. Optimization of coagulation process for treatment of olive oil mill wastewater using Moringa oleifera as a natural coagulant, CCD combined with RSM for treatment optimization. Process Saf. Environ. Prot. 2022, 162, 406–418. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ncube, B.; Madala, N.E.; Nyakudya, T.T.; Moyo, H.P.; Sibanda, M.; Ndhlala, A.R. Scribbling the cat: A case of the “miracle” plant, Moringa oleifera. Plants 2019, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.; Musnad, H.A.; Burgstaller, H. The tree that purifies water: Cultivating multipurpose Moringaceae in the Sudan. Unasylva 1986, 38, 23–28. [Google Scholar]
- Delelegn, A.; Sahile, S.; Husen, A. Water purification and antibacterial efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018, 7, 25. [Google Scholar] [CrossRef]
- Kumar, S.; Gopal, K. Screening of plant species for inhibition of bacterial population of raw water. J. Environ. Sci. Health Part A 1999, 34, 975–987. [Google Scholar] [CrossRef]
- Madsen, M.; Schlundt, J.; El Fadil, E.O. Effect of water coagulation by seeds of Moringa bacterial concentrations. J. Trop. Med. Hyg. 1988, 90, 101–109. [Google Scholar] [CrossRef]
- Narayasamy, S.; Saud, H.M. Water phytoremediation by sedimentation using Moringa oleifera seed powder to remove water turbidity in Malaysia. J. Agric. Chem. Environ. 2014, 3, 74–79. [Google Scholar] [CrossRef][Green Version]
- Amagloh, F.K.; Benang, A. Effectiveness of Moringa oleifera seed as coagulant for water purification. Afr. J. Agric. Res. 2009, 4, 119–123. [Google Scholar]
- Meybeck, M.; Chapman, D.V.; Helmer, R. Global Freshwater Quality: A First Assessment; Blackwell: Oxford, UK, 1990. [Google Scholar]
- DWAF. Department of Water and Sanitation. A Strategic Framework for Water Services; Department of Water Affairs and Forestry: Pretoria, South Africa, 2004. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical: Vienna, Austria, 2020. [Google Scholar]
- Elsergany, M. The Potential Use of Moringa peregrina Seeds and Seed Extract as a Bio-Coagulant for Water Purification. Water 2023, 15, 2804. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor-Basulto, D.L.; Astudillo-Sánchez, P.D.; del Real-Olvera, J.; Bandala, E.R. Wastewater treatment using Moringa oleifera Lam seeds: A review. J. Water Process Eng. 2018, 23, 151–164. [Google Scholar] [CrossRef]
- Du Plessis, E.; Duvenage, S.; Korsten, L.; Sigge, G. Measurement of Water Pollution Determining the Sources and Changes of Microbial Contamination and Impact on Food safety from Farming to Retail Level for Fresh Vegetables. Report to the Water Research Commission, WRC Report No. 2706/1/21: Pretoria, South Africa. 2021. Available online: https://wrc.org.za/mdocs-posts/measurement-of-water-pollution-determining-the-sources-and-changes-of-microbial-contamination-and-impact-on-food-safety-from-farming-to-retail-level-for-fresh-vegetables/ (accessed on 21 July 2024).
