Substrate and Doping Effects on the Growth Aspects of Zinc Oxide Thin Films Developed on a GaN Substrate by the Sputtering Technique
Abstract
1. Introduction
2. Experimental Techniques
2.1. Preparation of Porous GaN Substrate
2.2. Preparation of Pure and N-Doped ZnO Films on GaN Substrate
2.3. Characterization Techniques
3. Results and Discussion
3.1. Structural and Optical Analysis of GaN Substrates
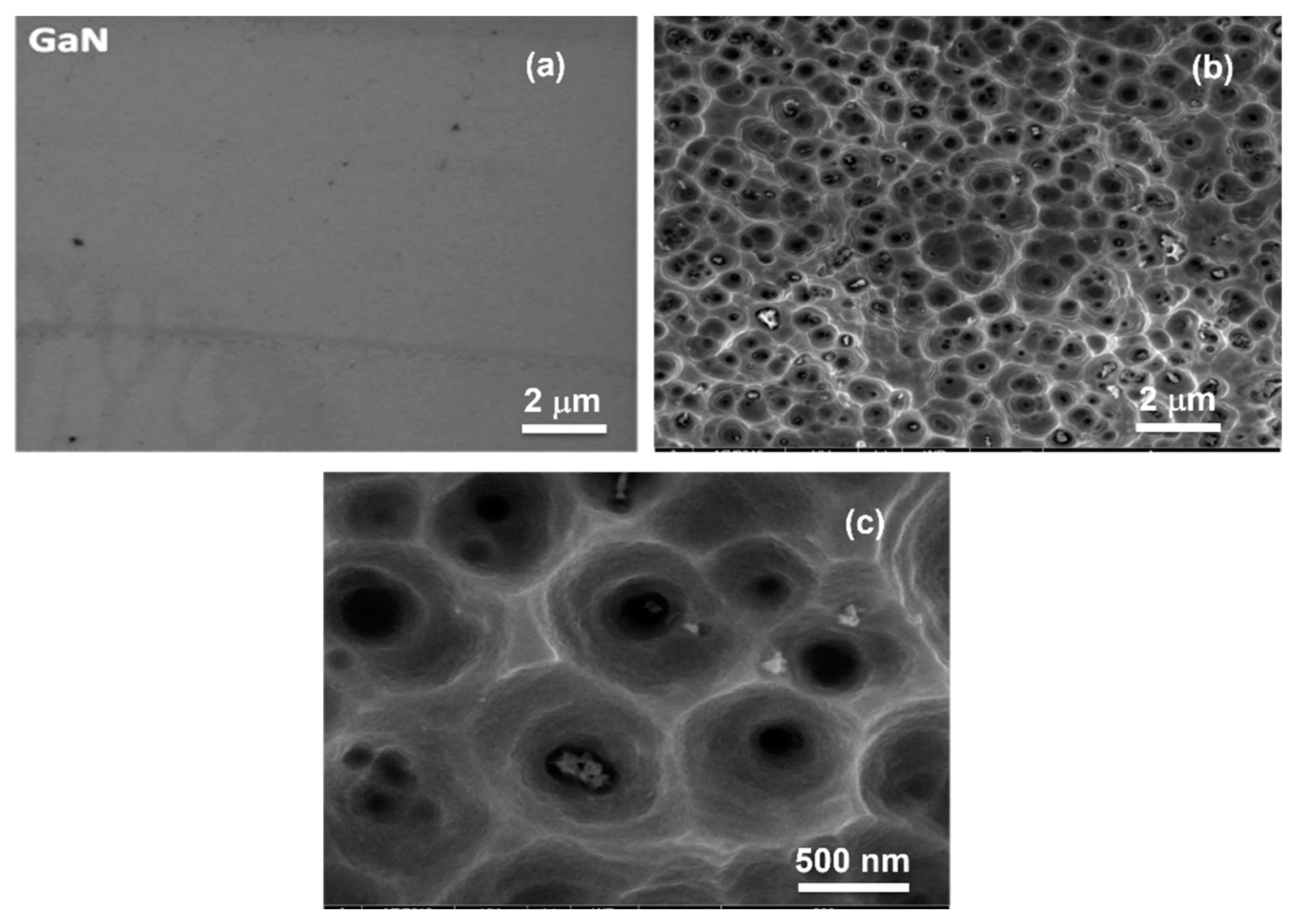
3.1.1. SEM Analysis of Pristine and Porous GaN Substrates
3.1.2. Raman Spectral Analysis of Pristine and Porous GaN Substrates
3.2. Structural, Morphological, and Electrical Properties of ZnO Films
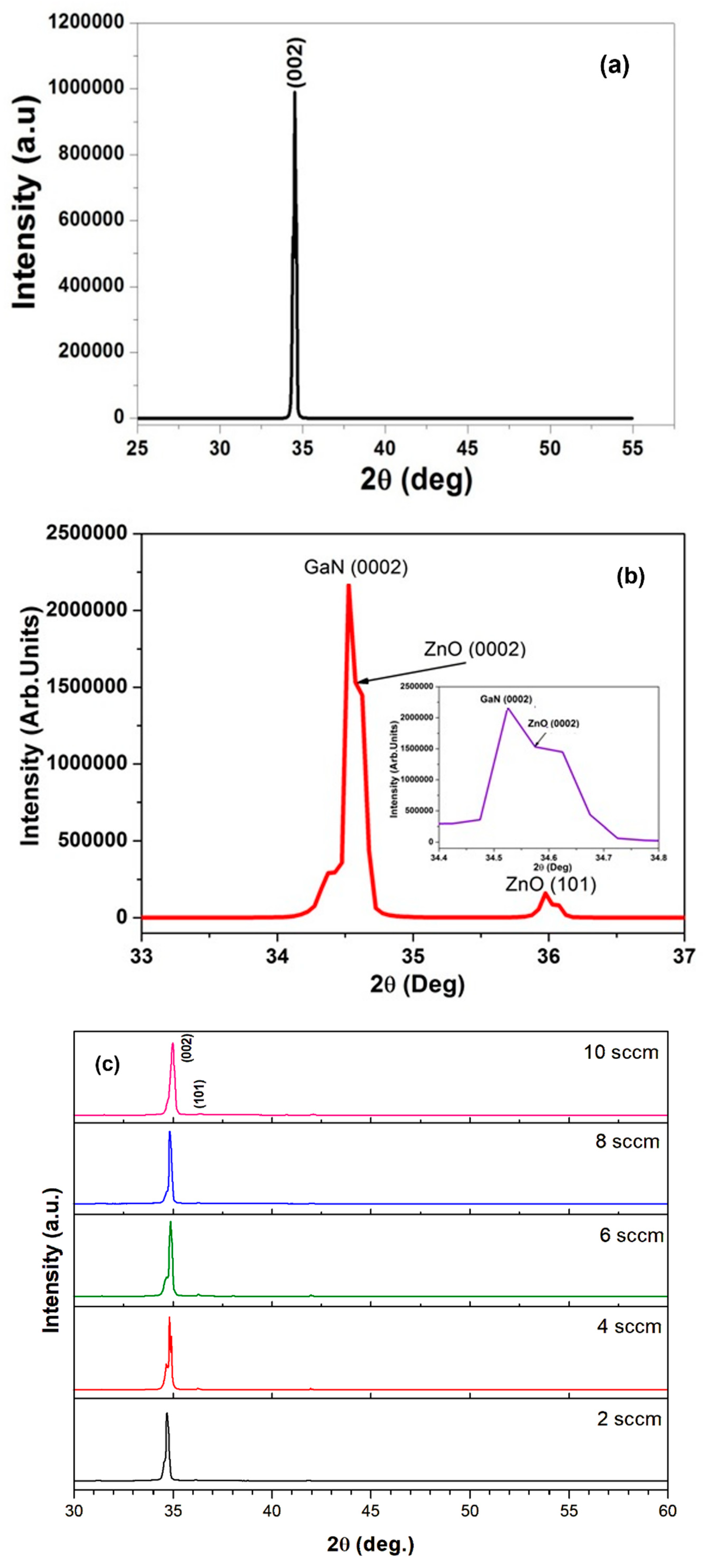
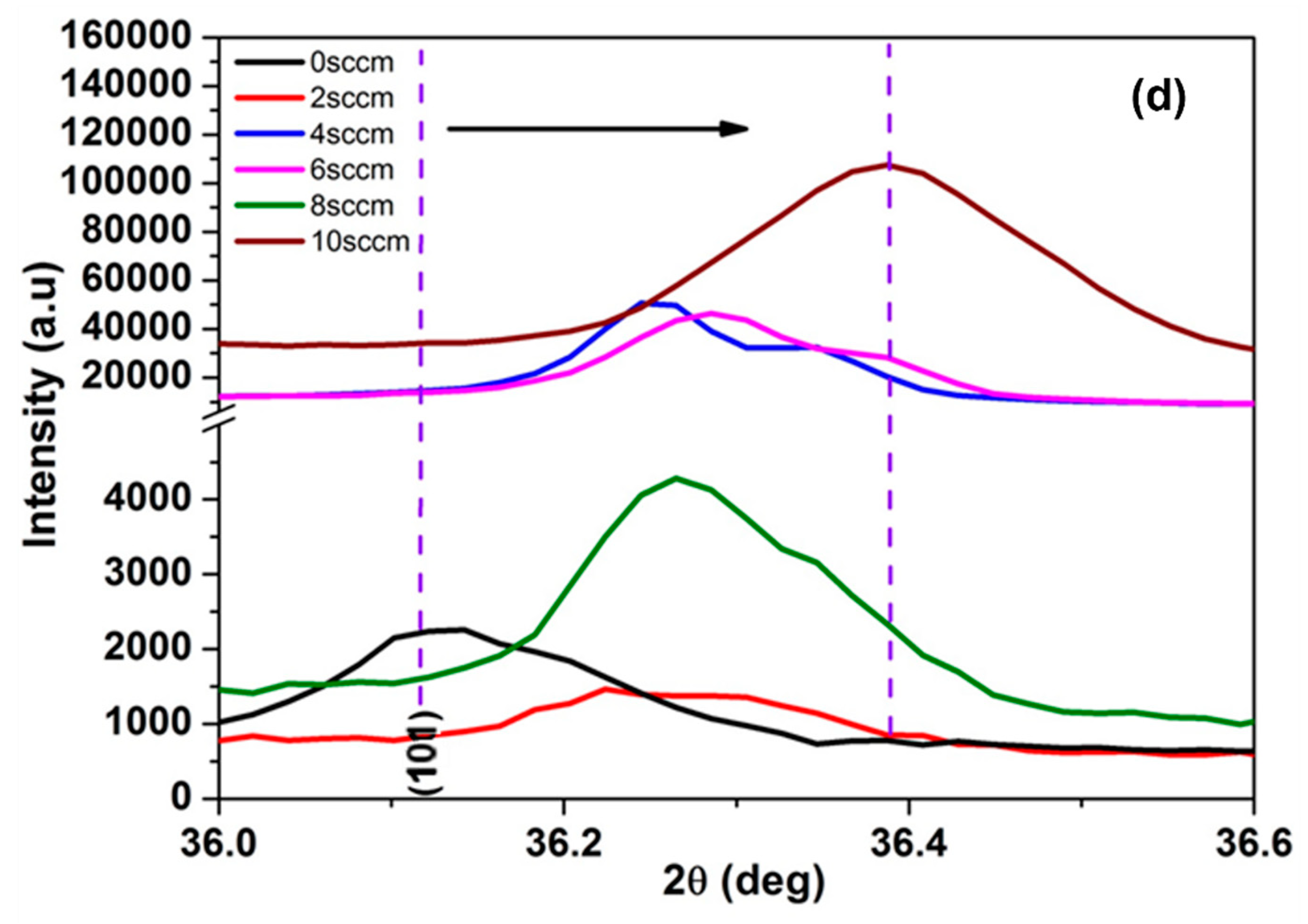
3.2.1. XRD Analysis of Pure and N-ZnO on Porous GaN Substrate
3.2.2. SEM Analysis of Pure and Nitrogen-Doped ZnO on GaN
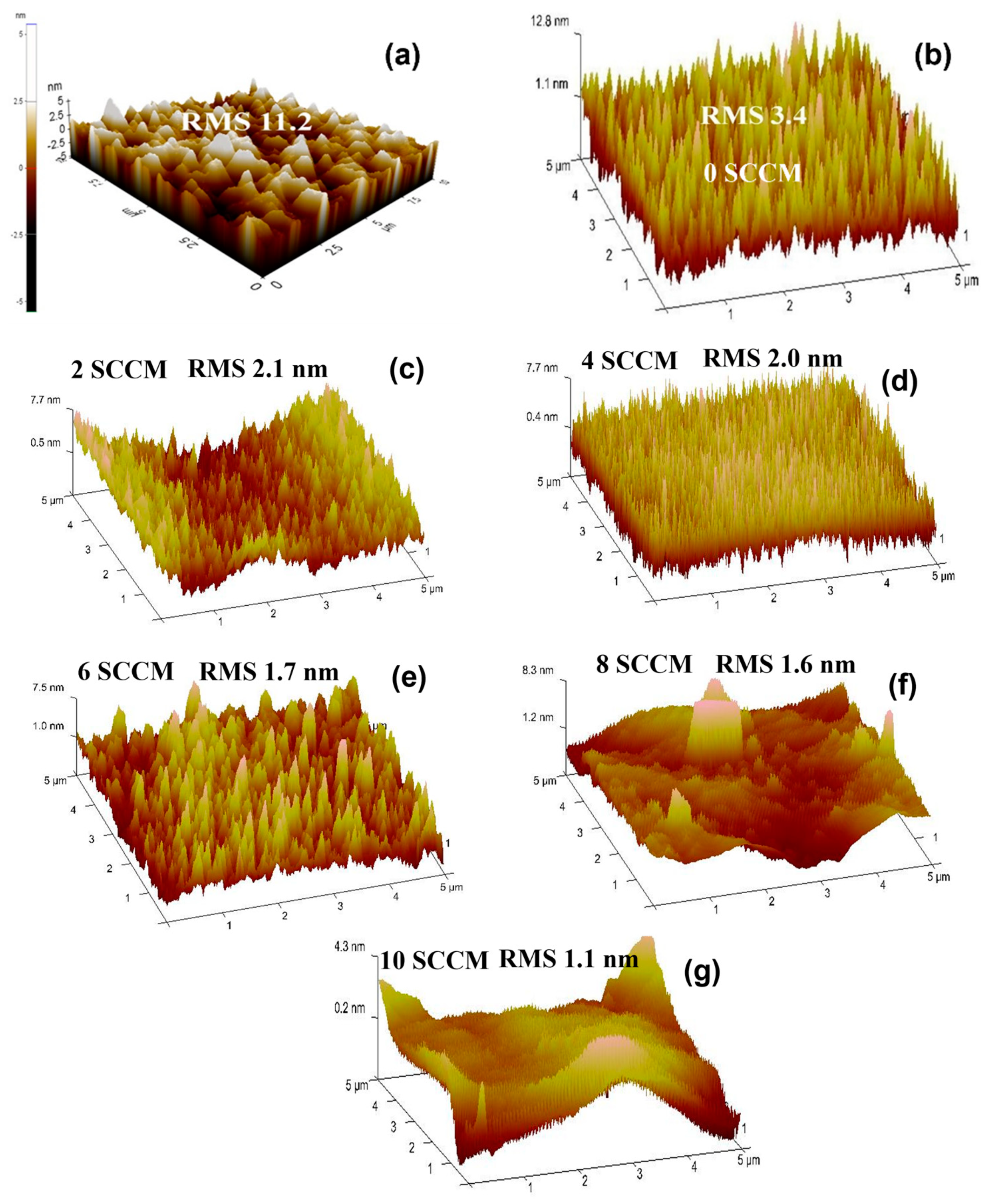
3.2.3. Atomic Force Microscopic Analysis of Pure ZnO and N-ZnO Films
3.2.4. Photoluminescence Analysis of ZnO Films on GaN Substrates
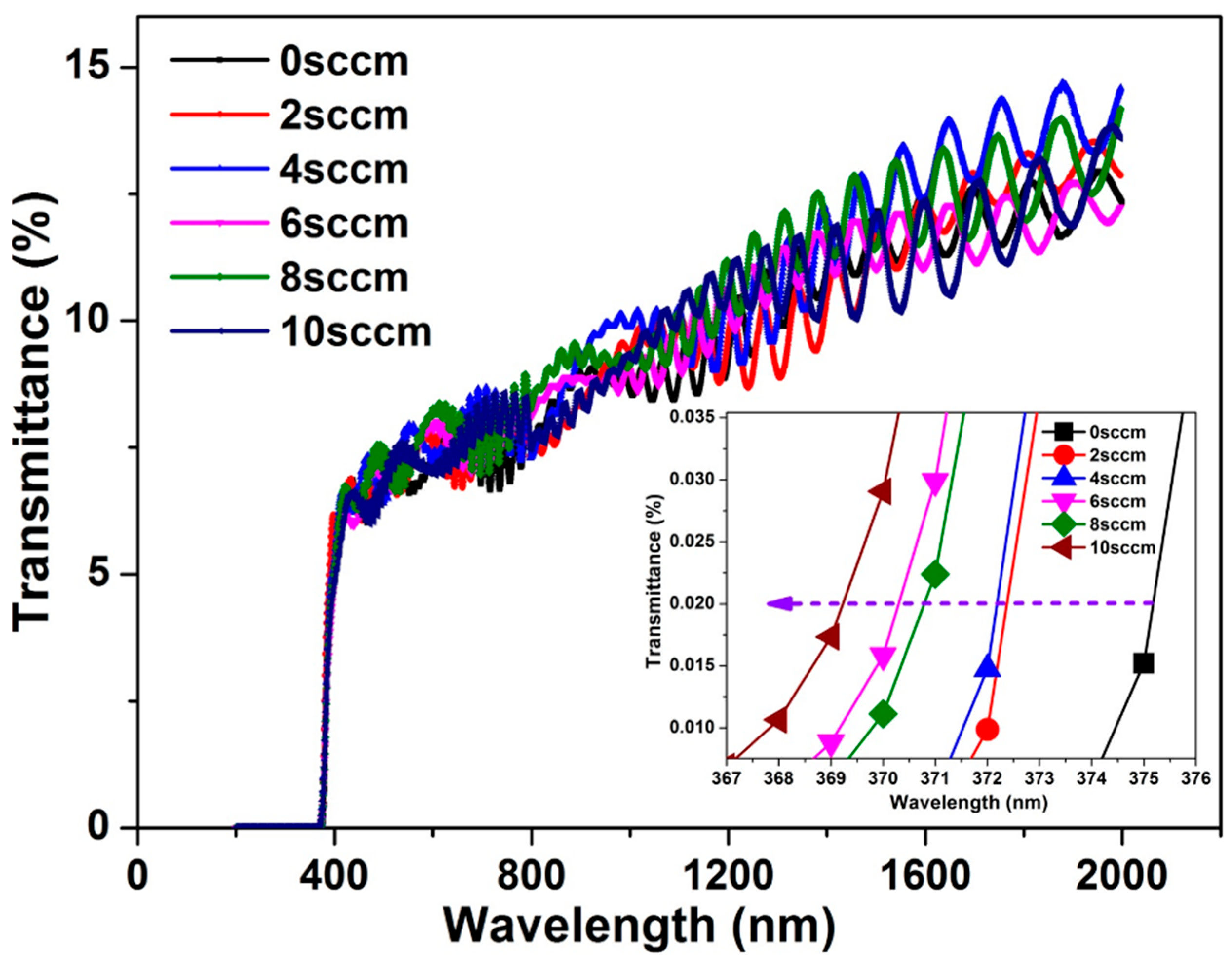
3.2.5. UV-Visible Spectra of ZnO Films on GaN Template
3.2.6. Electrical Properties of ZnO Films on GaN Substrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nagaraj, G.; Mohammed, M.K.; Shekargoftar, M.; Sasikumar, P.; Sakthivel, P.; Ravi, G.; Dehghanipour, M.; Akin, S.; Shalan, A.E. High-performance perovskite solar cells using the graphene quantum dot–modified SnO2/ZnO photoelectrode. Mater. Today Energy 2021, 22, 100853. [Google Scholar] [CrossRef]
- Khiari, M.; Gilliot, M.; Lejeune, M.; Lazar, F.; Hadjadj, A. Preparation of Very Thin Zinc Oxide Films by Liquid Deposition Process: Review of Key Processing Parameters. Coatings 2022, 12, 65. [Google Scholar] [CrossRef]
