Investigation of the Factors and Mechanisms Affecting the Foaming of Triethylene Glycol in Natural Gas Purification
Abstract
1. Introduction
2. Materials and Methods
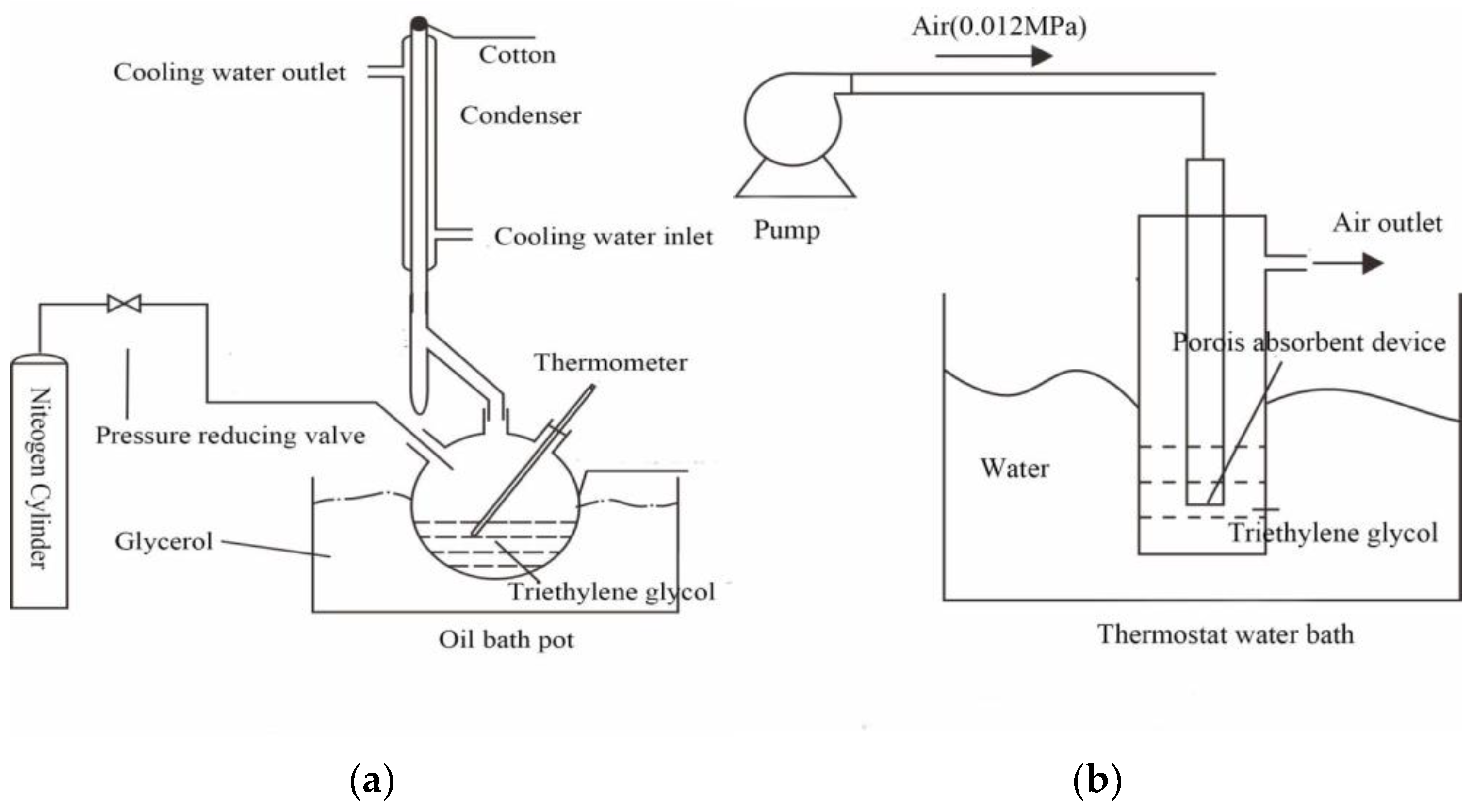
2.1. Experimental Apparatus and Drugs
2.2. Experimental Methods
2.2.1. Triethylene Glycol Regeneration Experiments
2.2.2. Measurement of Foaming Performance
2.2.3. Measurement Experiments
3. Results
3.1. The Influence of Impurities on the Properties of Triethylene Glycol
3.2. Effect of Water-Soluble Heat-Stable Salts on Triethylene Glycol Foaming Bulleted Lists Look Like This
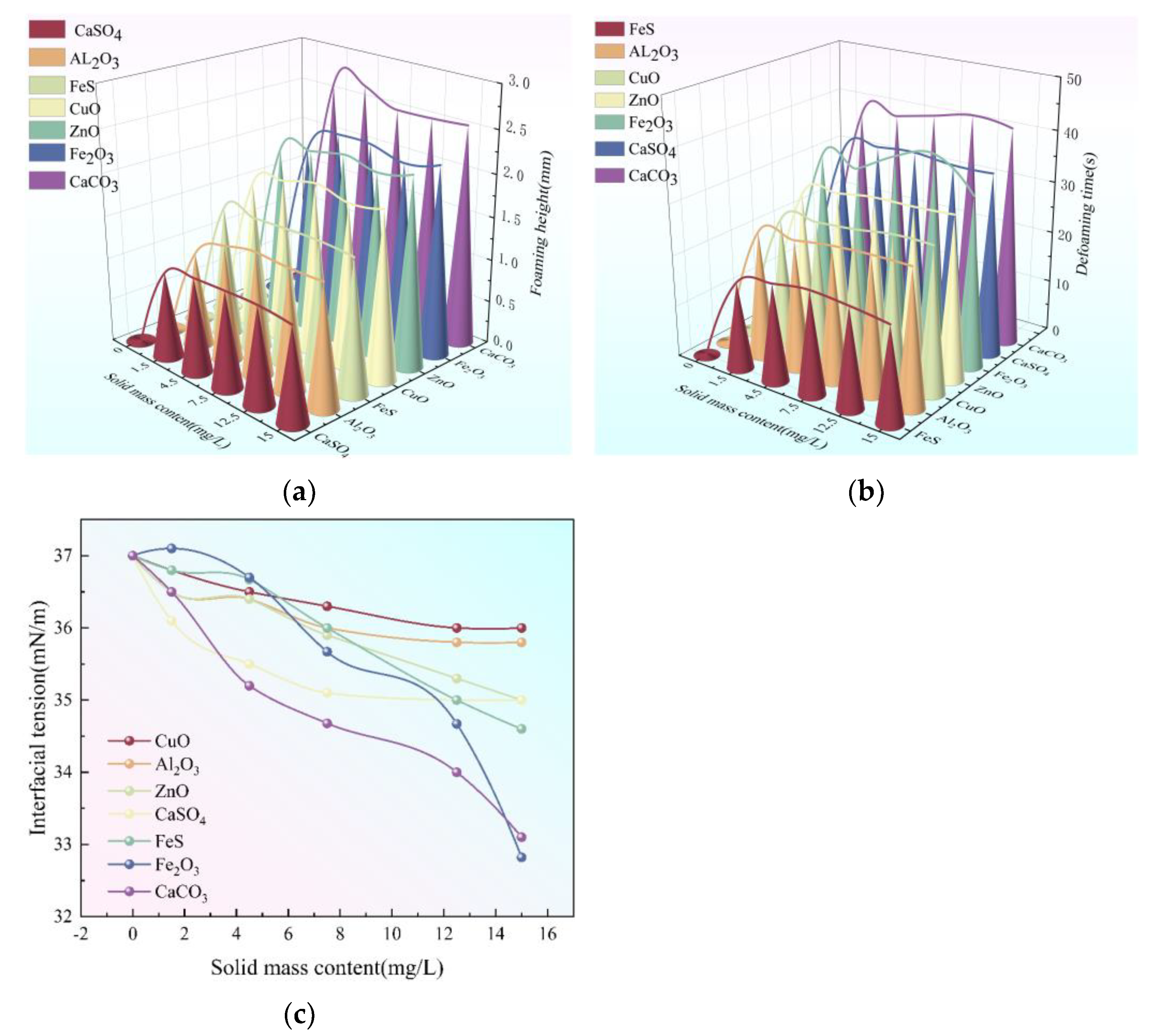
3.3. Effect of Solid Impurities on the Foaming of Triethylene Glycol
3.4. Effect of Corrosion Inhibitor and Foam Inhibitor on the Foaming of Triethylene Glycol
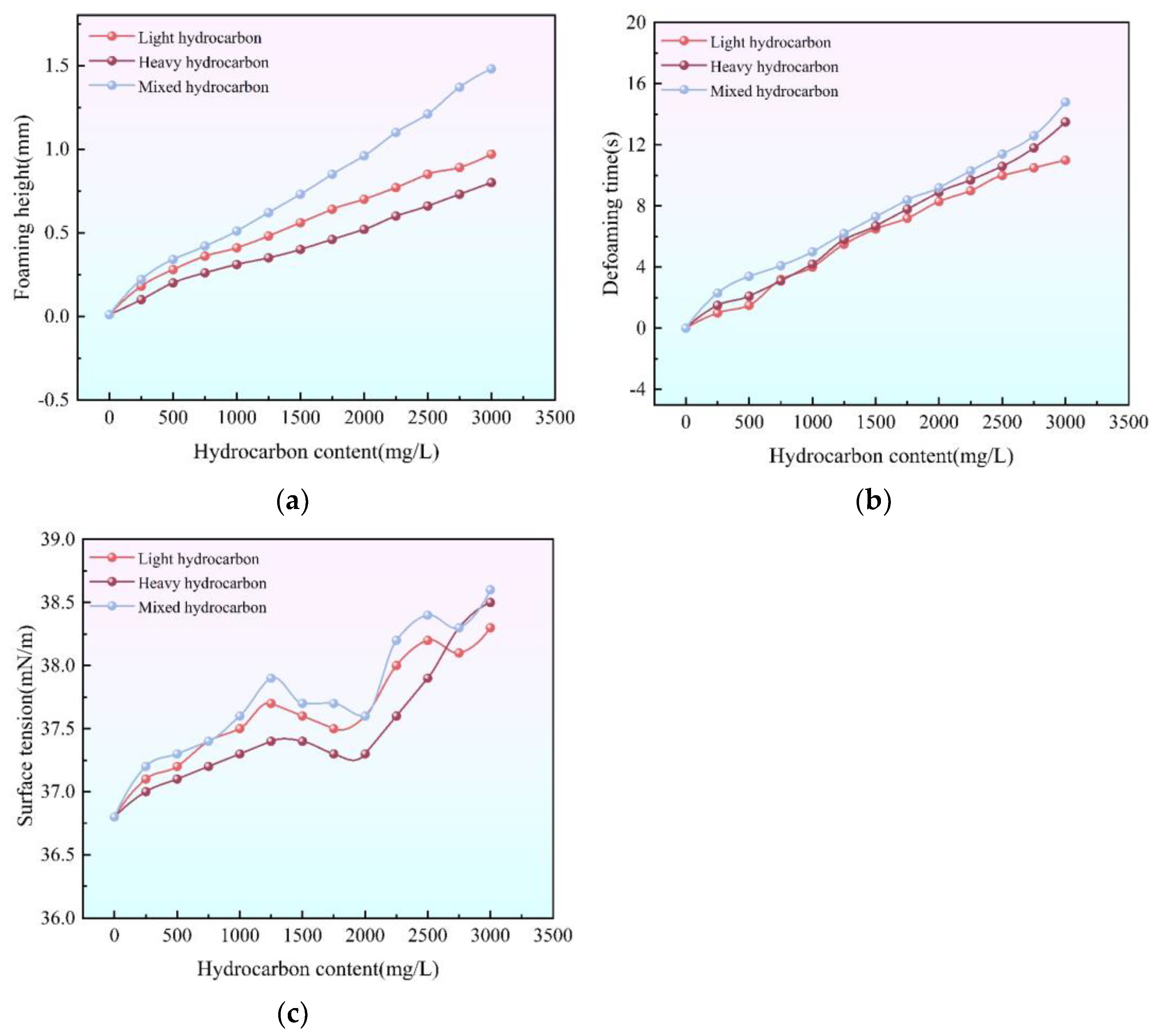
3.5. Impact of Hydrocarbons on Foaming in Triethylene Glycol Dehydration Plants
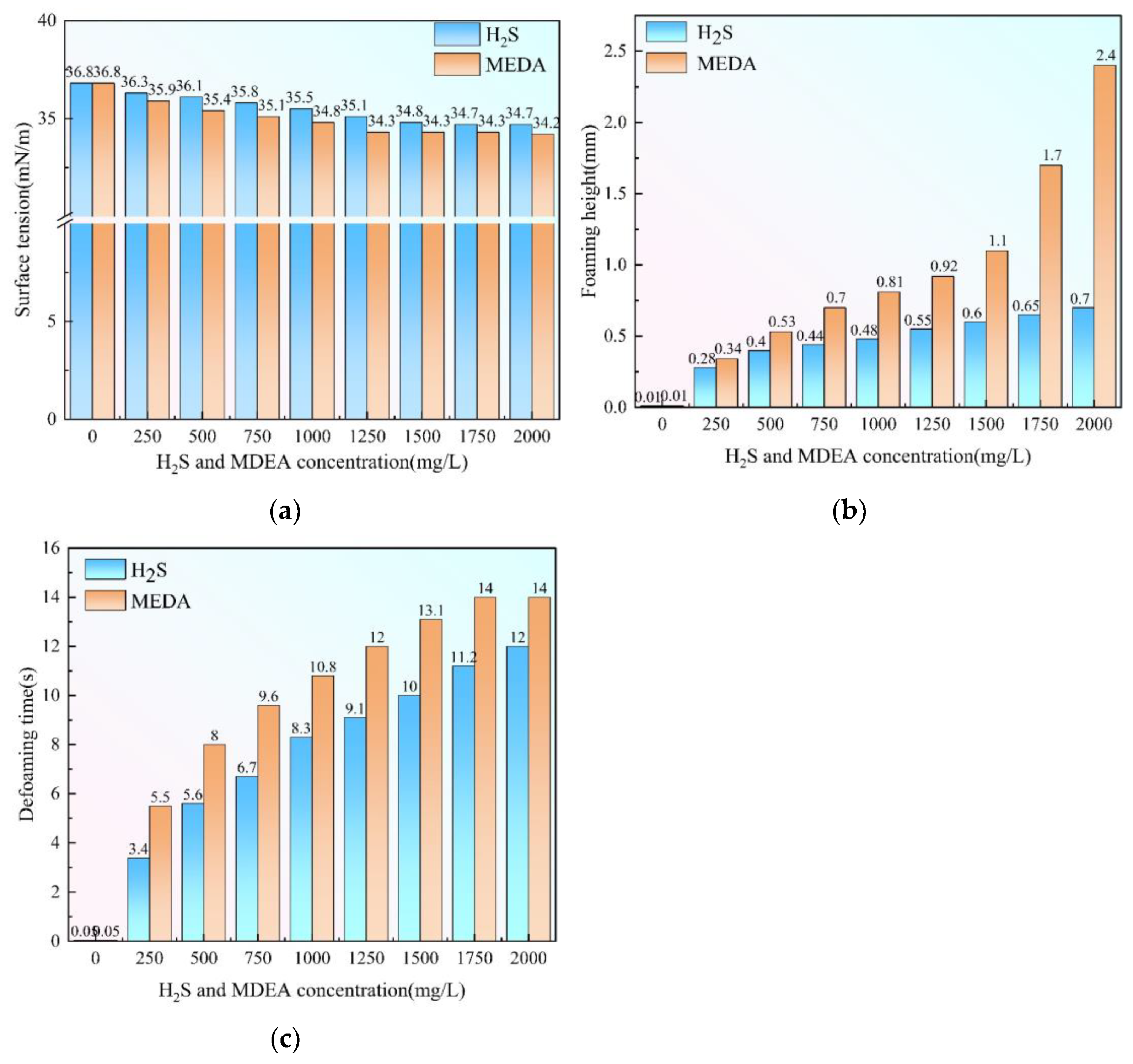
3.6. Effect of Hydrogen Sulfide and MDEA on Triethylene Glycol Foaming
