Abstract
This study investigates the thermophysical properties of multi-walled carbon nanotube (MWCNT) nanofluids dispersed in a water–ethylene glycol (50:50%) mixture. The nanofluids were prepared using a two-step method involving ultrasonication and high-pressure homogenization. The stability of the nanofluids was assessed using UV-Vis spectrophotometry over a period of 30 days. The results indicated a maximum decrease of 10% in the relative concentration, with no visible agglomeration or sedimentation. Thermal conductivity, viscosity, and density were experimentally measured at different temperatures and volumetric concentrations (0.025%, 0.05%, and 0.1%). The thermal conductivity of the nanofluids increased with both concentration and temperature, showing an enhancement of up to 10% at 50 °C for 0.1% vol. MWCNTs. The viscosity measurements revealed a maximum increase of 11% at 80 °C, while the density showed a slight increase with nanoparticle concentration and a decrease with temperature. The models proposed for estimating thermal conductivity (maximum deviation 1.5%) and viscosity (maximum deviation 3%) were found to be suitable, exhibiting good agreement with the experimental results. The results align with previous studies, reinforcing the role of Brownian motion and nanoparticle interactions in heat transfer enhancement. This study provides insights into the stability and thermophysical behavior of MWCNT nanofluids, contributing to their potential applications in thermal management systems.
1. Introduction
Nanofluids, defined as colloidal suspensions of nanoparticles in base fluids, have gained significant attention due to their superior thermophysical properties. Among various nanomaterials, multi-walled carbon nanotubes (MWCNTs) exhibit exceptional thermal conductivity, mechanical strength, and stability, making them promising candidates for heat transfer applications. The dispersion of MWCNTs in water–ethylene glycol (50:50%) mixtures is particularly relevant for industrial cooling and automotive applications due to the enhanced thermal performance of such fluids. However, ensuring the long-term stability and understanding the thermophysical behavior of these nanofluids remains a challenge.
Numerous studies have analyzed the stability and thermophysical properties of MWCNT-based nanofluids under various conditions. Regarding stability, ref. [1] provided a comprehensive discussion on the chemical and physical methods used to prepare stable CNT nanofluids, as well as the techniques employed to analyze stability and the likely causes of instability. Among chemical methods, covalent and non-covalent functionalization are prominent. In terms of physical methods, homogenization, stirring, and fine crushing are the most commonly used, often in combination to enhance dispersion stability. The authors concluded that maintaining long-term stability is a crucial factor for validating the application of nanofluids as thermal fluids.
Ref. [2] investigated the effects of CNT functionalization (CNT, CNT-OH, and CNT-COOH) on the thermal conductivity of nanofluids. Both their experimental and simulation results indicated that functionalization improves dispersion stability but may significantly reduce the axial thermal conductivity of the carbon nanotubes—and therefore the nanofluids. Furthermore, ref. [3] evaluated the stability of CNT-based nanofluids (50 mg/L) with various surfactants, including Triton-X, SDBS, SDS, PVP, CTAB, and PCE. Surfactant concentrations ranged from 0.005% to 0.1%, aiming to identify the influence of the surfactant type and concentration on nanofluid colloidal stability. Their experimental and numerical results showed that there is an optimal surfactant concentration that enhances nanoparticle dispersion in the base fluid. Surpassing this optimal concentration led to suspension instability due to micelle formation and interaction.
Regarding thermophysical properties, the literature suggests that factors such as nanoparticle concentration, morphology (shape and size), temperature, and suspension stability significantly affect these properties [4]. Recently, ref. [5] investigated the effect of MWCNT length on thermal conductivity and viscosity. At a concentration of 0.25 wt% MWCNTs with lengths between 1–3 μm, a thermal conductivity increase of up to 30% was observed. However, with MWCNTs measuring 25 μm, the enhancement dropped to 10%. This behavior was attributed by [6] to the higher surface area per unit volume in shorter nanotubes. In contrast, MWCNT length had no significant effect on dynamic viscosity. Ref. [7] analyzed the influence of MWCNT concentration, ranging from 0.0005 to 0.004 wt%, on thermal conductivity and dynamic viscosity. The results showed that both properties increased with nanoparticle concentration within the 20–50 °C temperature range. Ref. [8] discussed the challenges still faced by carbon-based nanofluids (MWCNTs, CNTs, graphene) in achieving industrial-scale applications. Key issues include long-term colloidal stability, the lack of reliable empirical correlations to estimate thermophysical properties, the limited reproducibility of experimental results, and high production costs.
This study aims to experimentally investigate the stability, thermal conductivity, viscosity, and density of MWCNT nanofluids at varying concentrations and temperatures. The results are compared with existing research to validate the observed trends and assess their practical implications.
Nanofluids have shown significant potential for enhancing heat transfer and improving energy efficiency across a wide range of industrial applications, including transportation, electronics cooling, energy storage, and tribology. Among these applications, solar energy has gained increasing attention, as nanofluids can enhance light absorption and heat transfer efficiency, improving energy generation profitability. In electronics, they are being explored for better thermal management in integrated circuits and microelectronic components. In tribology, nanoparticle-enhanced lubricants reduce friction and wear, extending components’ lifespan. In the automotive sector, nanofluids improve engine cooling systems and lubrication, reducing mechanical losses and enhancing energy efficiency. Table 1 lists key studies in these fields.

Table 1.
Selected works in the literature in different applications on nanofluids.
Beyond engineering, nanofluids have promising applications in medicine, biotechnology, and agriculture. They are used in radiation therapies and disease detection (e.g., cancer), genetic therapy, protein detection, and as fertilizers and pesticides, reducing toxic waste and promoting sustainability. They are also applied in cosmetics, food processing, and chemical industries, offering efficient and innovative alternatives [18].
2. Methods
Nanofluid stability is crucial for performance. The choice of nanoparticles and base fluid directly influences thermophysical properties. Ideally, nanofluids should provide high heat transfer rates with minimal pumping energy while avoiding corrosion, aggregation, and chemical interactions [19]. Achieving long-term stability remains challenging due to the risk of nanoparticle agglomeration and sedimentation, making careful synthesis analysis essential.
2.1. Nanofluid Synthesis Methods
The two primary synthesis methods are the one-step and two-step methods.
2.1.1. One-Step Method
This method simultaneously synthesizes and disperses nanoparticles into the base fluid using techniques such as physical vapor deposition (PVD), chemical reduction, and vacuum-submerged arc nanoparticle synthesis (SANSS). PVD evolved from the VEROS method, initially proposed by [20] and later modified by [21,22] for metallic nanofluid production. Chemical reduction techniques were introduced by [23] for copper nanofluid synthesis and by [24] for copper oxide nanofluids.
