Optimized Torrefaction of Corn Straw in a Screw Reactor: Energy Balance Analysis and Biochar Production Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Calculations, and Calibration of the Experimental Platform
2.1.1. Overall Design of the Experimental Platform
2.1.2. Calculations for Experimental Platform Design
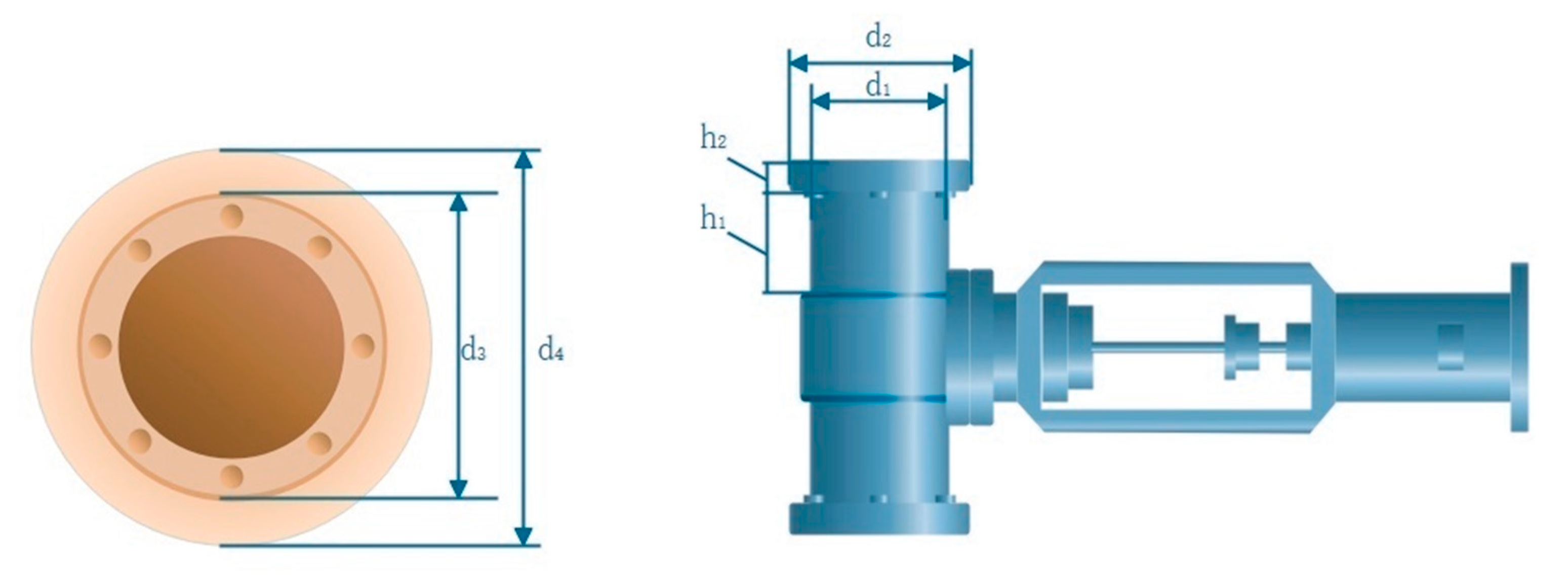
Calculation of Spiral Parameters
Heat Dissipation Loss Calculation
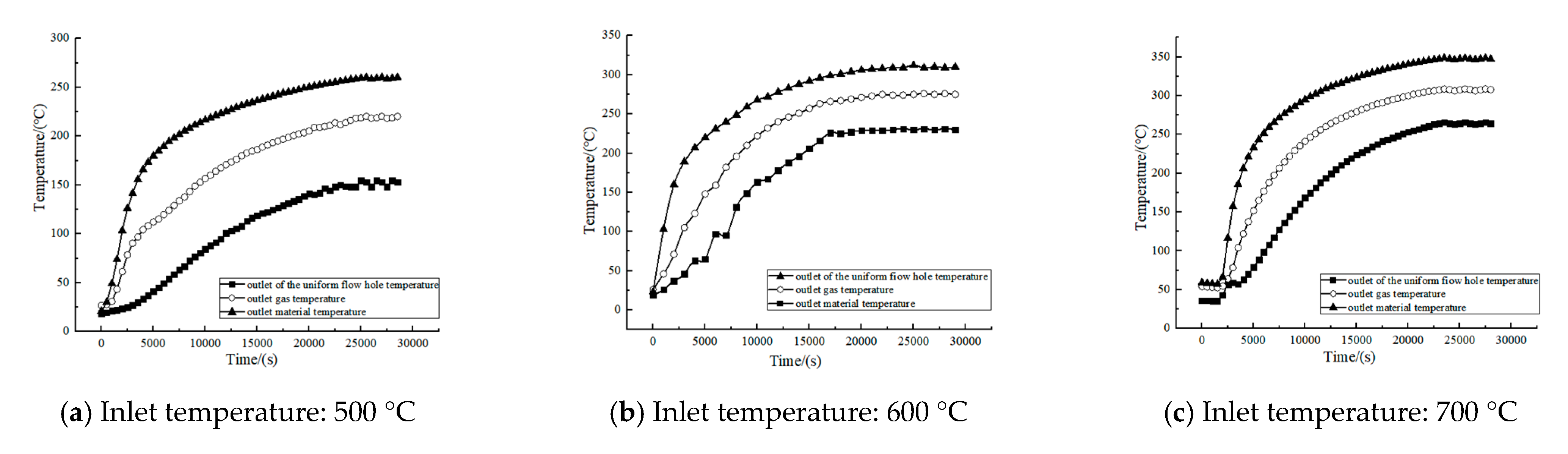
2.1.3. No-Load Thermal State Experiment
2.1.4. The Testing Method for Assessing the Compliance Rate of Torrefaction Products
3. Results and Discussion
3.1. Experimental Study on the Torrefaction of Corn Straw
3.1.1. Results of Torrefaction Product Conformity Rate Testing
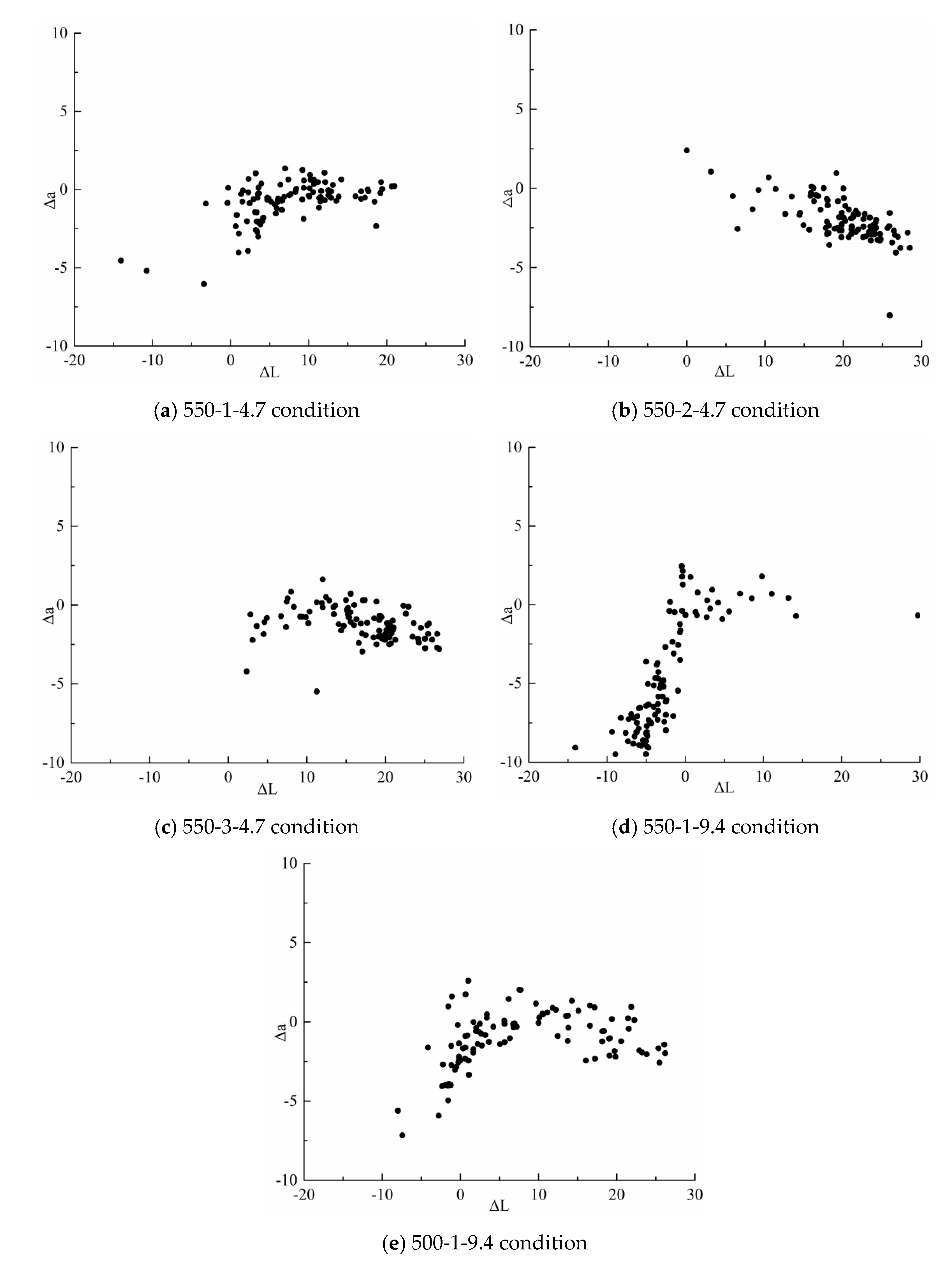
3.1.2. Results of Compliance Rate Determination After Optimization
3.2. Energy Balance Study of Corn Straw Torrefaction Process
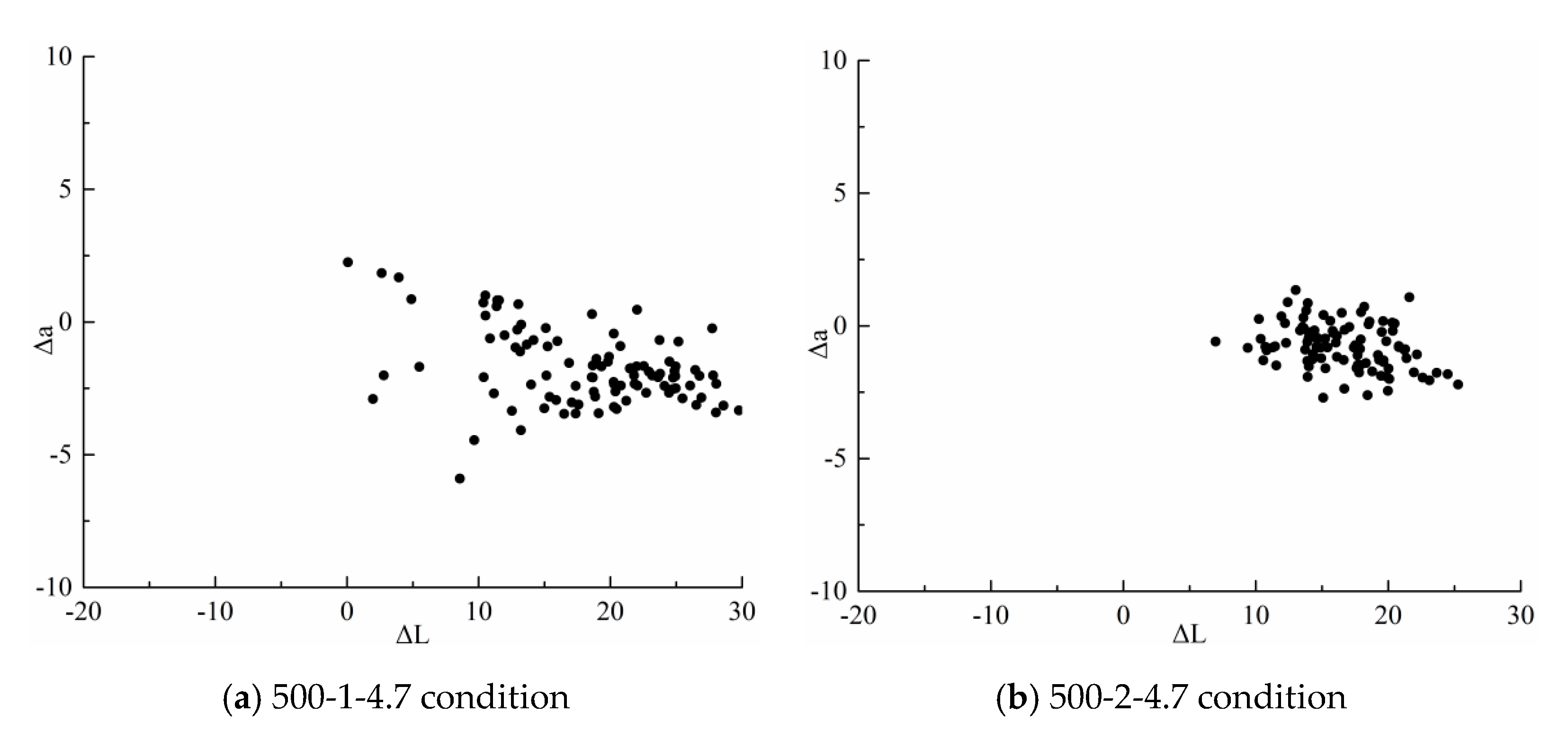
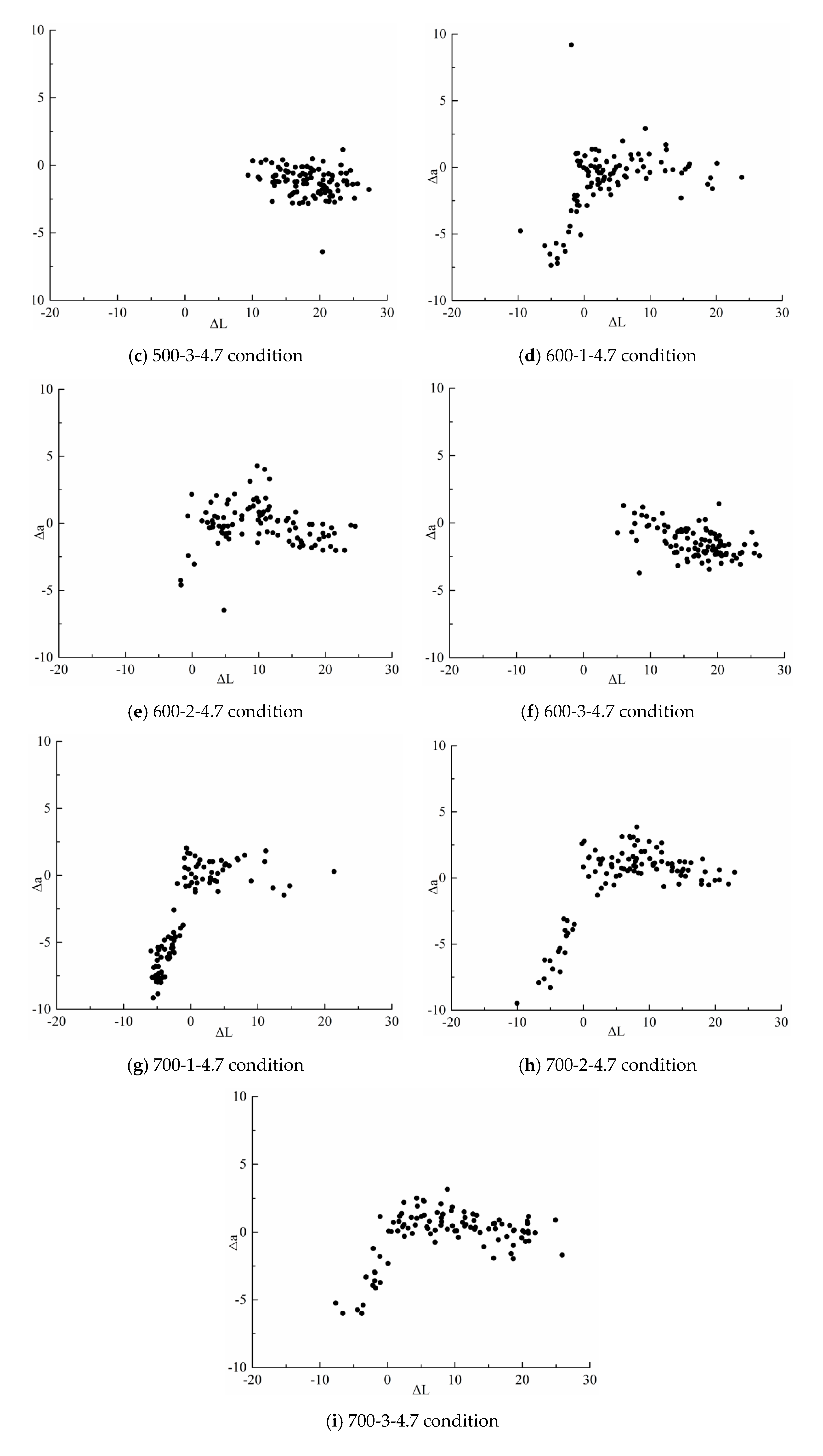
3.2.1. Measurement of Physicochemical Properties and Yield of Torrefaction Products
3.2.2. Discussion on the Energy Balance of the Corn Straw Torrefaction Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| D | Outer diameter of the spiral | ν | Kinematic viscosity |
| d | Diameter of the spiral shaft | ∅ | Radiant heat flux |
| S | Pitch of the spiral | Tw | Wall temperature |
| n | Maximum operational rotational speed of the spiral shaft | L | Brightness coordinate in color space |
| K | Comprehensive coefficient | ΔL | Color difference in the brightness coordinate |