3.7. Measures for Impurity Control and Foaming Inhibition in TEG Dehydration System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Dou, L.; Wang, H.; Liu, D.; Ba, D.; Zhao, Q.; Zhao, S.; Li, Q.; Liao, X.; Qu, J.; et al. The Future of Sustainable Development Driven by Natural Gas—Overview of the 28th World Natural Gas Conference. Nat. Gas Ind. 2022, 42, 1–6. [Google Scholar]
- Zhou, Q. The Position of China’s Petroleum and Natural Gas in the World. Pet. Nat. Gas Geol. 2020, 41, 888. [Google Scholar]
- Alcheikhhamdon, Y.; Hoorfar, M. Natural gas quality enhancement: A review of the conventional treatment processes, and the industrial challenges facing emerging technologies. J. Nat. Gas Sci. Eng. 2016, 34, 689–701. [Google Scholar] [CrossRef]
- Eldemerdash, U.N.; Abdrabou, M.; El-Sheltawy, S.T.; Abdelghany, A. Assessment of chemical enhancement and energy consumption of natural gas dehydration processes. Gas Sci. Eng. 2024, 123, 205226. [Google Scholar] [CrossRef]
- Gonfa, G.; Kidanu, A.; Prabhu, S.V.; Gomadurai, C. Introduction to natural gas dehydration methods and technologies. In Advances in Natural Gas: Formation, Processing, and Applications; Natural Gas Dehydration, Rahimpour, M.R., Makarem, M.A., Meshksar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 4, pp. 3–27. [Google Scholar]