This method minimizes oxidation and aggregation, enhancing stability. However, its limitations—high cost, low scalability, and difficulty in controlling nanoparticle concentration and size—restrict its industrial application.
2.1.2. Two-Step Method
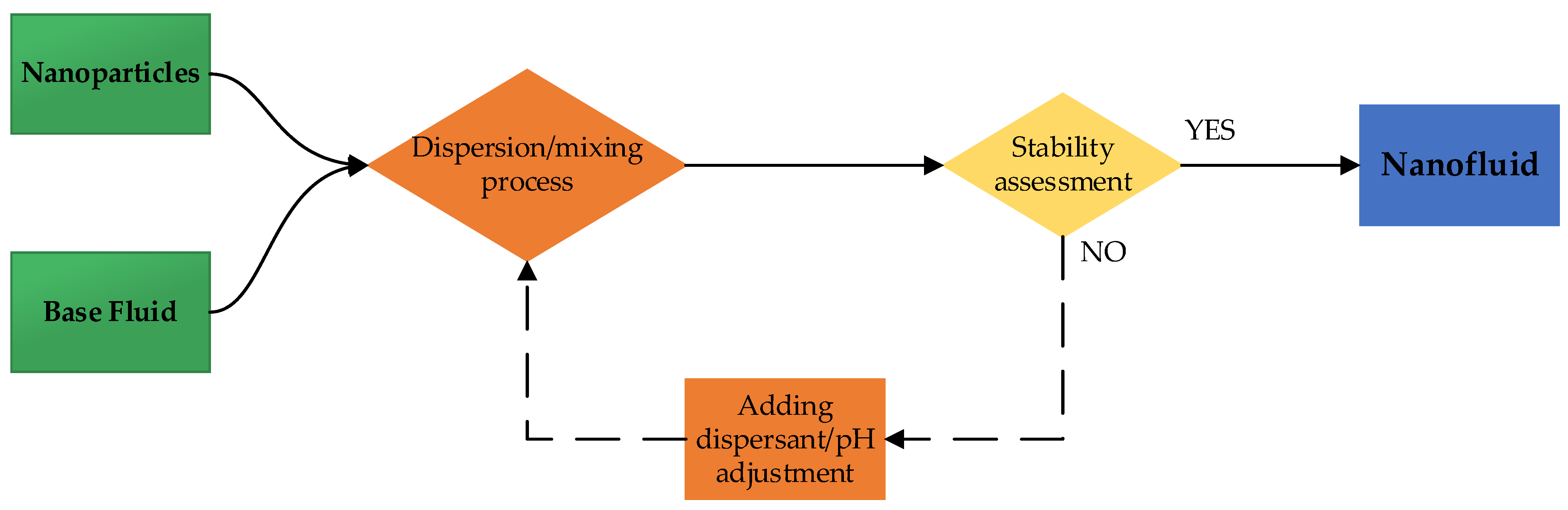
The two-step method is the most widely used due to lower operational costs and better control over the nanoparticle concentration [25]. It involves first synthesizing dry nanoparticles and then dispersing them into the base fluid (Figure 1). While transportation, storage, and drying costs are significant, this approach enables large-scale production with adjustable concentrations.
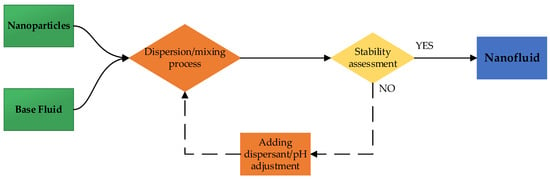
Figure 1.
Schematic diagram of two-step method for nanofluid preparation.
To achieve homogeneous dispersion, techniques such as ultrasonic agitation [26], high-pressure homogenization [16], and those reported in [27] are commonly used. However, stability remains a challenge due to nanoparticle aggregation. Surface treatments and surfactants are often employed to mitigate this effect and prolong nanofluid stability.
2.2. Nanofluid Stability
Adding nanoparticles to base fluids, like water or ethylene glycol, enhances their thermal conductivity but also increases the tendency for agglomeration and sedimentation, affecting their stability. Key influencing factors include the nanoparticle type, preparation method, pH, surfactant use, and surface treatment [28]. High surface energy and Brownian-motion-driven collisions are the primary contributors to colloidal instability.
Stability Mechanisms of Nanofluids
The dispersion of nanoparticles in a base fluid is governed by various microscopic forces. Gravity induces sedimentation due to density differences, while Van der Waals forces counteract this effect, promoting dispersion [29]. According to [28], nanoparticle aggregation depends on the balance between attractive Van der Waals forces and repulsive electrostatic interactions. Ref. [29] highlighted that the nanoparticles’ shape also influences aggregation, with plate-like or rod-shaped particles aggregating more rapidly than spherical ones due to their larger surface area.
Two primary mechanisms enhance repulsive forces to stabilize nanofluids: steric and electrostatic stabilization. Steric stabilization involves coating nanoparticles with polymers, creating a repulsive barrier that prevents aggregation [30]. This method allows the stable dispersion of different nanoparticles in a base fluid, even at high or low concentrations. Electrostatic stabilization, on the other hand, relies on surface charge repulsion between equally charged nanoparticles [31]. However, this approach is less effective in saline or non-aqueous environments and does not allow the redispersion of agglomerated nanoparticles.
2.3. Thermophysical Properties of Nanofluids
Thermophysical properties are critical for understanding nanofluid behavior in thermal systems. Experimental and numerical studies primarily focus on thermal conductivity and dynamic viscosity [25,32]. Although regression models exist for specific conditions, no universal model accurately predicts nanofluid properties.
2.3.1. Thermal Conductivity
Thermal conductivity significantly influences the heat transfer efficiency in nanofluid applications. Various factors affect it, including the nanoparticle characteristics (size, shape, material), concentration, base fluid, temperature, aggregation, surfactants, and PH. Studies show that enhancing the conductivity of a highly conductive base fluid (e.g., water) is more challenging than improving that of a low-conductivity fluid (e.g., oil or ethylene glycol) [33,34]. Increasing the nanoparticle concentration generally improves thermal conductivity, though some studies suggest a nonlinear relationship at higher concentrations [35,36]. Additionally, shape influences conductivity, with cylindrical nanoparticles often exhibiting superior performance compared to spherical ones [37].