- Mo, G.; Cui, Y.; Yin, J.; Gao, P. Influence of ZnO Film Deposition Parameters on Piezoelectric Properties and Film-to-Substrate Adhesion on a GH4169 Superalloy Steel Substrate. Micromachines 2022, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.R.; Voigt, J.A.; Liu, J.; Mckenzie, B.; Mcdermott, M.J.; Rodriguez, M.A.; Konishi, H.; Xu, H. Complex and Oriented ZnO Nanostructures. Nat. Mater. 2003, 2, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Rekha, S.M.; Neelamana, H.V.; Bhat, S.V. Recent Advances in Solution-Processed Zinc Oxide Thin Films for Ultraviolet Photodetectors. ACS Appl. Electron. Mater. 2023, 5, 4051–4066. [Google Scholar] [CrossRef]
- Shinde, S.S.; Shinde, P.S.; Bhosale, C.H.; Rajpure, K.Y. Optoelectronic properties of sprayed transparent and conducting indium doped zinc oxide thin films. J. Phys. D Appl. Phys. 2008, 41, 105109. [Google Scholar] [CrossRef]
- Ponja, S.D.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Highly conductive and transparent gallium doped zinc oxide thin films via chemical vapor deposition. Sci. Rep. 2020, 10, 638. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Wu, H.; Yang, J.; Wang, Y.; Zhang, J.; Bao, Q.; Wang, M.; Ma, Z.; Tress, W.; et al. A Transparent Electrode Based on Solution-Processed ZnO for Organic Optoelectronic Devices. Nat. Commun. 2022, 13, 4387. [Google Scholar] [CrossRef]
- Liu, L.; Mei, Z.; Tang, A.; Azarov, A.; Kuznetsov, A.; Xue, Q.-K.; Du, X. Oxygen vacancies: The origin of n-type conductivity in ZnO. Phys. Rev. B 2016, 93, 235305. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, physics, and applications. ChemPhysChem 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, F.; Wang, L.; Jia, Y.; Sun, Q. First-principles study of negative thermal expansion in zinc oxide. J. Appl. Phys. 2013, 114, 063508. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.C.; Xu, C.S.; Qiu, Y.Q.; Zhao, L.; Rong, X.M. Effect of nitrogenized Si (111) substrates on the quality of ZnO films grown by pulsed laser deposition. J. Phys. D Appl. Phys. 2009, 42, 035307. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.M.C.; Pimentel, A.C.M.B.G.; Gonçalves, A.M.F.; Marques, A.J.S.; Martins, R.F.P.; Pereira, L.M.N. Wide-bandgap high-mobility ZnO thin-film transistors produced at room temperature. Appl. Phys. Lett. 2004, 85, 2541–2543. [Google Scholar] [CrossRef]
- Chen, Y.; Ko, H.J.; Hong, S.K.; Yao, T. Layer-by-layer growth of ZnO epilayer on Al2O3(0001) by using a MgO buffer layer. Appl. Phys. Lett. 2000, 76, 559. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Vasanthi, V.; Sivanantham, N.; Saathvika, L.; Gopalakrishnan, N. Metal oxide charge transport materials for light emitting diodes—An overview. Mat. Sci. Semicond. Process. 2023, 165, 107679. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties, and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Hanada, T. Basic Properties of ZnO, GaN, and Related Materials. In Oxide and Nitride Semiconductors. Advances in Materials Research; Yao, T., Hong, S.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 12. [Google Scholar]
- Chao, C.H.; Wei, D.H. Synthesis and characterization of high c-axis ZnO thin film by plasma enhanced chemical vapor deposition system and its UV photodetector application. J. Vis. Exp. 2015, 3, 53097. [Google Scholar] [CrossRef]
- Hamdani, F.; Yeadon, M.; Smith, D.J.; Tang, H.; Kim, W.; Salvador, A.; Botchkarev, A.E.; Gibsor, J.M.; Polyakov, A.Y.; Skowronski, M.; et al. Microstructure and optical properties of epitaxial GaN on ZnO (0001) grown by reactive molecular beam epitaxy. J. Appl. Phys. 1998, 83, 983–990. [Google Scholar] [CrossRef]
- Dong, J.J.; Zhang, X.W.; Yin, Z.G.; Wang, J.X.; Zhang, S.G.; Liu, X. Ultraviolet electroluminescence from ordered ZnO nanorod array/p-GaN light emitting diodes. Appl. Phys. Lett. 2012, 100, 171109. [Google Scholar] [CrossRef]
- Look, D.C. Recent advances in ZnO materials and devices. Mater. Sci. Eng. B 2001, 80, 383–387. [Google Scholar] [CrossRef]