- De Klerk, A.; DeKlerk, L.; Chamier, J.; Wepener, V. Seasonal variations of water andsediment quality parameters in endorheic reedpans on the Mpumalanga Highveld. Water Sa 2012, 38, 663–672. [Google Scholar] [CrossRef]
- Rosmawanie, M.; Mohamed, R.; Al-Gheethi, A.; Pahazri, F.; Amir-Hashim, M.; Nur-Shaylinda, M. Sequestering of pollutants frompublic market wastewater using Moringa oleifera and Cicer arietinum flocculants. J. Environ. Chem. Eng. 2018, 6, 2417–2428. [Google Scholar]



| Parameter | Symbol | Method | Units |
|---|---|---|---|
| pH | pH | pH probe | |
| Electrical Conductivity | E C | E C meter | mS/m |
| Nitrate | IC | mg/L | |
| Nitrite | IC | mg/L | |
| Chloride | IC | mg/L | |
| Fluoride | IC | mg/L | |
| Sulfate | IC | mg/L | |
| Phosphate | IC | mg/L | |
| Bicarbonate | Titration | mg/L | |
| Sodium | Na | ICP-OES | mg/L |
| Potassium | K | ICP-OES | mg/L |
| Calcium | Ca | ICP-OES | mg/L |
| Magnesium | Mg | ICP-OES | mg/L |
| Boron | B | ICP-OES | mg/L |
| Total coliforms | TC | Viable plate count | CFU/mL |
| Escherichia coli | EC | Viable plate count | CFU/mL |
| Salmonella | Sal | Viable plate count | CFU/mL |
| Shigella | Shi | Viable plate count | CFU/mL |
| Parameter | Moretele Site | Mamelodi Site | Tierpoort Site | SANS 241:2015 Limit | WHO Limit for Drinking Water | Conformity | |
|---|---|---|---|---|---|---|---|
| pH | Unfiltered | 6.98 ± 0.02 a | 6.77 ± 0.04 a | 6.76 ± 0.3 a | ≥5 to ≤9.7 | 6.5–8.5 | Conforms |
| Large | 6.16 ± 0.04 a | 6.71 ± 0.01 b | 6.37 ± 0.07 a | ||||
| Medium | 6.64 ± 0.03 a | 5.61 ± 0.01 b | 6.62 ± 0.02 a | ||||
| Fine | 6.02 ± 0.08 a | 6.01 ± 0.01 a | 6.23 ± 0.03 b | ||||
| EC (mS/m) | Unfiltered | 42.2 ± 0 b | 34.35 ± 0.15 a | 17.8 ± 0.2 c | ≤170 | - | Conforms |
| Large | 99.75 ± 0.25 b | 80.38 ± 0.37 a | 17.85 ± 0.25 c | ||||
| Medium | 108.5 ± 0.5 b | 92.5 ± 0.5 a | 107.5 ± 0.5 b | ||||
| Fine | 70.5 ± 0.5 b | 65.55 ± 0.55 a | 23.9 ± 0.1 c | ||||
| (mg/L) | Unfiltered | 12.05 ± 0.05 a | 4.26 ± 0.53 b | 0.85 ± 0.01 c | <49 | 50 | Conforms |
| Large | 0.35 ± 0.01 a | 0.34 ± 0.02 a | 0.63 ± 0.01 b | ||||
| Medium | 11.45 ± 1.45 a | 0.21 ± 0.01 b | 0.57 ± 6.01 b | ||||
| Fine | 10.5 ± 0.5 a | 3.80 ± 0.8 b | 0.52 ± 0.01 c | ||||
| (mg/L) | Unfiltered | 0.07 ± 0.05 b | 4.7 ± 0.24 a | 0.05 ± 0.02 b | <3 | <3 | Non-Conforms |
| Large | 0.02 ± 0.01 a | 0.02 ± 0.00 a | 0.03 ± 0.0 b | ||||
| Medium | 0.02 ± 0.01 b | 0.02 ± 0.00 a | 0.01 ± 0.00 a | ||||
| Fine | 0.05 ± 0.01 b | 0.03 ± 0.01 a | 0.02 ± 0.10 a | ||||
| Cl− (mg/L) | Unfiltered | 36.85 ± 0.15 b | 17.35 ± 0.05 a | 8.57 ± 0.17 c | ≤300 | ≤500 | Conforms |
