Carbon Dioxide Capture by Alkaline Water with a Semi-Batch Column and Ultra-Fine Microbubble Generator
Abstract
:1. Introduction
2. Materials and Methods
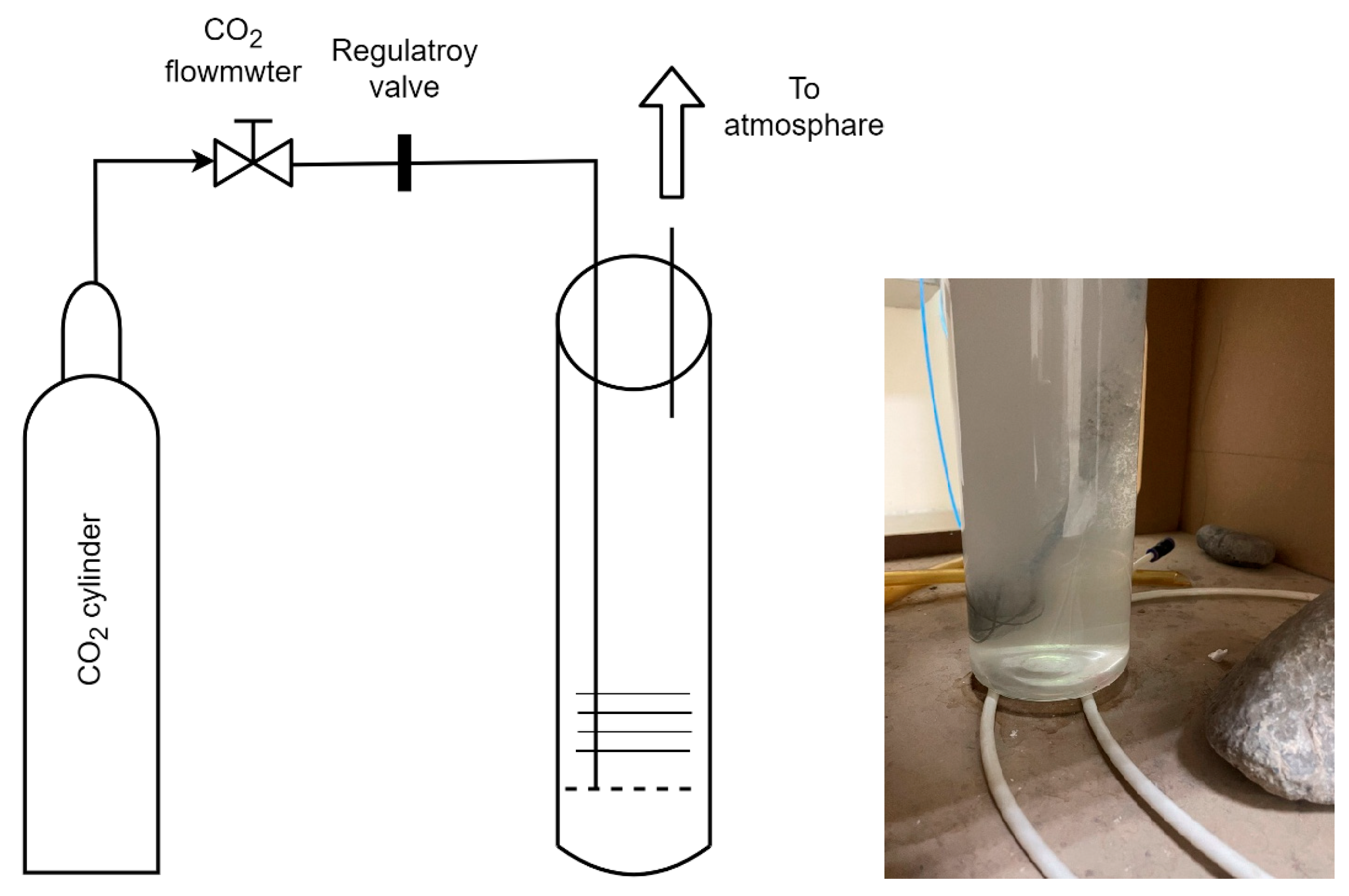
2.1. Semi-Batch Bubble Column
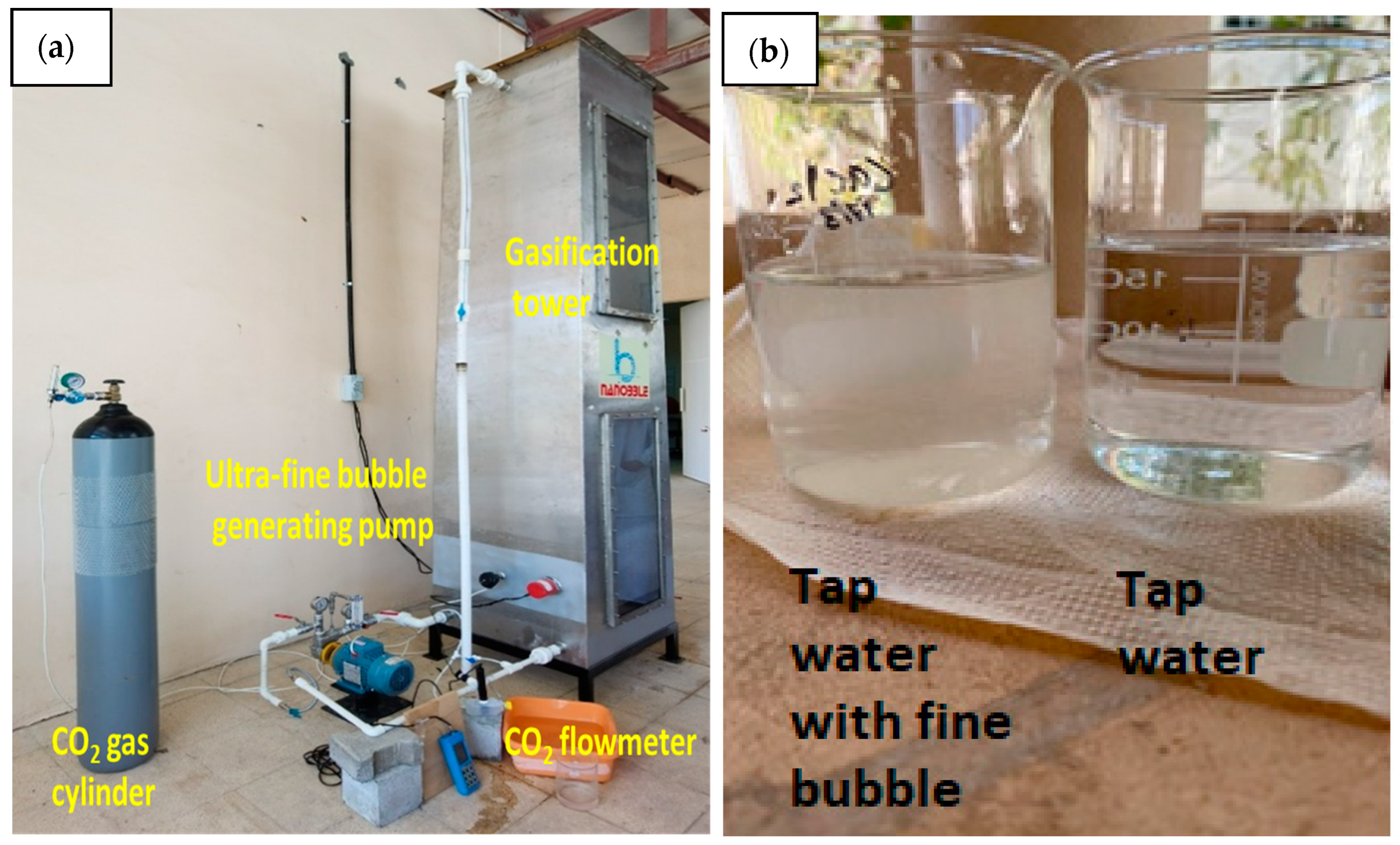
2.2. Ultra-Fine Micro Bubble Column
2.3. Characterization
2.4. Testing
3. Results and Discussion
3.1. Solution Dynamics of Treated Water—Effect of Variable Water Sources
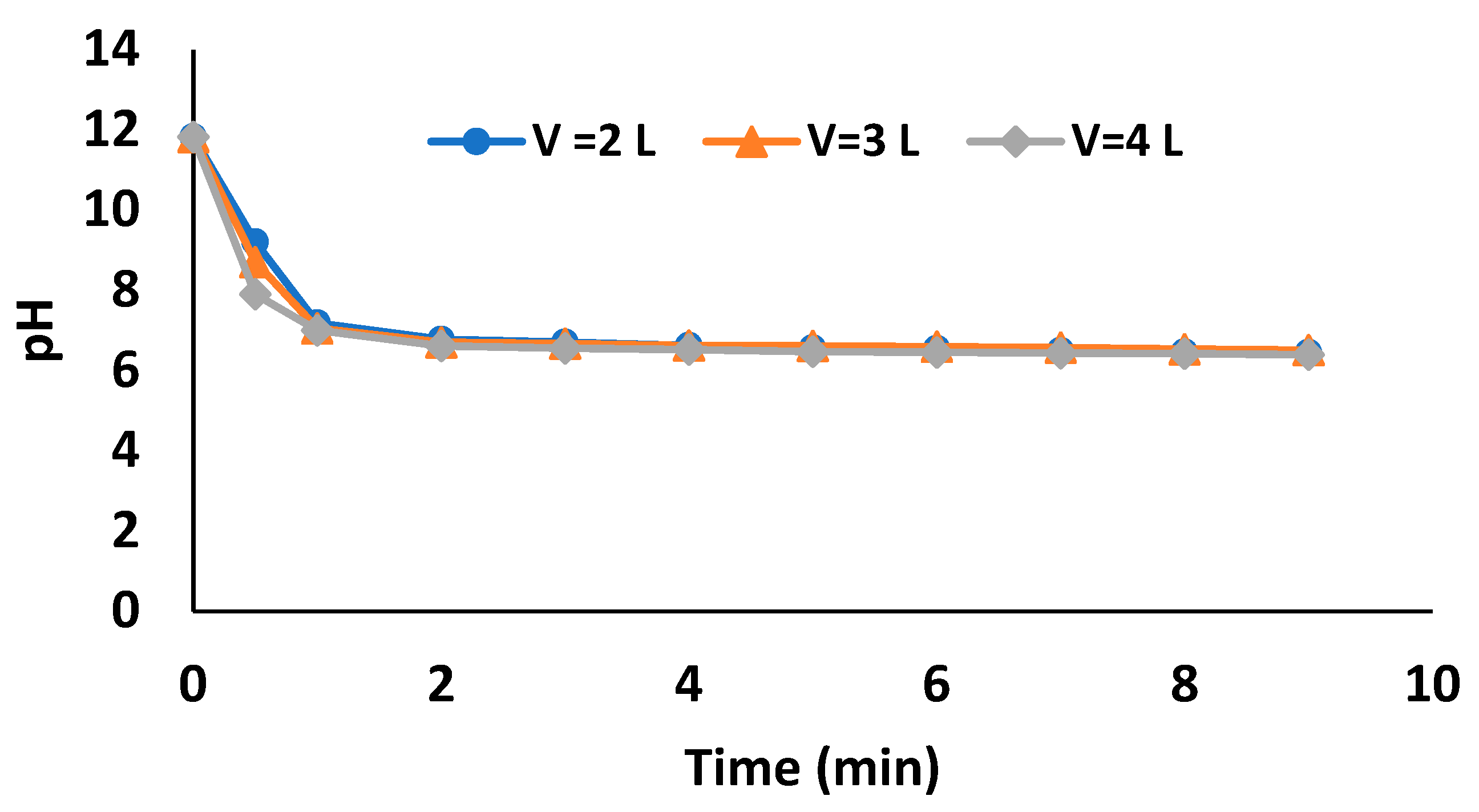
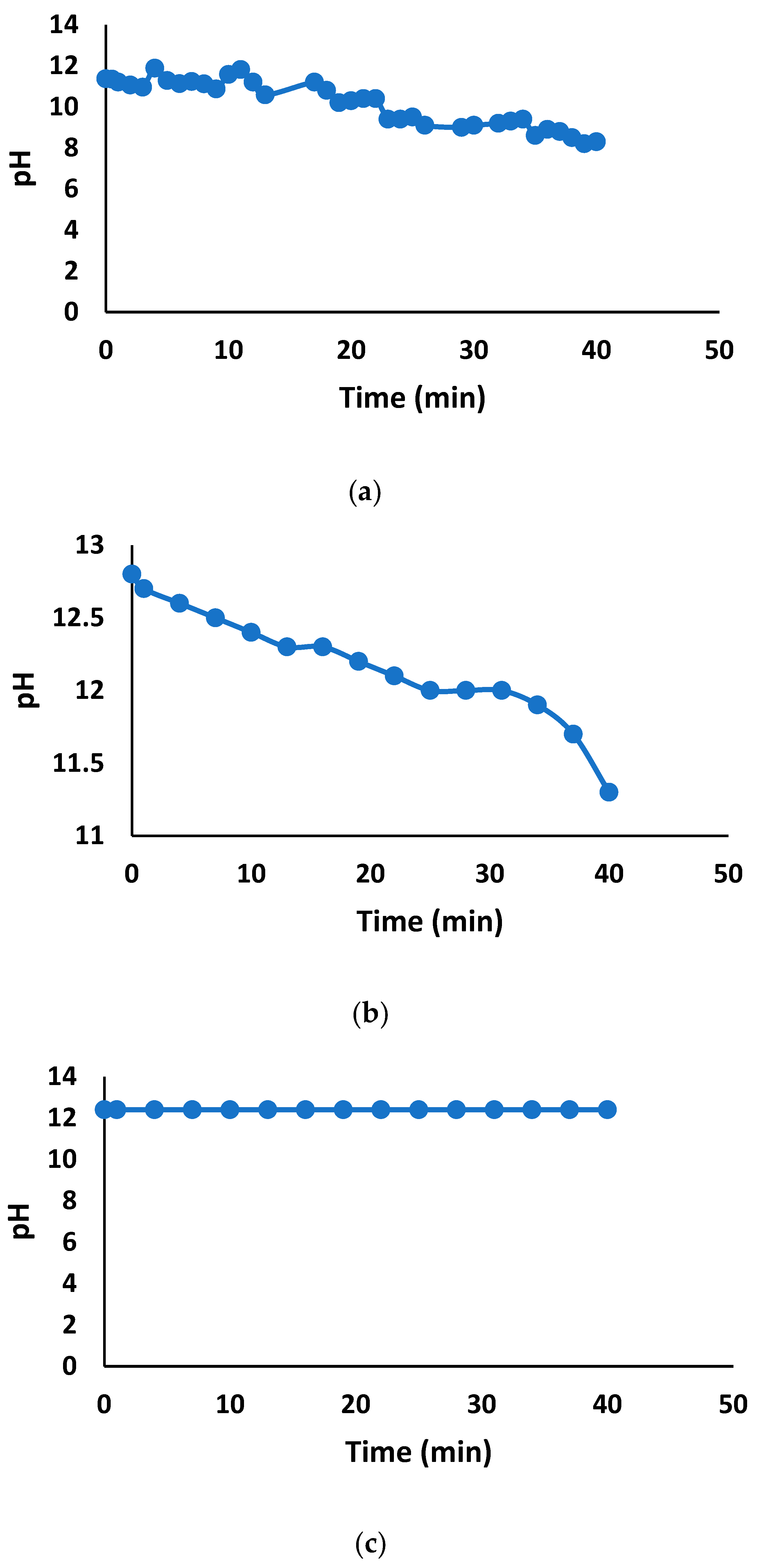
3.1.1. Tap Water Solutions—Batch-Scale Study
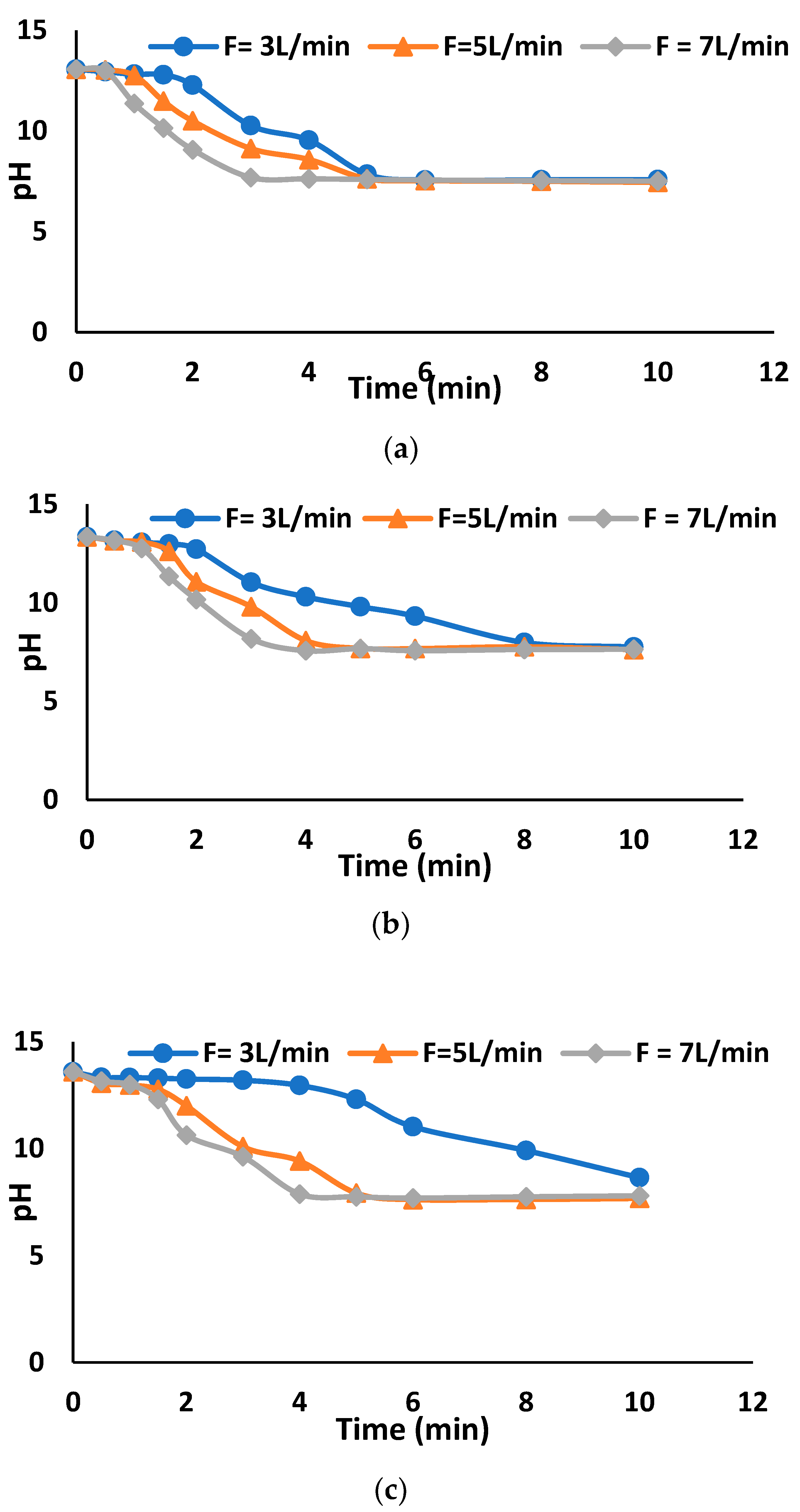
3.1.2. Tap Water Solutions—Pilot-Scale Study
3.1.3. Deionized Water Solutions
3.2. Mechanism of CO2 Mitigation Through Neutralization Reaction
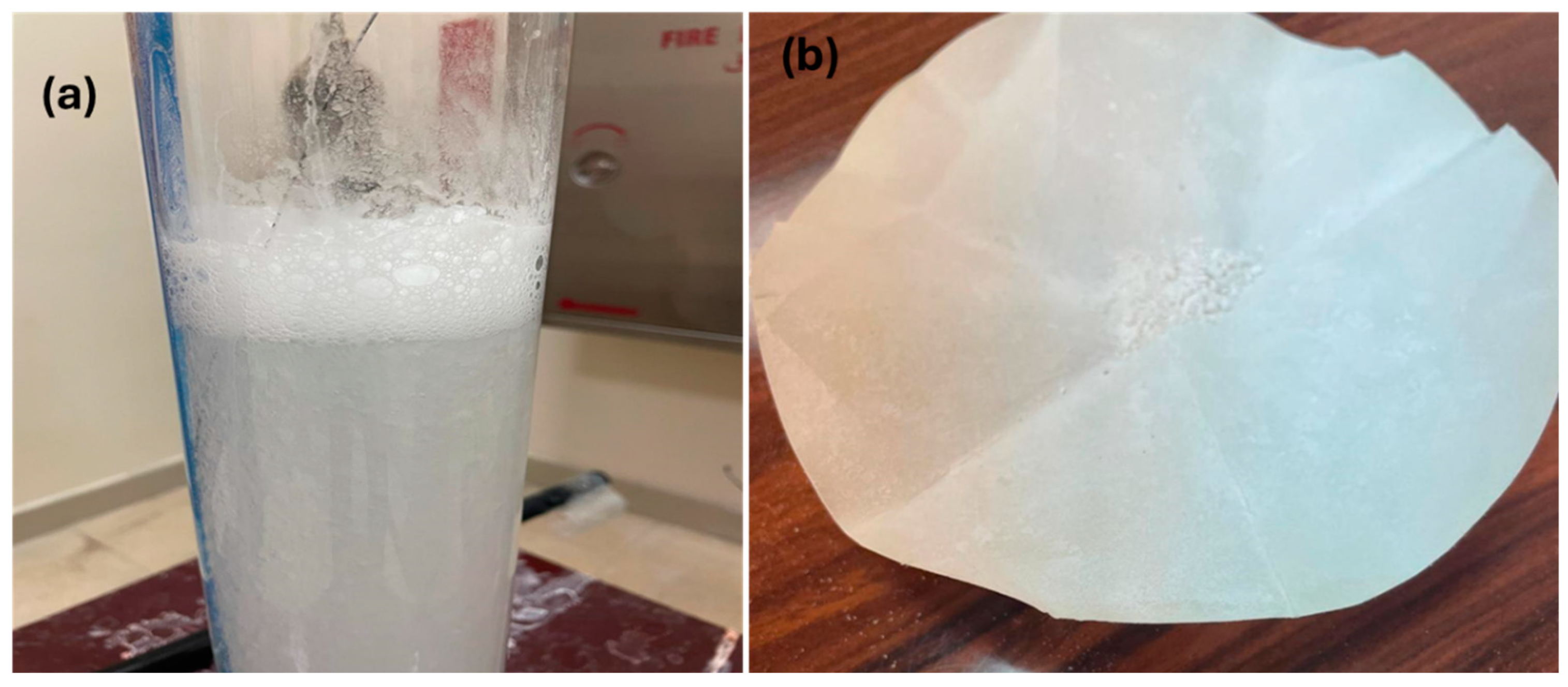
3.2.1. Precipitate Formation in Tap Water vs. Deionized Water
Tap Water
- Formation of carbonate ions
- Formation of calcium carbonate (CaCO3)
- Formation of magnesium carbonate (MgCO3):
Deionised Water
3.2.2. pH Variation of Treated Water
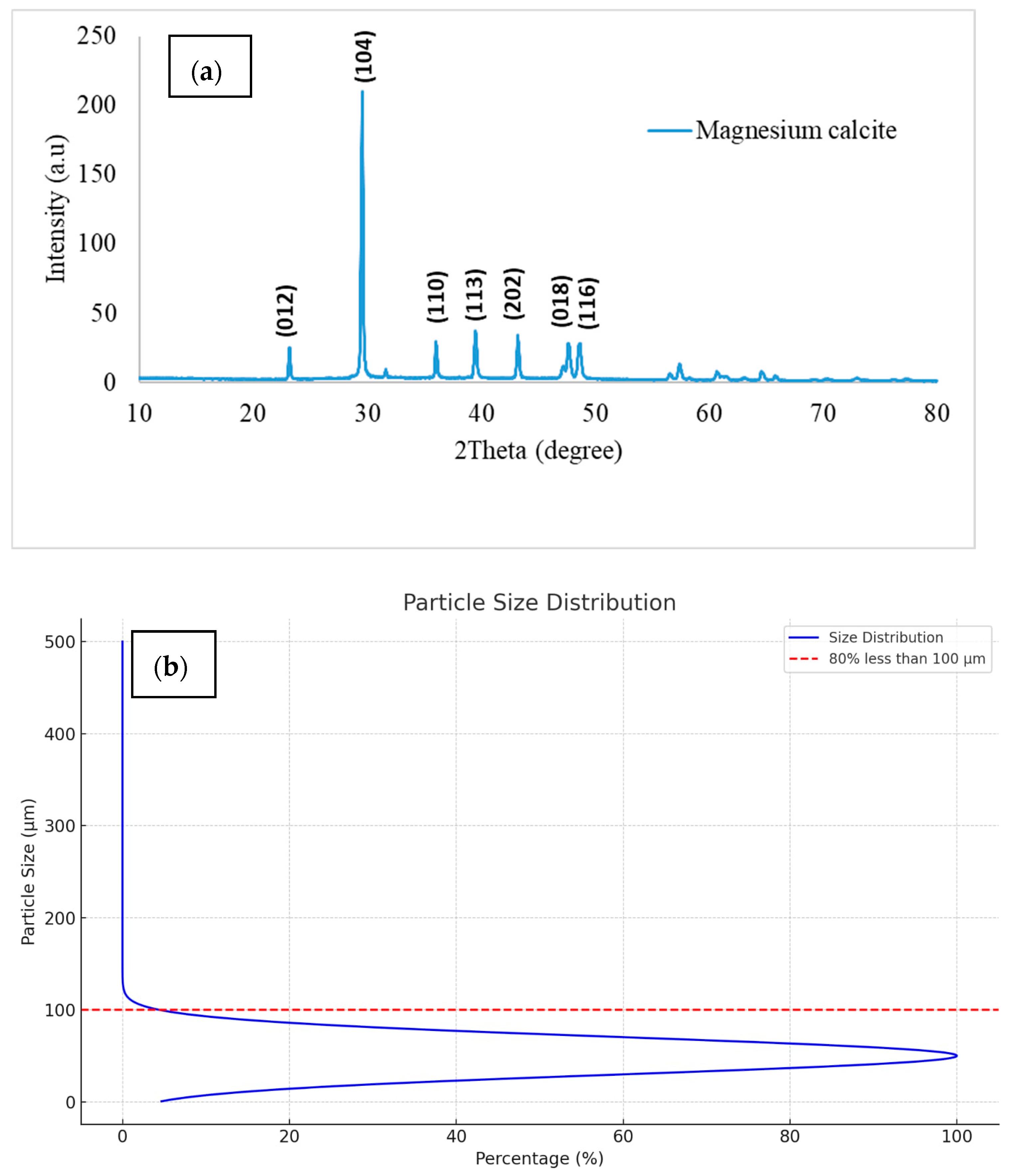
3.3. Material Characterization of the Precipitates
3.4. Water Analysis of Treated Water