Temperature also plays a role, with some studies reporting an increase in thermal conductivity with rising temperature, while others observe a decrease depending on nanoparticle properties [38,39]. The use of surfactants to prevent aggregation can also impact thermal conductivity, either enhancing or reducing it based on their concentration and type [40,41]. Excessive surfactant use may negatively affect conductivity, emphasizing the need to optimize its concentration [42,43].
2.3.2. Dynamic Viscosity
Viscosity represents a fluid’s internal resistance to flow and is crucial in determining the friction factor and convective heat transfer coefficient in nanofluids [44]. Reliable viscosity data are essential for designing heat transfer equipment, considering the pressure drop and heat exchange performance.
Adding nanoparticles generally increases viscosity, potentially raising pumping power requirements. Ref. [38] observed that increasing the FeC/water nanofluid concentration also increased viscosity, though its influence diminished above 55 °C. Similarly, ref. [45] found that MgO nanofluids showed up to a 71% viscosity increase with a higher nanoparticle concentration, possibly due to aggregation.
Temperature significantly affects viscosity, with a nonlinear decrease as temperature rises [46]. This reduction is linked to weakened intermolecular adhesion and enhanced Brownian motion [25]. Nanoparticle morphology also plays a role; smaller particles increase the solid–liquid contact area, raising viscosity. Furthermore, refs. [47,48] found that non-spherical Al2O3 nanoparticles (cylindrical or sheet-like) caused greater viscosity increases than spherical ones due to a greater shear interaction.
Surfactants influence viscosity depending on their type and concentration. Ref. [49] observed viscosity increases of between 15.79% and 22.81% in graphene/water nanofluids with different surfactants, highlighting their impact on nanofluid properties.
2.3.3. Density
Nanofluid density depends mainly on the nanoparticle concentration; higher concentrations increase the density. Other factors include the nanoparticles’ properties (size, shape, material), temperature, pressure, and surfactants. Ref. [50] proposed a widely used mixing rule for density estimation (Equation (1)), with deviations of below 0.6%.
Ref. [51] found that adding SiC nanoparticles increased the density, but temperature reduced it. Ref. [52] confirmed a linear density increase in TiO2 and Al2O3 nanofluids, while SiO2-based nanofluids showed lower increments, emphasizing the role of the base fluid.
2.3.4. Specific Heat Capacity
Specific heat measures the energy required to raise a substance’s temperature and is key for thermal management. Nanoparticles often reduce specific heat compared to the base fluid, as observed by [53] in graphene/water nanofluids, where increasing the concentration led to a slight decrease (~1.52%). However, ref. [54] reported increased specific heat in MWCNT/water-EG nanofluids, attributing it to the high surface energy of carbon nanotubes.
Several models predict nanofluid specific heat, including [50,55], considering nanoparticle and base fluid properties. These models are also adaptable for hybrid nanofluids [56].
3. Experimental Methodology
3.1. Nanofluid Dispersion
MWCNT nanofluids were prepared using a two-step method, involving ultrasonication and high-pressure homogenization to achieve uniform dispersion. A pre-dispersed mixture of functionalized carbon nanotubes (MWCNTs) in water was acquired from Nanostructured & Amorphous Materials, Inc. (NanoAmor) and subsequently diluted in the base fluid to achieve the desired concentrations. Table 2 presents the data provided by the manufacturer.

Table 2.
Characteristics of the multi-walled carbon nanotube (MWCNTS) used in nanofluid preparation.
The base fluid consisted of a water–ethylene glycol mixture (50:50% vol.). MWCNT concentrations of 0.025%, 0.05%, and 0.1% vol. were selected for the analysis. Table 3 presents all the samples produced for the tests carried out in the present work, as well as their abbreviations, which will be used throughout the text.

Table 3.
A description of the prepared nanofluid samples.
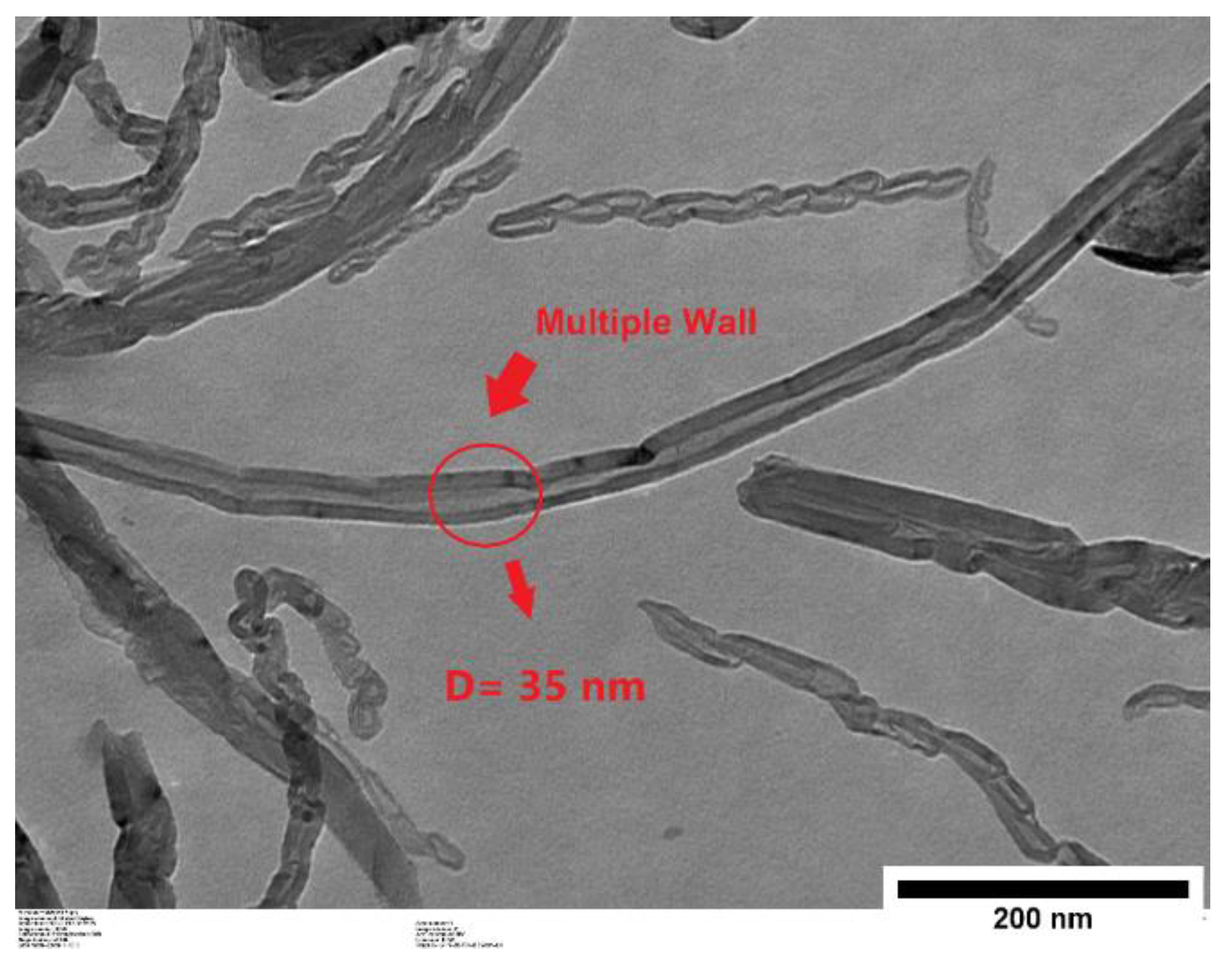
The morphology of the nanoparticles was analyzed using a HT-7700 transmission electron microscope (TEM) (Hitachi, Tokyo, Japan). The sample preparation involved ultrasonic dispersion in an alcoholic medium, followed by depositing a droplet of the dispersed sample onto a copper grid. The sample was then dried under an airflow before imaging.