| Large | 65.3 ± 0.05 b | 50.5 ± 0.5 a | 8.91 ± 0.01 c | ||||
| Medium | 72.94 ± 0.05 b | 56.5 ± 1.5 a | 12.5 ± 0.5 c | ||||
| Fine | 64.5 ± 0.5 b | 50.81 ± 0.19 a | 9.3 ± 0.01 c | ||||
| F− (mg/L) | Unfiltered | 0.29 ± 0.05 b | 0.22 ± 0.10 a | 0.16 ± 0.01 c | ≤1.5 | 1.5 | Conforms |
| Large | 0.05 ± 0.03 a | 0.05 ± 0.01 a | 0.15 ± 0.03 b | ||||
| Medium | 0.06 ± 0.01 a | 0.06 ± 0.02 a | 0.22 ± 0.01 b | ||||
| Fine | 0.36 ± 0.10 b | 0.15 ± 0.03 a | 0.27 ± 0.02 c | ||||
| (mg/L) | Unfiltered | 30.6 ± 0.1 b | 27.15 ± 0.05 a | 9.61 ± 0.02 c | ≤250/≤500 | - | Conforms |
| Large | 26.55 ± 0.35 a | 25.75 ± 49.25 a | 11.46 ± 0.46 b | ||||
| Medium | 29.5 ± 0.5 a | 25.5 ± 0.5 b | 8.05 ± 0.5 c | ||||
| Fine | 19.5 ± 0.5 b | 13.7 ± 0.7 a | 8.05 ± 0.05 c | ||||
| (mg/L) | Unfiltered | 1.9 ± 0.03 b | 0.75 ± 0.01 a | 0.1 ± 0.0 c | - | - | Conforms |
| Large | 0.76 ± 0 ab | 0.13 ± 2.12 a | 0.1 ± 0.0 b | ||||
| Medium | 0.95 ± 0.05 b | 0.05 ± 0.5 a | 0.1 ± 0 c | ||||
| Fine | 0.14 ± 0.01 b | 0.58 ± 0.16 a | 0.1 ± 0 c | ||||
| (mg/L) | Unfiltered | 149.5 ± 0.5 b | 140.5 ± 0.5 a | 86 ± 0.6 c | - | - | Conforms |
| Large | 110.5 ± 0.5 a | 140.2 ± 1.5 a | 38.62 ± 38.62 a | ||||
| Medium | 102.5 ± 0.5 b | 98.5 ± 0.5 a | 2.5 ± 0.5 a | ||||
| Fine | 192.5 ± 0.5 b | 128.5 ± 0.5 a | 111.45 ± 0.45 c | ||||
| Na (mg/L) | Unfiltered | 31.65 ± 0.05 b | 15.05 ± 0.05 a | 5.92 ± 0.02 c | ≤200 | 20 | Conforms |
| Large | 20.51 ± 0.49 a | 15.2 ± 0.02 a | 3.9 ± 0.3 b | ||||
| Medium | 13.1 ± 0.2 b | 13.5 ± 0.5 b | 2.5 ± 0.5 a | ||||
| Fine | 9. 3 ± 1 b | 9. 2 ± 2 b | 2.2 ± 0 a | ||||
| K (mg/L) | Unfiltered | 7.28 ± 0.20 b | 3.32 ± 0.02 a | 1.89 ± 0.02 a | ≤50 | 3000 | Conforms |
| Large | 22.1 ± 4.89 c | 3.31 ± 0.02 a | 2.12 ± 0.12 a | ||||
| Medium | 16.5 ± 0.50 b | 13.95 ± 0.05 b | 19.5 ± 0.5 c | ||||
| Fine | 19.35 ± 0.05 c | 8.1 ± 0.01 b | 2 ± 0.10 a | ||||
| Ca (mg/L) | Unfiltered | 28.65 ± 0.05 b | 27.7 ± 0.03 b | 14.65 ± 0.05 a | ≤150 | - | Conforms |
| Large | 28.95 ± 3.43 b | 27.85 ± 0.05 b | 15.5 ± 0.5 a | ||||
| Medium | 31.5 ± 0.5 c | 28.01 ± 1.10 b | 31 ± 1.13 c | ||||
| Fine | 29.55 ± 0.64 b | 30.08 ± 0.5 c | 15.8 ± 0.14 a | ||||
| Mg (mg/L) | Unfiltered | 14.4 ± 0.01 b | 17.45 ± 0.15 a | 9.7 ± 0.11 c | ≤70 | ≤50 | Conforms |
| Large | 20.02 ± 1.17 a | 17.45 ± 0.05 a | 9.95 ± 0.03 c | ||||
| Medium | 29.5 ± 0.5 b | 21.5 ± 1.5 a | 29.5 ± 0.5 b | ||||
| Fine | 29.55 ± 0.35 b | 18.75 ± 0.25 a | 8 ± 0.01 c | ||||
| B (mg/L) | Unfiltered | 0.04 ± 0.03 b | 0.02 ± 0.01 a | 0.01± 0.00 c | <2400 | 2.4 | Conforms |
| Large | 0.03 ± 0.01 b | 0.01 ± 0.14 a | 0.01 ± 0.00 a | ||||
| Medium | 0.03 ± 0.02 b | 0.01 ± 0.11 a | 0.01 ± 0.00 a | ||||