3.4.1. Water Alkalinity Testing
Treated Tap Water
Treated Deionized Water
Untreated Tap Water
3.4.2. Water Conductivity Testing
3.5. Comparision of Process Efficiency with the Reported Literature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, J.; Zhu, Y.; Zhang, Z.; Yang, W. Modification and improvement of microalgae strains for strengthening CO2 fixation from coal-fired flue gas in power plants. Bioresour. Technol. 2019, 291, 121850. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Q.; Qi, Y.; Chen, G.; Song, Y.; Kansha, Y.; Kitamura, Y. Absorption-microalgae hybrid CO2 capture and biotransformation strategy—A review. Int. J. Greenh. Gas Control. 2019, 88, 109–117. [Google Scholar] [CrossRef]
- Tu, R.; Jin, W.; Han, S.-F.; Zhou, X.; Wang, J.; Wang, Q.; He, Z.; Ding, W.; Che, L.; Feng, X. Enhancement of microalgal lipid production in municipal wastewater: Fixation of CO2 from the power plant tail gas. Biomass Bioenergy 2019, 131, 105400. [Google Scholar] [CrossRef]
- Nugroho, M.A.; Susanto, Y.B.; Kamilah, V.L.; Prameswari, R. Carbon Dioxide (CO2) Absorption Process Using Sodium Hydroxide (NaOH) IPTEK. J. Eng. 2023, 9, 30–34. [Google Scholar]