- Inoki, C.K.; Kuan, T.S.; Lee, C.D.; Sagar, A.; Feenstra, R.M.; Koleske, D.D.; Adesida, I. Growth of GaN on porous SiC and GaN substrates. J. Electron. Mater. 2003, 32, 855–860. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Maros, A.; Faleev, N.; Smith, D.J. Strain relaxation in low-mismatched GaAs/GaAs1-xSbx/GaAs heterostructures. Acta Mater. 2019, 162, 103–115. [Google Scholar] [CrossRef]
- Norton, D.P.; Heo, Y.W.; Ivill, M.P.; Ip, K.; Pearton, S.J.; Chisholm, M.F.; Steiner, T. ZnO: Growth, doping, and processing. Mater. Today 2004, 7, 34–40. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanopiezotronics. Adv. Mater. 2007, 19, 889–892. [Google Scholar] [CrossRef]
- Shafiqul, I.M.; Deep, R.; Lin, J.; Yoshida, T.; Fujita, Y. The Role of nitrogen dopants in ZnO nanoparticle-based light emitting diodes. Nanomaterials 2022, 12, 358. [Google Scholar] [CrossRef]
- Cerrato, E.; Privitera, A.; Chiesa, M.; Salvadori, E.; Paganini, M.C. Nitrogen-doped Zinc oxide for photo-driven molecular hydrogen production. Int. J. Mol. Sci. 2022, 23, 5222. [Google Scholar] [CrossRef]
- Musiliyu, K.A.; Ogunmola, E.D.; Ajayi, A.A.; Abodunrin, O.W. Effect of concentration on the properties of nitrogen-doped zinc oxide thin films grown by electrodeposition. Mater. Renew. Sustain. Energy 2023, 12, 23–29. [Google Scholar] [CrossRef]
- Wang, X.; Xin, S.; Tao, B.; Huang, B.; Subramaniam, V.; Ramachandran, K.; Zhang, F.; Shang, S.; Liu, X. Study of the Preparation and Performance of Mg-Doped Si/Al2O3/ZnO Nanowall Heterojunction UV Detector. ACS Appl. Nano Mater. 2024, 7, 21401–21409. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Ashokkumar, M. Comparative assessment of transition metals doping effects on structural, optical, optical conductivity, and photocatalytic features of ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2024, 35, 1614. [Google Scholar] [CrossRef]