| Fine | 0.02 ± 0.03 b | 0.01 ± 0.02 b | 0.01 ± 0.00 c |
| Parameter | Moretele Site | Mamelodi Site | Tierpoort Site | SANS 241:2015 Limit | WHO Limit for Drinking Water | Conformity | |
|---|---|---|---|---|---|---|---|
| pH | Unfiltered | 7.63 ± 0.01 b | 7.51 ± 0.01 a | 7.73 ± 0.01 c | ≥5 to ≤9.7 | ≤6.5–8.5 | Conforms |
| Large | 6.14 ± 0.12 ab | 5.57 ± 0.32 b | 7.73 ± 0.02 c | ||||
| Medium | 7.19 ± 0.06 a | 7.03 ± 0.02 a | 7.5 ± 0.24 a | ||||
| Fine | 6.06 ± 0.04 a | 5.35 ± 0.02 b | 6.01± 0.14 a | ||||
| EC (mS/m) | Unfiltered | 74.78 ± 1.93 a | 70.53 ± 0.47 a | 21.5 ± 1.5 b | ≤170 | - | N/A |
| Large | 100.3 ± 0.7 b | 80.75 ± 0.75 a | 21.9 ± 1.9 c | ||||
| Medium | 71.8 ± 1.7 a | 66.45 ± 1.25 a | 22.15 ± 1.65 b | ||||
| Fine | 74.1 ± 0.9 b | 109.5 ± 0.5 a | 94.3 ± 5.5 ab | ||||
| (mg/L) | Unfiltered | 27.5 ± 1.5 b | 2.16 ± 0.01 a | 0.7 ± 0.05 a | <49 | <50 | Conforms |
| Large | 39.55 ± 1.15 a | 0.35 ± 0.01 b | 0.64 ± 0.11 b | ||||
| Medium | 36.25 ± 1.05 a | 0.30 ± 0.65 b | 0.54 ± 0.00 b | ||||
| Fine | 0.88 ± 0.03 a | 33.05± 1.05 b | 0.21± 0.0 a | ||||
| (mg/L) | Unfiltered | 2.9 ± 0.01 b | 0.7 ± 0.01 a | 0.02 ± 0.01 c | <3 | <3 | Conforms |
| Large | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | ||||
| Medium | 0.02 ± 0.02 a | 0.02 ± 0.02 a | 0.02 ± 0.02 a | ||||
| Fine | 0.02 ± 0.02 a | 0.02 ± 0.02 a | 0.02 ± 0.02 a | ||||
| (mg/L) | Unfiltered | 71.2 ± 1.7 b | 52.75 ± 0.35 a | 8.89 ± 0.39 c | ≤300 | <500 | Conforms |
| Large | 65.35 ± 1.15 b | 52 ± 1.1 a | 8.91 ± 0.41 c | ||||
| Medium | 64.55 ± 1.35 b | 50.65 ± 1.25 a | 9.66 ± 0.34 c | ||||
| Fine | 11.35 ± 0.45 c | 73.55 ± 1.05 b | 58.4 ± 0.5 a | ||||
| F (mg/L) | Unfiltered | 0.31 ± 0.02 a | 0.26 ± 0.01 a | 0.15 ± 0.03 b | ≤1.5 | ≤1.5 | Conforms |
| Large | 0.02 ± 0.01 a | 0.03 ± 0.02 a | 0.16 ± 0.04 b | ||||
| Medium | 0.36 ± 0.01 b | 0.16 ± 0.01 a | 0.28 ± 0.01 c | ||||
| Fine | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.03 ± 0.01 a | ||||
| (mg/L) | Unfiltered | 27.5 ± 1.5 b | 2.16 ± 0.01 a | 0.7 ± 0.05 a | ≤250 | ≤500 | Conforms |
| Large | 296.5 ± 2.5 b | 196.5 ± 1.5 a | 11.95 ± 1.85 c | ||||
| Medium | 60.8 ± 0.2 b | 64.55 ± 0.45 a | 13.35 ± 0.45 c | ||||
| Fine | 170.50 ± 5.5 b | 133 ± 0.5 a | 130 ± 0.5 ab | ||||
| (mg/L) | Unfiltered | 8.7 ± 0.34 b | 3.88 ± 0.07 a | 0.1 ± 0.00 c | - | Conforms | |
| Large | 23.4 ± 0.4 b | 10.65 ± 0.55 a | 0.1 ± 0.00 c | ||||
| Medium | 7.21 ± 0.22 b | 4.75 ± 0.2 a | 0.1 ± 0.00 c | ||||
| Fine | 41.75 ± 0.05 b | 24.75 ± 0.15 a | 0.10 ± 1.25 c | ||||
| (mg/L) | Unfiltered | 171.5 ± 11.5 a | 204 ± 4.03 a | 92.25 ± 5.35 b | Conforms | ||