| Ψ | Fill factor | a | Red–green coordinate in color space |
| γ | Unit volume mass of the material | Δa | Color difference in the red-green coordinate |
| C | Tilt angle coefficient | b | Yellow–blue coordinate in color space |
| Q | Flow rate | Δb | Color difference in the yellow-blue coordinate |
| T | Temperature | h | Height |
| β | Heating rate | MY | Mass yield |
| Ea | Activation energy | EY | Energy yield |
| A | Pre-exponential factor | TGA | Thermogravimetric Analyzer |
| l | Length over which the fluid passes the wall | DTG | Differential Thermogravimetry |
| ρ | Density of air | HHV | Higher Heating Value |
| cp | Specific heat at constant pressure | LHV | Lower Heating Value |
| λ | Thermal conductivity | Mad | Moisture content |
| ε | Emissivity (painted surface) | Aad | Ash content |
| Pr | Prandtl number | Vad | Volatile matter content |
| g | Gravitational acceleration | FCad | Fixed carbon content |
| αv | Coefficient of volume expansion | Cad | Carbon content |
| ΔE | Total color difference | Had | Hydrogen content |
| Δt | Temperature difference between the wall and the fluid | Oad | Oxygen content |
| σ | Stefan–Boltzmann constant | Nad | Nitrogen content |
| Nu | Nusselt number | Sad | Sulfur content |
| Gr | Grashof Number | MFC | Mass flow computer |
References
- Liu, X.; Tu, S.; Liu, J.; Liu, Z. Emission Forecasting from Open Burning of Crop Straw and Policy Analysis: The Case for China. Energy Rep. 2023, 9, 2568. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, K.; Zhu, W.; Zhang, L.; Zhao, Z.; Xu, L.; Yang, J.; Shen, B. Effects of Oxygen Deficient and Flue Gas Torrefaction on the Surface Property and Structural Feature of Typical Agriculture Waste: Rice Husk. Fuel 2022, 327, 125211. [Google Scholar] [CrossRef]