- Ma, C.; Liu, X.; Zheng, H.; Wu, X.; Zhang, C. Research on the regulation method of triglycol dehydration system based on sensitivity analysis. Oil Nat. Gas Chem. Ind. 2021, 50, 1–8. [Google Scholar]
- Yang, Y.; Chen, Q.; Li, Z. Application of triglycol dehydration technology in Yuanba gas field purification device. Oil Nat. Gas Chem. Ind. 2021, 50, 9–14+19. [Google Scholar]
- Wang, Y.; San, C.; Wu, K. Research on the evaluation of the dehydration effect of shale gas triglycol dehydration device. Oil Nat. Gas Chem. Ind. 2020, 49, 1–7. [Google Scholar]
- Guo, P.; Ran, W.; Liu, H. Research progress on the prediction of natural gas hydrate generation conditions. Sci. Technol. Eng. 2018, 18, 133–139. [Google Scholar]
- Zhao, H.; Xia, Y.; Zhang, D.; Niu, M.; Ma, H.; Li, W. Research on the influence of impurities on the dehydration ability of triglycol. Nat. Gas Chem. Ind. (C1Chemistry Chem. Ind.) 2016, 41, 47–51. [Google Scholar]
- Li, C.; Li, Y.; Ran, S.; Chen, S.; Wu, M.; Yang, Y. Design and Application of Amine Liquid Online Deep Resurrection Device. Pet. Nat. Gas Chem. Ind. 2024, 53, 26–30. [Google Scholar]
- Chen, Q.; Peng, X.; Wu, M.; Zhang, X.; Huang, H. Analysis of Potential Bubbling Substances in Upstream Operations of Natural Gas Purification Plants. Pet. Nat. Gas Chem. Ind. 2022, 51, 33–37. [Google Scholar]
- Gao, C.; Liu, R.; Yang, J.; Zhao, H.; Wei, W.; Xie, J. Experimental study on removal of hydrocarbon impurities in MDEA and optimization of filtration system. Pet. Nat. Gas Chem. Ind. 2023, 52, 19–24. [Google Scholar]
- Jiang, J.; Yue, Y.; Chen, S. Analysis of the influence of process parameters on the safe and stable operation of MDEA desulfurization unit. Pet. Nat. Gas Chem. Ind. 2019, 48, 28–33. [Google Scholar]
- Alhseinat, E.; Pal, P.; Ganesan, A.; Banat, F. Effect of MDEA degradation products on foaming behavior and physical properties of aqueous MDEA solutions. Int. J. Greenh. Gas Control 2015, 37, 280–286. [Google Scholar] [CrossRef]