As shown in Figure 2, TEM imaging reveals the multi-walled structure of the carbon nanotubes. Additionally, ImageJ 1.53k software was used to process the images and determine the nanoparticles’ diameters.

Figure 2.
TEM image of multi-walled carbon nanotube.
The nanoparticle size distribution was analyzed using dynamic light scattering (DLS) with a Malvern-Zetasizer ZS90 (Malvern Panalytical, Malvern, Worcestershire, UK). In this method, the diffusion coefficient is determined based on intensity fluctuations, following the principles of light scattering theory, as discussed by [57]. This principle is essential for calculating particle size using the Stokes–Einstein equation:
where RH is the hydrodynamic radius, DF is the translational diffusion coefficient, kb is Boltzmann’s constant, T is the absolute temperature, and μ is the fluid viscosity.
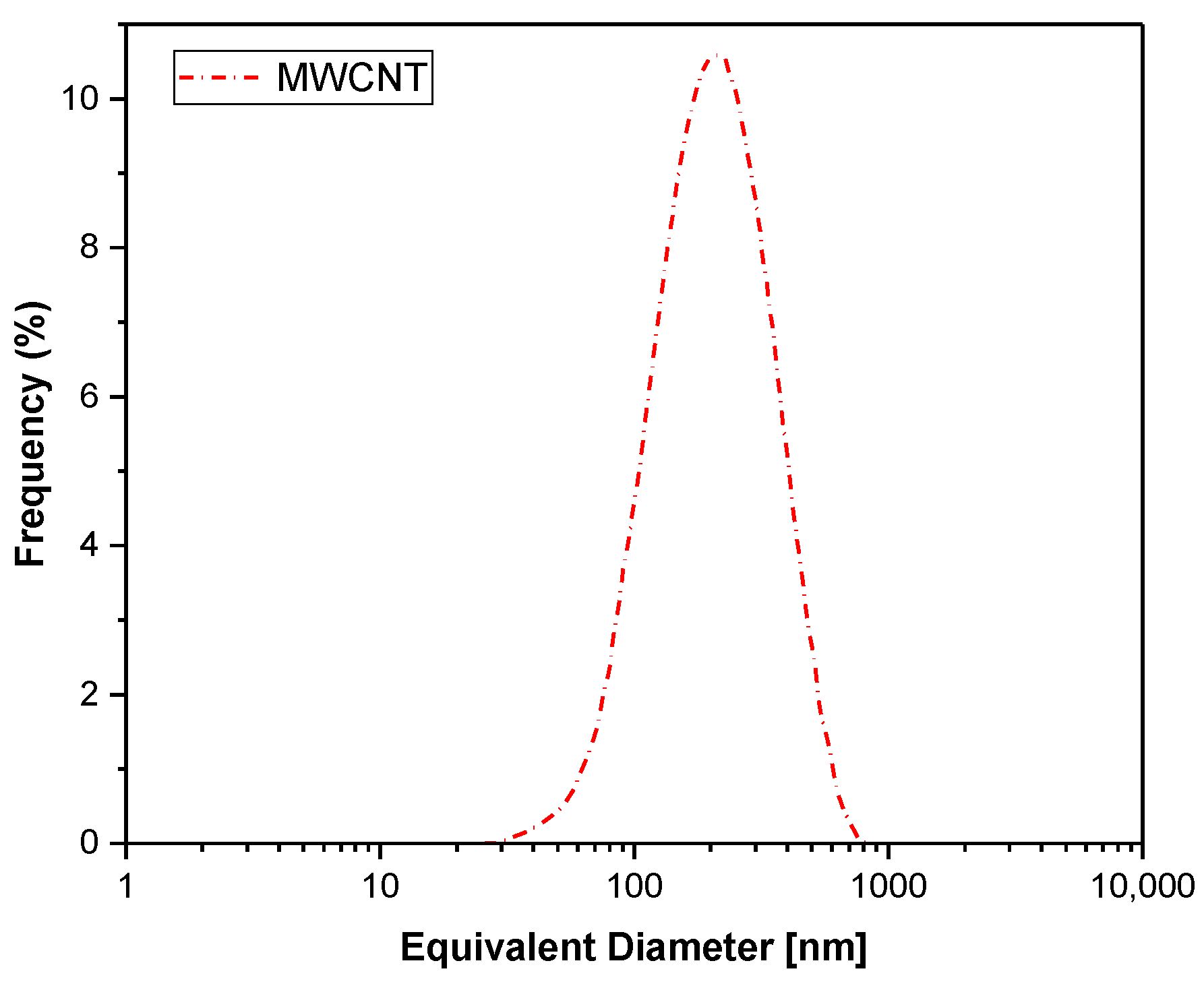
Figure 3 shows that the equivalent diameter was approximately 300 nm. The discrepancy between the measured values and the manufacturer’s data can be attributed to statistical variations in the sample or potential measurement errors associated with the tubular shape of carbon nanotubes. As noted by [58], methods used to assess nanotube nanoparticle dimensions often provide approximate, rather than exact, values. Due to their high aspect ratio (length/diameter), assuming a spherical shape for a single nanotube is not valid. However, MWCNT agglomerates can be approximated as globally spherical structures.