- Guo, S.; Liu, L.; Zhao, D.; Zhao, C.; Li, X.; Li, G. Optimization of Briquette Fuels by Co-Torrefaction of Residual Biomass and Plastic Waste Using Response Surface Methodology. Molecules 2023, 28, 2568. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, S.; Zhao, Y.; Wang, Z.; Ma, J.; Xu, L.; Yang, J.; Shen, B. Investigation on the fuel quality and hydrophobicity of upgraded rice husk derived from various inert and oxidative torrefaction conditions. Renew. Energ. 2022, 189, 1234–1248. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. A Review on Torrefied Biomass Pellets as a Sustainable Alternative to Coal in Power Generation. Renew. Sustain. Energy Rev. 2014, 40, 394–402. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, M.; Jiang, E. A Novel Continuous Reactor with Shaftless Spiral for Chemical Looping Gasification and the Control of Operating Parameters. J. Energy Inst. 2020, 93, 2084–2095. [Google Scholar] [CrossRef]
- Porat, A.; Careaga, F.; Briens, L.; Briens, C. Design and development of a rotating heater pyrolysis reactor. Can. J. Chem. Eng. 2020, 100, 25–37. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, J.; Yao, Z.; Cong, H.; Wang, J.; Zhang, X. Equipment for Biomass Continuous Grading Pyrolysis. Trans. Chin. Soc. Agric. Mach. 2016, 47, 220–226. [Google Scholar] [CrossRef]