- Perumal, R.; Hassan, Z. Effect of nitrogen doping on structural, morphological, optical and electrical properties of radio frequency magnetron sputtered zinc oxide thin films. Phys. B Condens. Matter 2016, 490, 16–20. [Google Scholar] [CrossRef]
- Seo, J.W.; Oh, C.S.; Cheong, H.S.; Yang, J.W.; Youn, C.J.; Lim, K.Y. UV-assisted electrochemical oxidation of GaN. J. Korean Phys. Soc. 2002, 41, 1017–1020. [Google Scholar]
- Yam, F.K.; Hassan, Z.; Ng, S.S. Porous GaN prepared by UV assisted electrochemical etching. Thin Solid Film. 2007, 515, 3469–3474. [Google Scholar] [CrossRef]
- Davydov, V.Y.; Kitaev, Y.E.; Goncharuk, I.; Smirnov, A.; Graul, J.; Semchinova, O.; Uffmann, D.; Smirnov, M.; Mirgorodsky, A.; Evarestov, R. Phonon dispersion and Raman scattering in hexagonal GaN and AlN. Phys. Rev. B 1998, 58, 12899. [Google Scholar] [CrossRef]
- Al-Heuseen, K.; Hashim, M.R.; Ali, N.K. Effect of different electrolytes on porous GaN using photo-electrochemical etching. Appl. Surf. Sci. 2011, 257, 6197–6201. [Google Scholar] [CrossRef]
- Zeng, Y.; Ning, J.; Zhang, J.; Jia, Y.; Yan, C.; Wang, B.; Wang, D. Raman analysis of E2 (High) and A1 (LO) phonon to the stress-free GaN grown on sputtered AlN/Graphene buffer layer. Appl. Sci. 2020, 10, 8814. [Google Scholar] [CrossRef]
- Abed, C.; Bouzidi, C.; Elhouichet, H.; Gelloz, B.; Ferid, M. Mg doping induced high structural quality of sol–gel ZnO nanocrystals: Application in photocatalysis. Appl. Surf. Sci. 2015, 349, 855–863. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Saleh, W.R.; Al-Sammarraie, A.M.A. Structural and optical properties of ZnO nanostructures synthesized by hydrothermal method at different conditions. Nano Hybrids Compos. 2022, 35, 75–83. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Arshi, N.; Anwar, M.S.; Koo, B.H. Morphological evolution between nanorods to nanosheets and room temperature ferromagnetism of Fe-doped ZnO nanostructures. Cryst. Eng. Comm. 2012, 14, 4016–4026. [Google Scholar] [CrossRef]