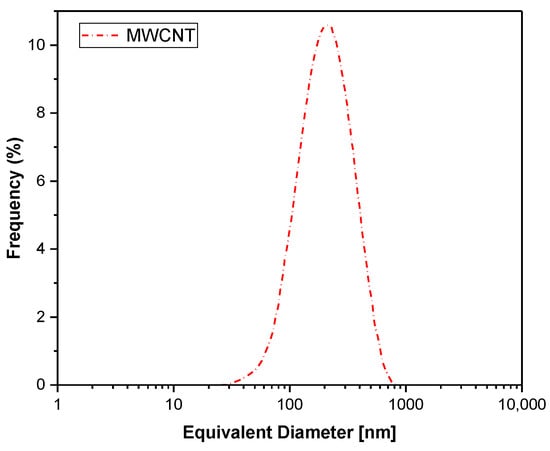
Figure 3.
The size distribution of the MWCNT nanoparticles employed in this study.
3.2. Stability Analysis
The stability of the nanofluids was evaluated using ultraviolet-visible (UV-Vis) spectrophotometry, which involves exposing the sample to different wavelengths of visible light. The sample can scatter or absorb this light depending on its composition. First applied to nanofluids by [59], this method allows for a quantitative assessment of sedimentation over time, based on the initial relationship between absorbance and nanoparticle concentration. This relationship follows Beer–Lambert’s law [60]:
where A represents absorbance, ε is the molar absorption coefficient, c is the nanoparticle concentration, and d is the optical path length through the sample.
The absorbance measurements were performed using a UV-Vis 1900 spectrophotometer (Shimadzu, Duisburg, Germany), which operates over a wavelength range of 190 nm to 1100 nm, with an absorbance measurement range of −4 to 4.
3.3. Measurement of Thermophysical Properties
3.3.1. Thermal Conductivity
The thermal conductivity of the nanofluids was experimentally measured using a THB-1 (LINSEIS, Selb, Germany) thermal property analyzer. This device employs the Transient Hot Bridge (THB) method, patented by Linseis Thermal Analysis.
The measurement probe applies a constant heat flux for a set period, recording the surrounding sample’s temperature variations. Assuming a homogeneous and isotropic medium, the thermal conductivity is determined based on the temperature–time relationship and the probe’s geometric characteristics, as described by the following equation:
where Po is the constant heat flux per unit time and area, L is the length of each bridge resistor, ΔT is the average temperature change, F(t) is a function dependent on sensor geometry, and km is the thermal conductivity of the material under analysis.
3.3.2. Dynamic Viscosity and Density
The dynamic viscosity of the nanofluid samples was measured using an Anton Paar Stabinger SVM™ 3000 rotational viscometer (Graz, Austria), equipped with concentric cylinders. The device operates based on a modified Couette system, which measures torque and rotational speed with a high resolution.
The Peltier cell integrated into the device allows for a wide viscosity range (0.2 to 20,000 mPa·s) and temperature range (−56 to 105 °C) in a single system. The device requires a small sample volume (~2.5 mL) and also includes a densitometer based on the oscillating U-tube principle. Both the viscosity and density measurements were performed simultaneously, optimizing the testing process.
3.4. Uncertainty Analysis
The uncertainty analysis was conducted following the procedure demonstrated by [52], which is commonly used to estimate experimental uncertainties. The primary factors considered in the uncertainty of thermal conductivity, viscosity, and density were temperature variations and the accuracy of the measuring instruments. Table 4 presents the calculated uncertainties associated with these thermophysical properties.

Table 4.
Uncertainties in thermophysical properties measurements.
The propagation of uncertainty in the calculated parameters was performed following the methodology described by Taylor and Kuyatt [61], available in the Engineering Equation Solver (EES) software (Professional V.9). According to the standard, assuming the individual measurements are independent and random (x1, x2, x3, …, xn), the uncertainty in a calculated variable (y) can be determined. In this study, the uncertainty was calculated considering both the effect of the standard deviation and the declared uncertainty from the equipment manufacturers.
4. Results and Discussion
4.1. Stability
To assess the stability of MWCNT nanofluids, quartz cuvettes were used, as carbon nanotube nanoparticles exhibit a peak absorption wavelength at 262 nm. The quartz cuvettes (model K22-135) are suitable for a 190–2500 nm wavelength range.
Due to the dark color of the carbon nanotube samples, dilution was necessary before measurement to avoid spectral saturation. Each sample was diluted at a 1:40 ratio with the base fluid to maintain an appropriate absorbance level within the spectrophotometer’s measurement range. All the measurements were conducted at room temperature (25–30 °C).
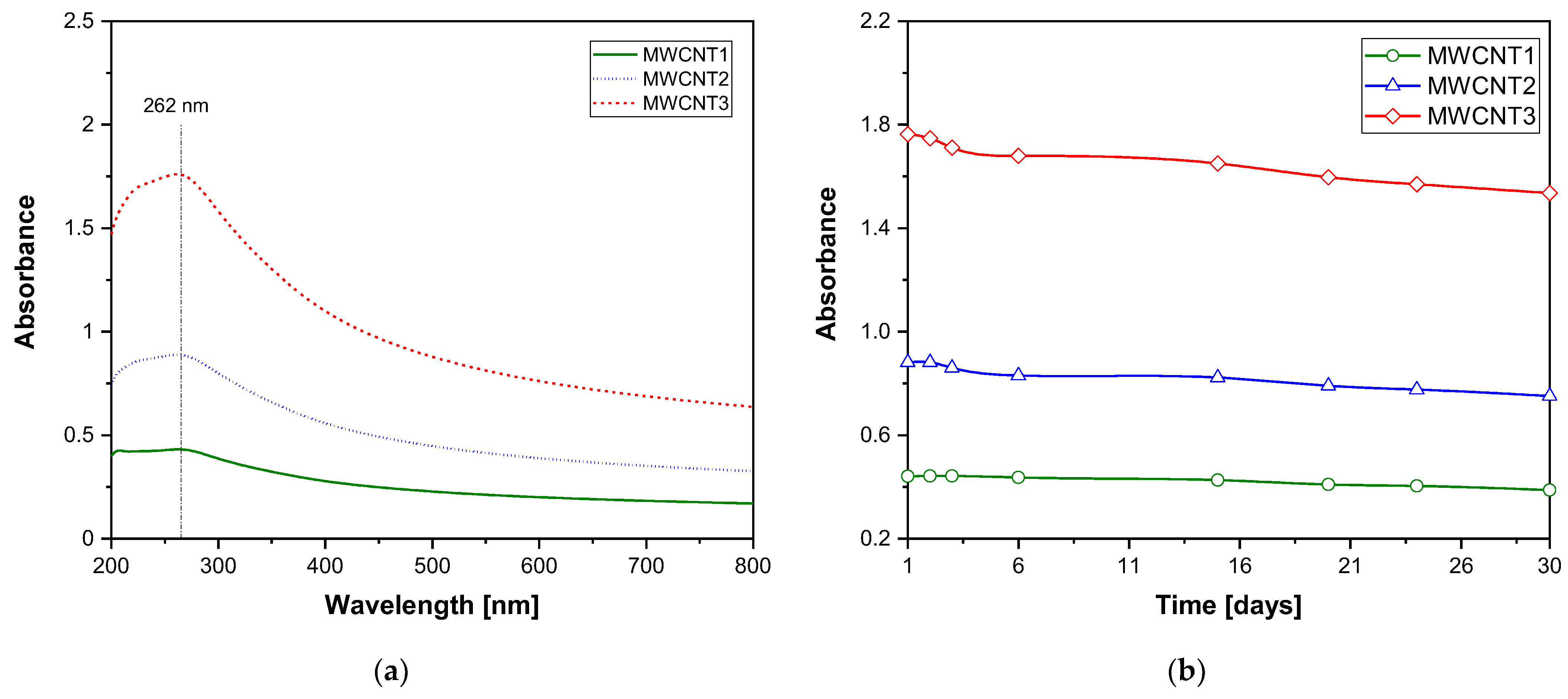
To evaluate the relative nanoparticle concentration over time, a linear regression was performed between the nanoparticle concentration and absorbance at the peak wavelength (262 nm), based on the initial-day data. Figure 4a illustrates this linear relationship, where the regression coefficient was 17.61.