- Qin, L.; Shao, Y.; Hou, Z.; Jiang, E. Effect of Temperature on the Physicochemical Characteristics of Pine Nut Shell Pyrolysis Products in a Screw Reactor. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2831–2843. [Google Scholar] [CrossRef]
- Aladin, A.; Alwi, R.S.; Syarif, T. Design of Pyrolysis Reactor for Production of Bio-Oil and Bio-Char Simultaneously. AIP Conf. Proc. 2017, 1840, 110010. [Google Scholar] [CrossRef]
- Isemin, R.L.; Kuzmin, S.N.; Konyakhin, V.V.; Milovanov, O.Y.; Mikhalev, A.V.; Muratova, N.S.; Nebyvayev, A.V.; Kokh-Tatarenko, V.S. Development of a Fluidized-Bed Reactor for Oxidative Torrefaction of Biowastes. Therm. Eng. 2020, 67, 626–633. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Z.; Zhao, L.; Song, J.; Jia, J. Torrefaction of Sorghum Straw Pellets in a Stationary Reactor with a Feeding Screw. Energy Fuels 2020, 34, 5997–6007. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biomass Fast Pyrolysis in a Shaftless Screw Reactor: A 1-D Numerical Model. Energy 2018, 157, 792–805. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of Fast Pyrolysis Temperature on Biochar Labile Fraction and Short-Term Carbon Loss in a Loamy Soil. Biomass Bioenergy 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, L.; Gao, W.; Xiao, X.; Liu, Y. Structure Optimization Design of Screw Conveyor Based on EDEM. J. Phys. Conf. Ser. 2022, 2200, 012014. [Google Scholar] [CrossRef]
- Xu, Q. Design and Simulation of Screw Drum Biomass Continuous Pyrolysis Device Feeder. Cumt 2017. [Google Scholar]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100885. [Google Scholar] [CrossRef]