- Kwak, H.S.; Kim, J.Y.H.; Woo, H.M.; Jin, E.; Min, B.K.; Sim, S.J. Synergistic effect of multiple stress conditions for improving microalgal lipid production. Algal Res. 2016, 19, 215–224. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, Z.; Lin, W. Comparison of removal efficiencies of carbon dioxide between aqueous ammonia and NaOH solution in a fine spray column. Energy Procedia 2011, 4, 512–518. [Google Scholar] [CrossRef]
- Ye, W.; Huang, J.; Lin, J.; Zhang, X.; Shen, J.; Luis, P.; Van der Bruggen, B. Environmental evaluation of bipolar membrane electrodialysis for NaOH production from wastewater: Conditioning NaOH as a CO2 absorbent. Sep. Purif. Technol. 2015, 144, 206–214. [Google Scholar] [CrossRef]
- Institute of Metallurgy and Ore Beneficiation; Kenzhaliyev, B.; Temirova, S.; Fisher, D.; Baltabekova, Z. Removal of hexavalent chromium from aqueous solutions by sorption with activated carbon. Review. Vestnik Kazn. 2024, 146, 8–16. [Google Scholar] [CrossRef]
- Abdykadyrov, A.; Abdullayev, S.; Tashtay, Y.; Zhunussov, K.; Marxuly, S. Purification of surface water by using the corona discharge method. Min. Miner. Deposits 2024, 18, 125–137. [Google Scholar] [CrossRef]