- Alhseinat, E.; Amr, M.; Jumah, R.; Banat, F. Removal of MDEA foam creators using foam fractionation: Parametric study coupled with foam characterization. J. Nat. Gas Sci. Eng. 2015, 26, 502–509. [Google Scholar] [CrossRef]
- Guo, B.; He, Z.; Liu, X.; Jiang, A.; Wang, L.; Liu, C. Analysis and recovery research on the failure cause of triglycol. Nat. Gas Ind. 2006, 9, 152–153+181. [Google Scholar]
- Jin, X.; Zhang, N.; Wu, X.; Chen, Z. Research on the impact of pollutants on the dehydration and foaming of triglycol. Nat. Gas Ind. 2005, 25, 97–98+105. [Google Scholar]
- Guo, H.; Li, D.; Lei, X.; Zhao, H.; Wang, X.; Gao, J.; Li, W. Inactivation analysis and degradation characteristics of triglycol solution. J. Chem. Eng. Coll. Univ. 2017, 31, 1127–1134. [Google Scholar]
- Jiang, H.; Yang, C.; Zhu, C. Process analysis and improvement of natural gas dehydration device. Nat. Gas Chem. Ind. (C1 Chem. Chem. Ind.) 2009, 34, 49–53+58. [Google Scholar]
- Li, M.; Xu, L.; Zhang, Y.; Fan, J.; Du, Y.; Pu, Y. The use of triglycol in natural gas dehydration production. Drill. Process 2005, 28, 107–108. [Google Scholar]