- Asadipour, H.; Alizadeh, M.; Ashrafizadeh, F. Evaluation of Graphene Transparent Coating on Copper for Color and Oxidation Control at Low Temperatures. Diam. Relat. Mater. 2022, 130, 109502. [Google Scholar] [CrossRef]
- Kačíková, D.; Kačík, F.; Čabalová, I.; Ďurkovič, J. Effects of Thermal Treatment on Chemical, Mechanical and Colour Traits in Norway Spruce Wood. Bioresour. Technol. 2013, 144, 669–674. [Google Scholar] [CrossRef]
- Ren, X.; Sun, R.; Meng, X.; Vorobiev, N.; Schiemann, M.; Levendis, Y.A. Carbon, Sulfur and Nitrogen Oxide Emissions from Combustion of Pulverized Raw and Torrefied Biomass. Fuel 2017, 188, 310–323. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Ma, J.; Kong, W.; Yuan, P.; Sun, R.; Shen, B. Analysis of Functionality Distribution and Microstructural Characteristics of Upgraded Rice Husk after Undergoing Non-Oxidative and Oxidative Torrefaction. Fuel 2022, 310, 122477. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Lim, C.J.; Grace, J.R. Oxidative Torrefaction of Spruce-Pine-Fir Sawdust in a Slot-Rectangular Spouted Bed Reactor. Energy Convers. Manag. 2018, 174, 276–287. [Google Scholar] [CrossRef]
- Liu, Y.; Rokni, E.; Yang, R.; Ren, X.; Sun, R.; Levendis, Y.A. Torrefaction of Corn Straw in Oxygen and Carbon Dioxide Containing Gases: Mass/Energy Yields and Evolution of Gaseous Species. Fuel 2021, 285, 119044. [Google Scholar] [CrossRef]
- Wang, L.; Sha, L.; Zhang, S.; Cao, F.; Ren, X.; Levendis, Y.A. Preparation of Activated Coke by Carbonization, Activation, Ammonization and Thermal Treatment of Sewage Sludge and Waste Biomass for SO2 Absorption Applications. Fuel Process. Technol. 2022, 231, 107233. [Google Scholar] [CrossRef]