- Darmana, D.; Henket, R.L.B.; Deen, N.G.; Kuipers, J.A.M. Detailed modelling of hydrodynamics, mass transfer and chemical reactions in a bubble column using a discrete bubble model: Chemisorption of CO2 into NaOH solution, numerical and experimental study. Chem. Eng. Sci. 2007, 62, 2556–2575. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, W.; Ren, J.; Xu, R. Photocatalytic reduction of CO2: A brief review on product analysis and systematic methods. Anal. Methods 2013, 5, 1086–1097. [Google Scholar] [CrossRef]
- Kordylewski, W.; Sawicka, D.; Falkowski, T. Laboratory tests on the efficiency of carbon dioxide capture from gases in naoh solutions. J. Ecol. Eng. 2013, 14, 54–62. [Google Scholar] [CrossRef]
- Tavan, Y.; Hosseini, S.H. A novel rate of the reaction between NaOH with CO2 at low temperature in spray dryer. Petroleum 2017, 3, 51–55. [Google Scholar] [CrossRef]
- Salmón, I.R.; Cambier, N.; Luis, P. CO2 capture by alkaline solution for carbonate production: A comparison between a packed column and a membrane contactor. Appl. Sci. 2018, 8, 996. [Google Scholar] [CrossRef]
- Pichler, T.; Stoppacher, B.; Kaufmann, A.; Siebenhofer, M.; Kienberger, M. Continuous Neutralization of NaOH Solution with CO2 in an Internal-Loop Airlift Reactor. Chem. Eng. Technol. 2021, 44, 38–47. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, G.; Liu, J.; Li, G.; Wang, Y. Highly efficient CO2 adsorption by imidazole and tetraethylenepentamine functional sorbents: Optimization and analysis using response surface methodology. J. Environ. Chem. Eng. 2021, 9, 105639. [Google Scholar] [CrossRef]
- Jagannathan, T.; Chandramouli, A.; Ramachandran, K.; Dev, S.; Devi, E.L.; Sharma, K.; Rajamani, C. Use of drone with sodium hydroxide carriers to absorb carbon dioxide from ambient air. J. Emerg. Investig. 2021, 4, 1–5. [Google Scholar] [CrossRef]
- Sadewo, R.P.; Hidhayati, N.; Ambarsari, L.; Anam, K. CO2 Sequestration Using Sodium Hydroxide and Its Utilization for Chlorella sorokiniana Biomass Production. Biosaintifika J. Biol. Biol. Educ. 2022, 14, 391–399. [Google Scholar] [CrossRef]
- Kozlowski, A.M.; Hasani, M. Cellulose interactions with CO2 in NaOH(aq): The (un)expected coagulation creates potential in cellulose technology. Carbohydr. Polym. 2022, 294, 119771. [Google Scholar] [CrossRef]