- Al-Aiderous, A. Troubleshooting Gas Dehydration Systems ULZ 001066 Using Data Analysis. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 15–18 November 2021. [Google Scholar]
- Arubi, T.I.M.; Duru, U.I. Optimizing Glycol Dehydration SystemFor Maximum Efficiency: A Case Study of a Gas Plant in Nigeria. In Proceedings of the CIPC/SPE Gas Technology Symposium 2008 Joint Conference, Calgary, AB, Canada, 16–19 June 2008; SPE: Calgary, AB, Canada, 2016; p. 113781. [Google Scholar]
- Soliman; Mohamed; Alwani; Abdo, M.; Aiderous, A.Y. Troubleshooting Glycol Loss in Gas De-hydration Systems Using Data Analysis at Upstream Operation. In Proceedings of the Abu Dhabi International Petroleum EXhibition & Conference, Abu Dhabi, United Arab Emirates, 11–14 November 2019. [Google Scholar]
- Dugstad, A.; Sirnes, H. Experimental Study of Black Powder Formation in Field TEG solutions. In Proceedings of the CORROSION 2011, Houston, TX, USA, 13–17 March 2011; p. 11083. [Google Scholar]
- GB/T 7462-1994; Surfactant, Determination of Foaming Power, Improvement Ross-Miles Law. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 1994.