- Guo, S.; Guo, T.; Che, D.; Liu, H.; Sun, B. Response Surface Analysis of Energy Balance and Optimum Condition for Torrefaction of Corn Straw. Korean J. Chem. Eng. 2022, 39, 1287–1298. [Google Scholar] [CrossRef]



| Conditions | Temperatures (°C) | Feed Rates (kg/h) | Gas Flow Rates (kg/h) |
|---|---|---|---|
| 1 | 500 | 1 | 4.7 |
| 2 | 500 | 2 | 4.7 |
| 3 | 500 | 3 | 4.7 |
| 4 | 600 | 1 | 4.7 |
| 5 | 600 | 2 | 4.7 |
| 6 | 600 | 3 | 4.7 |
| 7 | 700 | 1 | 4.7 |
| 8 | 700 | 2 | 4.7 |
| 9 | 700 | 3 | 4.7 |
| 10 | 500 | 1 | 9.4 |
| 11 | 550 | 1 | 9.4 |
| 12 | 600 | 1 | 9.4 |
| 13 | 700 | 1 | 9.4 |
| Operating condition | 500-1-4.7 | 500-2-4.7 | 500-3-4.7 |
| Compliance rate(%) | 0 | 0 | 0 |
| Operating condition | 600-1-4.7 | 600-2-4.7 | 600-3-4.7 |
| Compliance rate(%) | 27 | 5 | 0 |
| Operating condition | 700-1-4.7 | 700-2-4.7 | 700-3-4.7 |
| Compliance rate(%) | 63 | 19 | 16 |
| Operating condition (Before optimization) | 550-1-4.7 | 550-2-4.7 | 550-3-4.7 | |||
| Compliance rate (%) | 6 | 0 | 0 | |||
| Operating condition (After optimization) | 500-1-9.4 | 550-1-9.4 | 600-1-9.4 | 700-1-9.4 | ||
| Compliance rate (%) | 23 | 81 | 100 | 100 | ||
| Operating Condition | Proximate Analyses | Ultimate Analyses | HHV (kJ/kg) | LHV (kJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Cad | Had | Oad | Nad | Sad | |||
| Raw material | 3.04 | 7.62 | 71.93 | 17.41 | 43.38 | 5.12 | 0.55 | 40.18 | 0.11 | 15.56 | 14.33 |