- Tu, M.-L.; Su, Y.-K.; Ma, C.-Y. Nitrogen-doped p-type ZnO films prepared from nitrogen gas radio-frequency magnetron sputtering. J. Appl. Phys. 2006, 100, 053705–053708. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [PubMed]
- Siva, N.; Sakthi, D.; Ragupathy, S.; Arun, V.; Kannadasan, N. Synthesis, structural, optical and photocatalytic behavior of Sn doped ZnO nanoparticles. Mater. Sci. Eng. B 2020, 253, 114497. [Google Scholar] [CrossRef]
- Hirai, M.; Kumar, A. Infrared Lattice Vibrations of Nitrogen-doped ZnO Thin Films. Mater. Res. Soc. Symp. Proc. 2007, 1035, 707. [Google Scholar] [CrossRef]
- Decremps, F.; Datchi, F.; Saitta, A.M.; Polian, A.; Pascarelli, S.; Di Cicco, A.; Baudelet, F. Local structure of condensed zinc oxide. Phys. Rev. B 2003, 68, 104101. [Google Scholar] [CrossRef]
- Kang, J.H.; Song, A.; Park, Y.J.; Seo, J.H.; Walker, B.; Chung, K.-B. Tungsten-doped zinc oxide and indium–zinc oxide films as high-performance electron-transport layers in N–I–P perovskite solar cells. Polymers 2020, 12, 737. [Google Scholar] [CrossRef]
- Dimitrov, D.; Tsai, C.-L.; Petrov, S.; Marinova, V.; Petrova, D.; Napoleonov, B.; Blagoev, B.; Strijkova, V.; Hsu, K.Y.; Lin, S.H. Atomic layer-deposited Al-doped ZnO thin films for display applications. Coatings 2020, 10, 539. [Google Scholar] [CrossRef]
- Saddique, M.; Khan, A.; Liao, H.; Yu, G.; Iqbal, I.; Lei, M.; Lang, R.; Mi, Z.; Chen, H.; Zong, H.; et al. Reduction of threading dislocations in GaN grown on patterned sapphire substrate masked with serpentine channel. Mater. Sci. Semicond. Process. 2021, 134, 106013. [Google Scholar] [CrossRef]