- Wu, X.; Kang, X. Methyldiethanolamine(MDEA)Research on the influencing factors and mechanism of desulfurization solution foaming. Nat. Gas Chem. Ind. (C1 Chem. Chem. Ind.) 2008, 33, 31–36. [Google Scholar]
- Xiao, F.; Bai, B.; Gao, F. The influence of inorganic salts and hydrogen sulfide on the dehydration of natural gas triglycol ULZ 001094. Oil Nat. Gas Chem. Ind. 2022, 51, 1–6. [Google Scholar]
- Weng, J.; Xia, Y.; Huang, C.; Chen, X.; Song, L.; Zhang, Z.; Xun, R. Research on the purification method of triglycol dehydration solution in Jingbian gas field. Oil Gas Chem. Ind. 2017, 46, 16–21. [Google Scholar]
- Long, X.; Zhou, Q.; Meng, J. Experimental study on the effect of water-soluble inorganic salts on the dehydration performance of triglycol. Oil Nat. Gas Chem. Ind. 2023, 52, 9–15. [Google Scholar]
- Dong, X.Q.; Xu, J.; Cao, C.B.; Sun, D.J.; Jiang, X. Aqueous foam stabilized by hydrophobically modified silica particles and liqui D paraffin droplets. Colloids Surf. A. 2010, 353, 181–188. [Google Scholar] [CrossRef]
- Zhao, G.; Fang, J.; Dai, C.; Yan, Y.; Yan, Z.; Qing, Y. Enhanced Foam Stability By Adding Dispersed Particle Gel: A New 3-Phase Foam Study. In Proceedings of the SPE Asia PAcific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar]
- Meng, J.; Yuan, L.; Liu, T.; Dong, B. The effect of inorganic salts on triglycol rheusity. Oil Gas Storage Transp. 2015, 34, 849–853. [Google Scholar]
- Jiang, N.; Yu, X.; Sheng, Y.; Zong, R.; Li, C. Role of salts in performance of foam stAbilized with sodium dodecyl sulfate. Chem. Eng. Sci. 2020, 216, 115474. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, G. Research on the forced defoaming of coal sludge flotation foam by polydimethylsiloxane. Clean Coal Technol. 2021, 27, 212–217. [Google Scholar]
- Wang, H.; Liang, H.; Liu, Y.; Bai, J.; He, Y.; Gao, W. Research on the dehydration and defoaming characteristics of foam-assisted deoxidation air-driven extraction liquid. Oil Gas Field Ground Eng. 2023, 42, 23–27. [Google Scholar]
- Wu, G. Research on the Mechanism of the Influence of Inorganic Salts on the Foam Stability of Surfactants and Their Composite Systems. Ph.D. Thesis, China University of Petroleum (East China), Qingdao, China, 2017. [Google Scholar]
- Ibrahim, T.K.; Abdulrahman, R.K.; Khalaf, F.H.; Kamal, I.M. The Impact of Stripping Gas Flow Rate on Triethylene Glycol Losses fromGlycol Regeneration Unit: Si Mulation study. J. Chem. Eng. Process Technol. 2017, 8, 1000337. [Google Scholar]
- Long, X.; Cheng, H.; Zhang, Q. Experimental research on the effect of solid impurities on the quality of triglycol for natural gas dehydration Study intensively. Nat. Gas Chem. Ind. (C1 Chem. Chem. Ind.) 2022, 47, 106–112. [Google Scholar]
- Fang, C.; Ma, J.; Duan, X. Analysis of the impact of shale gas well return discharge on dehydration devices at different stages. Nat. Gas Oil 2021, 39, 7–11. [Google Scholar]
- Wu, J.; Wu, X.; Zhang, N.; Wang, X. Changqing Gas Field Second Purification Plant MDEA Desulfurization Solution Foaming Cause (II)—Foaming Causes and Treatment Measures. Nat. Gas Ind. 2005, 4, 171–174+26. [Google Scholar]