| 500-1-4.7 | 2.22 | 8.19 | 72.10 | 17.49 | 43.64 | 5.16 | 0.48 | 40.23 | 0.08 | 17.27 | 16.05 |
| 500-2-4.7 | 1.78 | 9.61 | 71.52 | 17.09 | 43.04 | 5.11 | 0.50 | 39.88 | 0.08 | 17.05 | 15.86 |
| 500-3-4.7 | 1.66 | 9.54 | 71.48 | 17.32 | 43.22 | 5.11 | 0.48 | 39.90 | 0.09 | 17.09 | 15.90 |
| 550-1-4.7 | 1.37 | 8.29 | 71.94 | 18.4 | 44.85 | 5.18 | 0.52 | 39.66 | 0.13 | 17.64 | 16.44 |
| 550-2-4.7 | 2.80 | 7.94 | 72.08 | 17.18 | 43.26 | 5.16 | 0.50 | 40.24 | 0.10 | 16.99 | 15.76 |
| 550-3-4.7 | 1.48 | 9.19 | 72.23 | 17.10 | 43.60 | 5.18 | 0.50 | 39.95 | 0.10 | 17.19 | 15.99 |
| 600-1-4.7 | 2.12 | 9.39 | 69.44 | 19.05 | 44.85 | 5.03 | 0.54 | 37.99 | 0.08 | 17.78 | 16.60 |
| 600-2-4.7 | 1.62 | 8.86 | 71.42 | 18.10 | 44.15 | 5.14 | 0.50 | 39.63 | 0.10 | 17.45 | 16.25 |
| 600-3-4.7 | 1.94 | 9.67 | 71.36 | 17.03 | 43.12 | 5.11 | 0.50 | 39.56 | 0.10 | 16.95 | 15.75 |
| 700-1-4.7 | 1.72 | 10.59 | 67.66 | 20.03 | 45.18 | 4.93 | 0.57 | 36.93 | 0.08 | 17.46 | 16.31 |
| 700-2-4.7 | 1.16 | 8.76 | 70.44 | 19.64 | 45.18 | 5.13 | 0.53 | 39.16 | 0.08 | 17.84 | 16.66 |
| 700-3-4.7 | 1.54 | 8.62 | 71.58 | 18.26 | 44.51 | 5.14 | 0.48 | 39.60 | 0.11 | 17.61 | 16.42 |
| 500-1-9.4 | 2.00 | 9.55 | 70.00 | 18.45 | 44.32 | 5.06 | 0.52 | 38.47 | 0.08 | 17.49 | 16.30 |
| 550-1-9.4 | 1.88 | 9.86 | 66.78 | 21.48 | 46.34 | 4.88 | 0.57 | 36.39 | 0.08 | 17.58 | 16.44 |
| 600-1-9.4 | 2.26 | 23.06 | 20.46 | 54.22 | 63.12 | 2.76 | 0.97 | 7.76 | 0.07 | 22.50 | 23.82 |
| 700-1-9.4 | 2.06 | 23.13 | 20.54 | 54.27 | 62.18 | 2.87 | 0.98 | 8.71 | 0.07 | 24.42 | 23.72 |