| Large | 90.36 ± 1.20 b | 77.25± 1.65 a | 110.5 ± 1.5 c | ||||
| Medium | 193.5 ± 1.5 b | 218.5 ± 1.5 a | 113.5 ± 1.5 c | ||||
| Fine | 104 ± 1.10 a | 99.9 ± 0.1 a | 32.15 ± 0.55 b | ||||
| Na (mg/L) | Unfiltered | 83.95 ± 1.35 b | 62.19 ± 2.01 a | 9.45 ± 0.53 c | ≤200 | ≤200 | Conforms |
| Large | 77.85 ± 0.65 b | 61.2 ± 1.1 a | 21.8 ± 1.3 c | ||||
| Medium | 94.5 ± 5.2 a | 94.55 ± 4.65 a | 22.6 ± 0.5 b | ||||
| Fine | 10.04 ± 0.05 c | 84.4 ± 0.9 b | 65.2 ± 0.7 a | ||||
| K (mg/L) | Unfiltered | 13.74 ± 1.34 a | 9.28 ± 0.25 a | 0.95 ± 0.03 b | ≤50 | 3000 | Conforms |
| Large | 15.95 ± 1.65 b | 39.1 ± 0.2 a | 2.26 ± 0.25 c | ||||
| Medium | 9.55 ± 0.05 b | 8.27 ± 0.01 a | 2.13 ± 0.02 c | ||||
| Fine | 14.52 ± 0.48 b | 20.3 ± 0.5 a | 3.25 ± 0.45 c | ||||
| Ca (mg/L) | Unfiltered | 39.55 ± 6.65 b | 30.48 ± 1.42 a | 20.3 ± 0.8 a | ≤150 | - | Conforms |
| Large | 41.5 ± 1.4 b | 34.15 ± 1.35 a | 25.05 ±0.75 a | ||||
| Medium | 40.55 ± 0.65 b | 46.8 ± 0.5 b | 35.35 ± 0.45 a | ||||
| Fine | 13.75 ± 0.85 c | 32.3 ± 0.6 b | 21.8 ± 0.5 a | ||||
| Mg (mg/L) | Unfiltered | 21.8 ± 1.1 ab | 22.05 ± 1.45 a | 13.8 ± 1 c | ≤70 | 0.08 | Conforms |
| Large | 21.25 ± 0.55 a | 28.85 ± 1.55 b | 17.15 ± 0.65 c | ||||
| Medium | 26 ± 0.8 a | 22.55 ± 0.35 a | 18.11 ± 0.31 b | ||||
| Fine | 21.2 ± 0.6 a | 30.7 ± 0.7 b | 23.35 ± 0.45 a | ||||
| B (mg/L) | Unfiltered | 0.07 ± 0.01 ab | 0.01 ± 0.00 b | 0.03 ± 0.01 a | <2400 | 2.4 | Conforms |
| Large | 0.05 ± 0.01 b | 0.01 ± 0.00 c | 0.02 ± 0.01 a | ||||
| Medium | 0.06 ± 0.01 b | 0.01 ± 0.00 c | 0.03 ± 0.01 a | ||||
| Fine | 0.05 ± 0.02 b | 0.01 ± 0.0 a | 0.01 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raphasha, D.G.; Ndhlala, A.R.; Tsvuura, Z. Investigating the Effectiveness of a Simple Water-Purifying Gadget Using Moringa oleifera Seeds as the Active Beads. Processes 2025, 13, 1172. https://doi.org/10.3390/pr13041172
Raphasha DG, Ndhlala AR, Tsvuura Z. Investigating the Effectiveness of a Simple Water-Purifying Gadget Using Moringa oleifera Seeds as the Active Beads. Processes. 2025; 13(4):1172. https://doi.org/10.3390/pr13041172
Chicago/Turabian StyleRaphasha, Dineo G., Ashwell R. Ndhlala, and Zivanai Tsvuura. 2025. "Investigating the Effectiveness of a Simple Water-Purifying Gadget Using Moringa oleifera Seeds as the Active Beads" Processes 13, no. 4: 1172. https://doi.org/10.3390/pr13041172
APA StyleRaphasha, D. G., Ndhlala, A. R., & Tsvuura, Z. (2025). Investigating the Effectiveness of a Simple Water-Purifying Gadget Using Moringa oleifera Seeds as the Active Beads. Processes, 13(4), 1172. https://doi.org/10.3390/pr13041172