Figure 4.
(a) UV-Vis absorbance spectra of MWCNT nanofluid on day 1. (b) Absorbance at 262 nm as a function of the aging time of the MWCNT nanofluid.
Figure 4b shows absorbance variation after 30 days. The results indicate that after one month, the relative concentration decreased by a maximum of 10% for sample MWCNT2. This finding aligns with the studies by [62,63], which also observed absorption peaks near 260 nm for carbon nanotube nanofluids.
Overall, a gradual concentration reduction over 30 days was observed, with a maximum decrease of 10%. However, no visible aggregation or sedimentation was detected in the samples, confirming that the nanofluids remained stable throughout the analysis period.
4.2. Thermal Conductivity Results
Thermal conductivity measurements were conducted for both pure ethylene glycol and the base fluid (H2O/EG 50:50 vol.%), within a temperature range of 30 °C to 80 °C, in 10 °C increments. Each reported value represents the average of ten measurements.
Table 5 compares the experimentally obtained results with values from the CoolProp database for water–ethylene glycol (50:50 vol.%) mixtures. The experimental variations remained within ±3% of the database values.

Table 5.
Experimental results and ASHRAE base values for thermal conductivity at different temperatures.
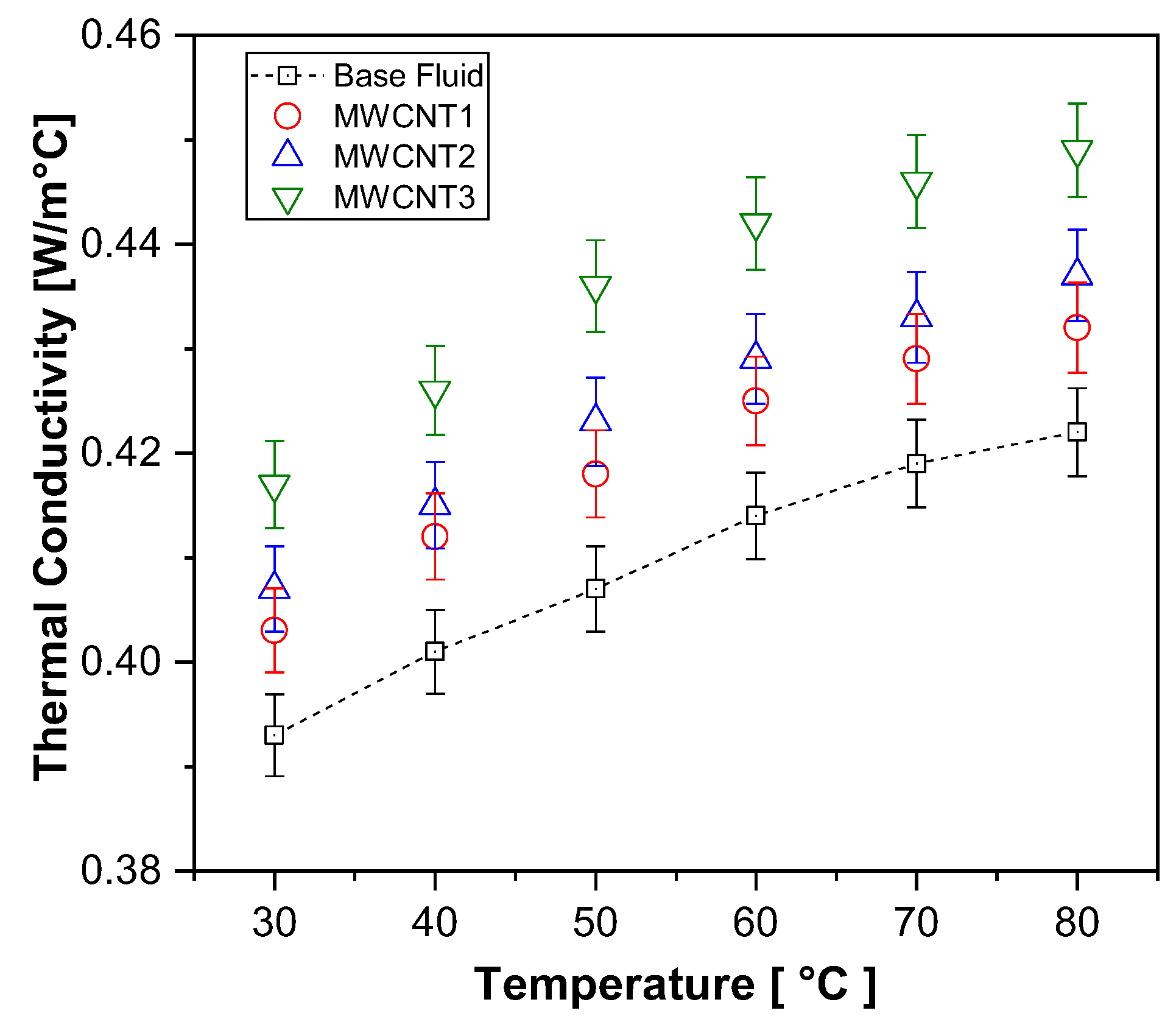
The thermal conductivity increments obtained for the carbon nanotube nanofluids, as shown in Figure 5, averaged 2.7%, 3.7%, and 6.4% for samples MWCNT1, MWCNT2, and MWCNT3, respectively. The results indicate a clear trend of increasing thermal conductivity with both higher concentrations and temperatures. According to [65] as the temperature increases, the surface energy of nanoparticles decreases, while the nanofluid viscosity is reduced, intensifying the Brownian motion. Consequently, as the temperature rises, the random movement of nanoparticles and their collisions also increase, thereby enhancing the thermal conductivity of the nanofluid.