- Lipps, W.C. Standard Methods for the Examination of Water and Wastewater, 24th ed.; WEF APHA AWWA, USA: Washington, DC, USA, 2022. [Google Scholar]
- Santos, H.S.S.; Nguyen, H.; Venâncio, F.Q.; Ramteke, D.; Zevenhoven, R.; Kinnunen, P. Mechanisms of Mg carbonates precipitation and implications for CO2 capture and utilization/storage. Inorg. Chem. Front. 2023, 10, 2507–2546. [Google Scholar] [CrossRef]
- Lucile, F.; Cézac, P.; Contamine, F.; Serin, J.-P.; Houssin, D.; Arpentinier, P. Solubility of Carbon Dioxide in Water and Aqueous Solution Containing Sodium Hydroxide at Temperatures from (293.15 to 393.15) K and Pressure up to 5 MPa: Experimental Measurements. J. Chem. Eng. Data 2012, 57, 784–789. [Google Scholar] [CrossRef]
- Altiner, M.; Yildirim, M. Production of precipitated calcium carbonate particles with different morphologies from dolomite ore in the presence of various hydroxide additives. Physicochem. Probl. Miner. Process. 2017, 53, 413–426. [Google Scholar] [CrossRef]




| Water Sample | Total Alkalinity Methyl Orange Alkalinity (as CaCO3) | Phenolphthalein Alkalinity (as CaCO3) | Total Hardness (as CaCO3) |
|---|---|---|---|