- Sivakumar, V.; Sivaganesh, D.; Gopal, J.N.; Muthuvinayagam, M.; Kim, J.M.; Kannan, P.K.; Saravanakumar, S. Enhancement of intrinsic green emission in phase pure ZnO. Phys. B Condens. Matter 2022, 644, 414155. [Google Scholar] [CrossRef]
- King, P.D.C.; Veal, T.D.; Fuchs, F.; Wang, C.Y.; Payne, D.J.; Bourlange, A.; Zhang, H.; Bell, G.R.; Cimalla, V.; Ambacher, O.; et al. Band gap, electronic structure, and surface electron accumulation of cubic and rhombohedral In2O3. Phys. Rev. B 2009, 79, 205211. [Google Scholar] [CrossRef]
- van der Pauw, L.J. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Res. Rep. 1958, 13, 1–9. [Google Scholar]
- Imasato, K.; Kang, S.D.; Ohno, S.; Snyder, G.J. Band engineering in Mg3Sb2 by alloying with Mg3Bi2 for enhanced thermoelectric performance. Mater. Horiz. 2018, 5, 59–64. [Google Scholar] [CrossRef]
- Keavney, D.J.; Buchholz, D.B.; Ma, Q.; Chang, R.P.H. Where does the spin reside in ferromagnetic Cu-doped ZnO? Appl. Phys. Lett. 2007, 91, 012501. [Google Scholar] [CrossRef]
- Erhart, P.; Albe, K.; Klein, A. First-principles study of intrinsic point defects in ZnO: Role of band structure, volume relaxation, and finite-size effects. Phys. Rev. B 2006, 73, 205203. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.H.; Zunger, A. Microscopic Origin of the Phenomenological Equilibrium “Doping Limit Rule” in n-Type III-V Semiconductors. Phys. Rev. Lett. 2000, 84, 1232–1235. [Google Scholar] [CrossRef]
- Hopoğlu, H.; Aydınoğlu, H.S.; Özer, A.; Tüzemen, E.S. Investigation of nitrogen doped ZnO thin films: Effects on their structural and optical properties. Opt. Mater. 2021, 122, 111685. [Google Scholar] [CrossRef]
- Wang, L.Y.; Mal′shukov, A.G.; Chu, C.S. Nonuniversality of the intrinsic inverse spin-Hall effect in diffusive systems. Phys. Rev. B 2012, 85, 165201. [Google Scholar] [CrossRef]




| Growth Parameters | Experimental Values |
|---|---|
| Substrate | Unintentionally doped GaN (n-type) |
| Metal oxide target | ZnO ceramic (99.999%) |