Figure 5.
Experimental results on thermal conductivity for MWCNT nanofluids and base fluid.
Although the observed increments for the carbon nanotube samples are noteworthy, they are still lower than most results reported in the literature, as analyzed by [39]. This discrepancy may be attributed to the presence of surfactants (ranging between 1.8% and 2% by weight) in the highly concentrated and functionalized solutions used to produce the carbon nanotube nanofluid samples. According to [28], while surfactants contribute to improved sample stability, they may also limit potential gains in thermal conductivity.
Regarding the effect of the increasing carbon nanotube concentration, the results were compared with those obtained by [36]. For functionalized MWCNT nanofluids with carboxyl (COOH) groups, the authors highlighted that a higher solid volume fraction, combined with enhanced nanotube interactions, further increases the thermal conductivity.
The maximum temperature evaluated in their study was 50 °C, where an enhancement of approximately 10% was observed for a 0.1 vol% concentration. A similar trend was reported by [66], but their findings were limited to volume concentrations of up to 0.125%. For higher concentrations, the formation of nanoparticle chains within the base fluid was found to reduce the surface-to-volume ratio, negatively impacting the thermal conductivity.
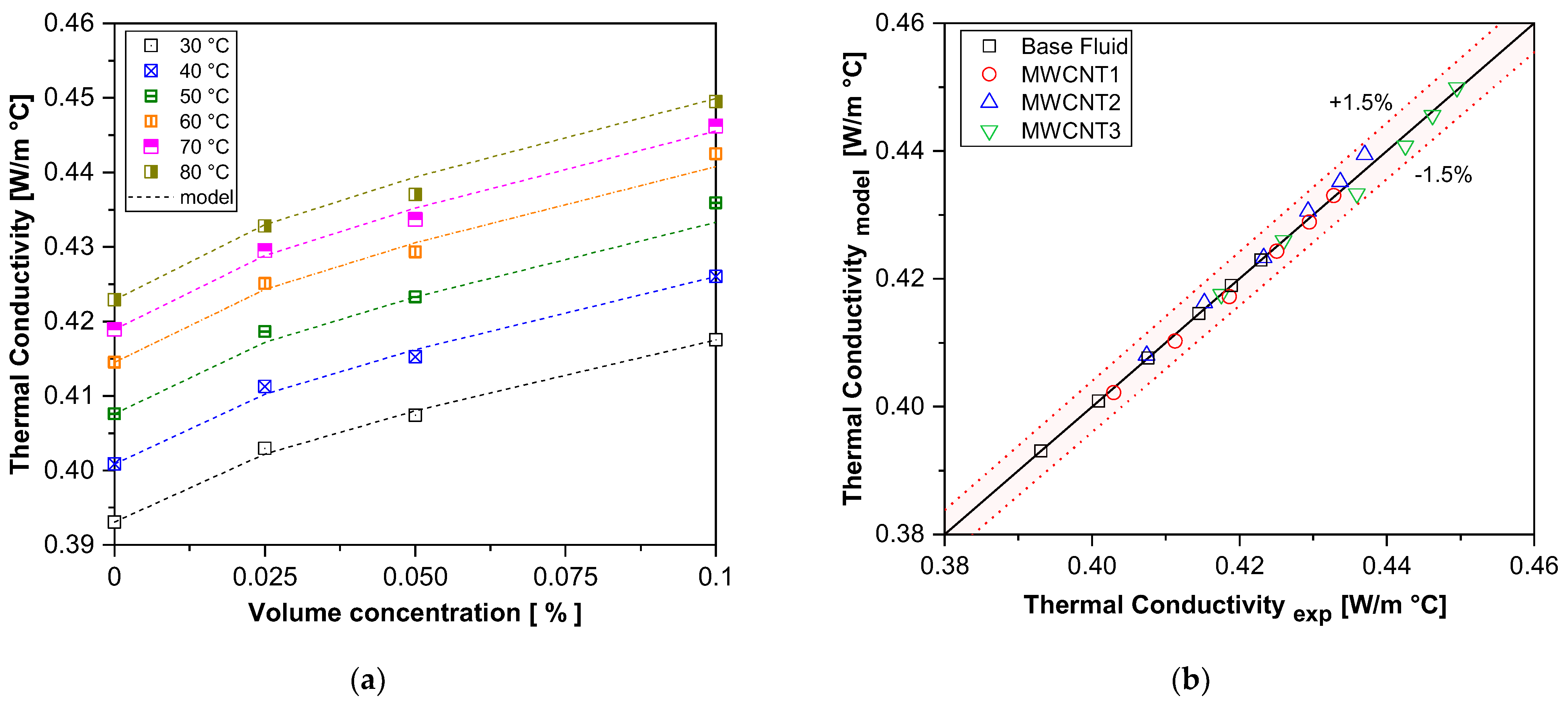
Due to the lack of a suitable model to predict the thermal conductivity of functionalized carbon nanotube nanofluids at temperatures up to 80 °C, a new correlation was developed based on experimental data. The proposed model, described by Equation (5), was derived using the curve-fitting method with the Marquardt–Levenberg algorithm, as suggested by [67], and implemented in MATLAB Version 8.5 (R2015a). This method optimizes the coefficients of the independent variables (volume concentration and temperature) to achieve the best fit, minimizing the sum of squared differences between experimental and predicted values:
where a = 7.81, b = 0.712, and c = 0.0259.
Figure 6 compares the experimental thermal conductivity results of carbon nanotube nanofluid samples with the values predicted by the proposed correlation in Equation (5). The new model was demonstrated to be accurate and applicable at higher temperatures, with a maximum deviation of 1.5% from the experimental data. This comparison highlights the significant contribution of the present study to the field of nanofluids, expanding the understanding and modeling of thermal conductivity.