| Untreated Tap Water | 369.80 mg/L | 0.00 mg/L | 340.40 mg/L |
| Treated Tap Water | 993.40 mg/L | 501.00 mg/L | 198.20 mg/L |
| Treated Deionized Water | 96.80 mg/L | 0.00 mg/L | 39.80 g/L |
| CO2 Absorption Efficiency (%) | NaOH Concentration (M) | CO2 Flow Rate (L/min) | Process Type | Key Findings | Reference |
|---|---|---|---|---|---|
| 85 | 0.5 | 10 | Air-lift reactor | Bulk neutralization | Pichler et al. (2021) [16] |
| Moderate | Not mentioned | Not mentioned | Euler-Lagrange model | Larger bubble size, limited mass transfer | Darmana et al. (2007) [10] |
| 85 | 0.5 | 140 | Dreschel washers | Higher NaOH concentrations improve efficiency | Kordylewski et al. (2013) [13] |
| Variable | Not specified | Not specified | Spray absorption | Inverse relation between CO2 flow rate and absorption | Nugroho et al. (2023) [4] |
| Significantly higher | 0.1–0.3 | 3–7 | Ultra-microbubble system | Enhanced gas-liquid interaction, dual environmental benefits | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almamari, S.A.; Al-Dawery, S.K.; Farooq, S.; Al Aisri, D.H.; Alrahbi, S.S.; Al Fazari, A.A.; Harharah, H.N.; Harharah, R.H.; Al Alawi, S.S.; Hayder, G. Carbon Dioxide Capture by Alkaline Water with a Semi-Batch Column and Ultra-Fine Microbubble Generator. Processes 2025, 13, 1259. https://doi.org/10.3390/pr13041259