| Base pressure | 3.0 × 10−5 milli bar |
| Working pressure | 2.0 × 10−2 milli bar |
| Deposition temperature | 300 K |
| Argon gas (atmosphere) | 10 sccm |
| Nitrogen gas (doping) | Gas flow rate, 2 sccm, 4 sccm, 6 sccm, 8 sccm, and 10 sccm, respectively. |
| Radio frequency (RF) sputtering power | 150 W |
| Film Sample | Nitogen Gas Flow (sccm) | RMS Values on Non-Porous GaN (nm) | RMS Values on Etched Porous GaN (nm) | Carrier Concentraion (cm−3) | Carrier Mobility (cm2 V−1 s−1) |
|---|---|---|---|---|---|
| Pure ZnO | 0 sccm | 11.2 | 3.4 | 4.71 × 1017 | 13.15 |
| N-doped ZnO | 2 sccm | - | 2.1 | 2.12 × 1018 | 2.5 |
| N-doped ZnO | 4 sccm | - | 2.0 | 8.54 × 1019 | 37.5 |
| N-doped ZnO | 6 sccm | - | 1.7 | 4.40 × 1021 | 1.0 |
| N-doped ZnO | 8 sccm | - | 1.6 | 5.29 × 1021 | 1.7 |
| N-doped ZnO | 10 sccm | - | 1.1 | 7.99 × 1018 | 29.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, R.; Saravanan, L.; Liu, J.-H. Substrate and Doping Effects on the Growth Aspects of Zinc Oxide Thin Films Developed on a GaN Substrate by the Sputtering Technique. Processes 2025, 13, 1257. https://doi.org/10.3390/pr13041257
Perumal R, Saravanan L, Liu J-H. Substrate and Doping Effects on the Growth Aspects of Zinc Oxide Thin Films Developed on a GaN Substrate by the Sputtering Technique. Processes. 2025; 13(4):1257. https://doi.org/10.3390/pr13041257
Chicago/Turabian StylePerumal, R., Lakshmanan Saravanan, and Jih-Hsin Liu. 2025. "Substrate and Doping Effects on the Growth Aspects of Zinc Oxide Thin Films Developed on a GaN Substrate by the Sputtering Technique" Processes 13, no. 4: 1257. https://doi.org/10.3390/pr13041257
APA StylePerumal, R., Saravanan, L., & Liu, J.-H. (2025). Substrate and Doping Effects on the Growth Aspects of Zinc Oxide Thin Films Developed on a GaN Substrate by the Sputtering Technique. Processes, 13(4), 1257. https://doi.org/10.3390/pr13041257