| Operating condition | 500-1-4.7 | 500-2-4.7 | 500-3-4.7 | 500-1-9.4 | 550-1-4.7 | 550-2-4.7 | 550-3-4.7 | 550-1-9.4 |
| MY (%) | 86.55 | 88.48 | 90.12 | 72.14 | 73.36 | 85.84 | 87.58 | 58.84 |
| EY (%) | 96.06 | 96.95 | 98.98 | 81.09 | 83.17 | 93.73 | 96.75 | 66.48 |
| Operating condition | 600-1-4.7 | 600-2-4.7 | 600-3-4.7 | 600-1-9.4 | 700-1-4.7 | 700-2-4.7 | 700-3-4.7 | 700-1-9.4 |
| MY (%) | 67.50 | 74.87 | 78.49 | 43.73 | 59.47 | 70.49 | 72.06 | 34.08 |
| EY (%) | 77.13 | 83.96 | 85.50 | 68.86 | 66.73 | 80.82 | 81.55 | 53.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Mu, J.; Zhang, X.; Ding, X.; Zheng, M.; Guo, T. Optimized Torrefaction of Corn Straw in a Screw Reactor: Energy Balance Analysis and Biochar Production Enhancement. Processes 2025, 13, 1302. https://doi.org/10.3390/pr13051302
Wang Y, Mu J, Zhang X, Ding X, Zheng M, Guo T. Optimized Torrefaction of Corn Straw in a Screw Reactor: Energy Balance Analysis and Biochar Production Enhancement. Processes. 2025; 13(5):1302. https://doi.org/10.3390/pr13051302
Chicago/Turabian StyleWang, Yulu, Jiyou Mu, Xin Zhang, Xueqiang Ding, Mingmin Zheng, and Tiankuo Guo. 2025. "Optimized Torrefaction of Corn Straw in a Screw Reactor: Energy Balance Analysis and Biochar Production Enhancement" Processes 13, no. 5: 1302. https://doi.org/10.3390/pr13051302
APA StyleWang, Y., Mu, J., Zhang, X., Ding, X., Zheng, M., & Guo, T. (2025). Optimized Torrefaction of Corn Straw in a Screw Reactor: Energy Balance Analysis and Biochar Production Enhancement. Processes, 13(5), 1302. https://doi.org/10.3390/pr13051302