Almamari SA, Al-Dawery SK, Farooq S, Al Aisri DH, Alrahbi SS, Al Fazari AA, Harharah HN, Harharah RH, Al Alawi SS, Hayder G. Carbon Dioxide Capture by Alkaline Water with a Semi-Batch Column and Ultra-Fine Microbubble Generator. Processes. 2025; 13(4):1259. https://doi.org/10.3390/pr13041259
Chicago/Turabian StyleAlmamari, Samiya A., Salam K. Al-Dawery, Saima Farooq, Dalal H. Al Aisri, Sumaya S. Alrahbi, Aisha A. Al Fazari, Hamed N. Harharah, Ramzi H. Harharah, Salim S. Al Alawi, and Gasim Hayder. 2025. "Carbon Dioxide Capture by Alkaline Water with a Semi-Batch Column and Ultra-Fine Microbubble Generator" Processes 13, no. 4: 1259. https://doi.org/10.3390/pr13041259
APA StyleAlmamari, S. A., Al-Dawery, S. K., Farooq, S., Al Aisri, D. H., Alrahbi, S. S., Al Fazari, A. A., Harharah, H. N., Harharah, R. H., Al Alawi, S. S., & Hayder, G. (2025). Carbon Dioxide Capture by Alkaline Water with a Semi-Batch Column and Ultra-Fine Microbubble Generator. Processes, 13(4), 1259. https://doi.org/10.3390/pr13041259