- Lai, X.; Zhang, Y.; Guo, L.; Yao, B.; Liu, J.; Yang, H. Research on the oil-repellent properties of ultra-low interface tension surfactants. Fine Petrochem. 2014, 31, 40–44. [Google Scholar]
- Parvaneh, R.; Riahi, S.; Lotfollahi, M.N. Experimental Evaluation of a Polymer Foam Agent on the Foam Stability, Concern to Surfactant, Nanop Article, and Salinity. SPE J. 2022, 27, 1462–1479. [Google Scholar] [CrossRef]
- Deng, L.; Li, G.; Cao, Y.; Wang, J. Research on the effect of flotation foaming agent on bubble merger behavior. J. China Min. Univ. 2017, 46, 410–414. [Google Scholar]
- Wang, C.; Yi, Y.; Sun, C.; Ding, F.; Mao, R.; Han, J. The influence of the properties of the gas/liquid interface membrane of the defoamer on its defoaming performance. Dly. Chem. Ind. 2017, 47, 13–17. [Google Scholar]
- Zhu, W.; Peng, X.; Ye, H. MDEA Desulfurization Solution Foaming Research. Pet. Nat. Gas Chem. Ind. 2015, 44, 22–27. [Google Scholar]
- Liu, Q.; Yang, C.; Tang, J. Analysis of the foaming characteristics of natural gas selective dethiamine solution formula. Nat. Gas Chem. Ind. (C1 Chem. Chem. Ind.) 2020, 45, 81–89. [Google Scholar]


| Experimental Setup | Provider |
|---|---|
| HH-W602 Super Constant Temperature Oil Bath | Tianjing Experimental Instrument Factory, Changzhou, Jiangsu, China |
| JJ2000B Rotating Drop Surface tension/Contact Angle Measuring Instrument | Shanghai Zhongchen Digital Technology Equipment Co, Shanghai, China. |
| Self-constructed experimental equipment for regeneration of triethylene glycol | self-build |
| Self-constructed triethylene glycol foaming equipment | self-build |
| Experimental Reagents | Supplier, Purity |
|---|---|
| C6H14O4, NaCl, KCl, MgCl2·6H2O, CaCl2, NaHCO3, BaCl2·2H2O, anhydrous Na2SO4, petroleum ether, gasoline | Chengdu Cologne Chemical Co., Ltd., Chengdu, Sichuan, China, analytical pure |
| Na2SO4, FeCl3·6H2O, CuO, Al2O3 | Tianjin Zhiyuan Chemical Reagent Co., Ltd., Tianjin, China, analytical pure |
| Fe2O3, FeS, CaSO4·H2O, CaCO3 | Xilong Science Co., Ltd., Chaoshan, Shantou, Chian, analytical pure |
| ZnO | Shandong Miguel Mirabella Chemical Co., yanggu, Shandong, China. |
| Corrosion inhibitor, defoamer | Longwangmiao natural gas purification plant use |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Huang, Q.; Li, X.; Wu, Q.; Yan, H.; Meng, J.; Long, X. Investigation of the Factors and Mechanisms Affecting the Foaming of Triethylene Glycol in Natural Gas Purification. Processes 2025, 13, 1261. https://doi.org/10.3390/pr13051261
Liang H, Huang Q, Li X, Wu Q, Yan H, Meng J, Long X. Investigation of the Factors and Mechanisms Affecting the Foaming of Triethylene Glycol in Natural Gas Purification. Processes. 2025; 13(5):1261. https://doi.org/10.3390/pr13051261
Chicago/Turabian StyleLiang, Hongyi, Qian Huang, Xin Li, Quan Wu, Han Yan, Jiang Meng, and Xueyuan Long. 2025. "Investigation of the Factors and Mechanisms Affecting the Foaming of Triethylene Glycol in Natural Gas Purification" Processes 13, no. 5: 1261. https://doi.org/10.3390/pr13051261
APA StyleLiang, H., Huang, Q., Li, X., Wu, Q., Yan, H., Meng, J., & Long, X. (2025). Investigation of the Factors and Mechanisms Affecting the Foaming of Triethylene Glycol in Natural Gas Purification. Processes, 13(5), 1261. https://doi.org/10.3390/pr13051261