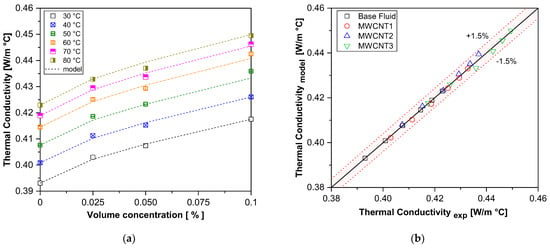
Figure 6.
A comparison of the experimental results with those of the proposed correlation for thermal conductivity. (a) Thermal conductivity vs. volume concentration; (b) experimental vs. model.
4.3. Dynamic Viscosity Results
Table 6 presents the validation measurements of dynamic viscosity, comparing the base fluid’s viscosity with reference data from the CoolProp software Version 6.1. The experimental values obtained for the base fluid’s dynamic viscosity showed discrepancies compared to the reference values, with average deviations of 5% and 4%.

Table 6.
Experimental results and ASHRAE base values for viscosity at different temperatures.
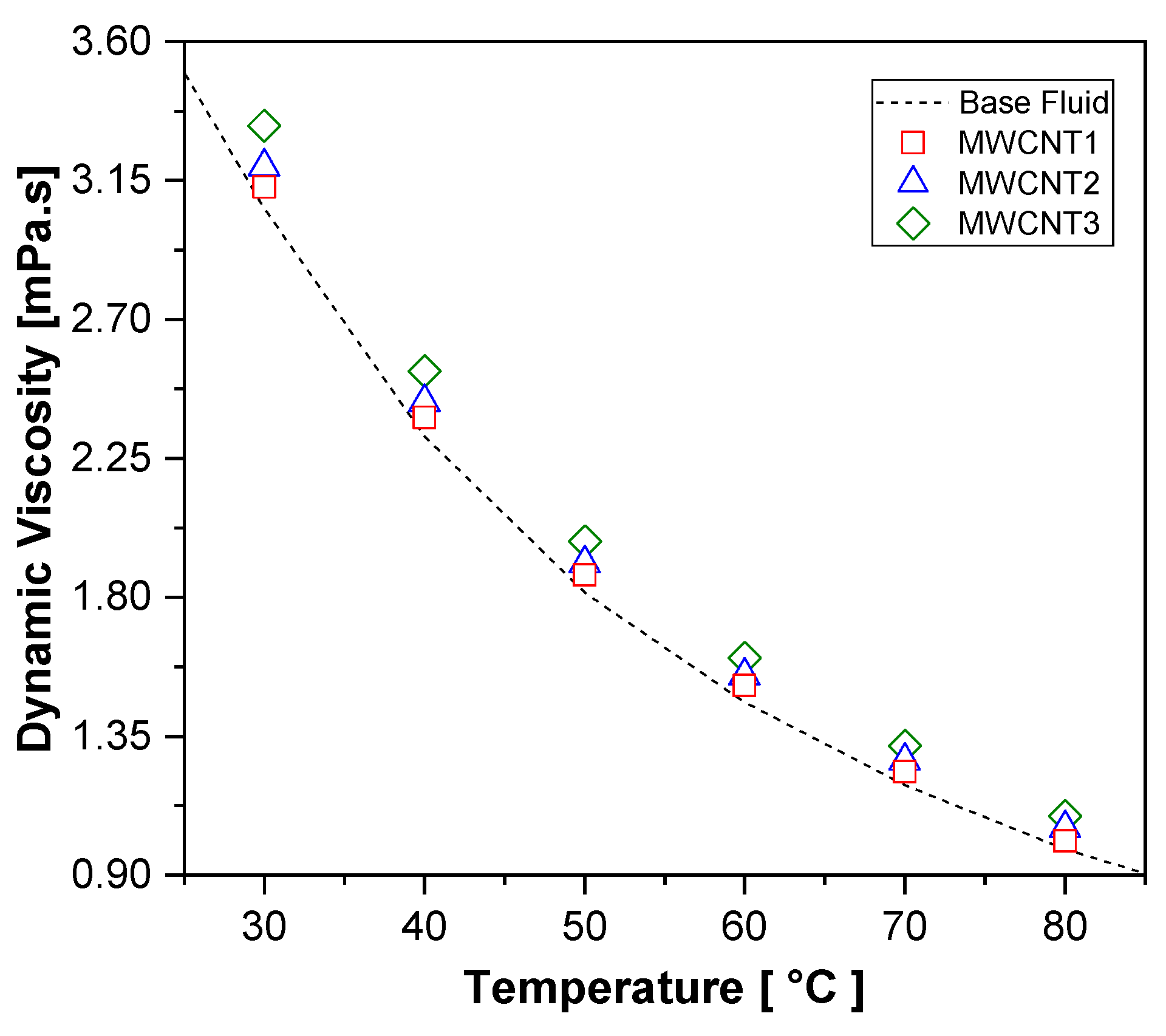
Figure 7 presents the experimental results for the dynamic viscosity of the nanofluid samples. It can be observed that viscosity decreases exponentially with increasing temperature for both the nanofluids and the base fluid. The highest increase in viscosity, compared to the base fluid, was observed for the MWCNT3 sample at 80 °C, reaching up to 11%. The MWCNT1 and MWCNT2 samples showed average increases of approximately 3% and 5.8%, respectively, across the evaluated temperature range.

Figure 7.
The experimental results for the dynamic viscosity of MWCNT nanofluids and the base fluid.
Overall, the dynamic viscosity of the nanofluids increased with the rising nanoparticle concentration. According to [68], this behavior at low concentrations is not solely dependent on the volume fraction of nanoparticles; factors such as particle shape and surfactant addition must also be considered, making it difficult to predict viscosity behavior. For instance, [39] reported viscosity increases of up to 9.2% for similar MWCNT concentrations, whereas [34] found a maximum increase of 2% for MWCNT/water–EG nanofluids with a 0.1 vol% concentration. These variations suggest that the concentration, surfactant type, and preparation methods significantly influence nanofluid viscosity. Moreover, as noted by [69], the stability of the nanofluid also has a direct impact due to potential agglomeration and sedimentation. These findings indicate that greater pumping power may be required to circulate MWCNT nanofluids. Additionally, the sensitivity of viscosity to temperature changes is a crucial factor in understanding the practical behavior of these fluids in real applications.
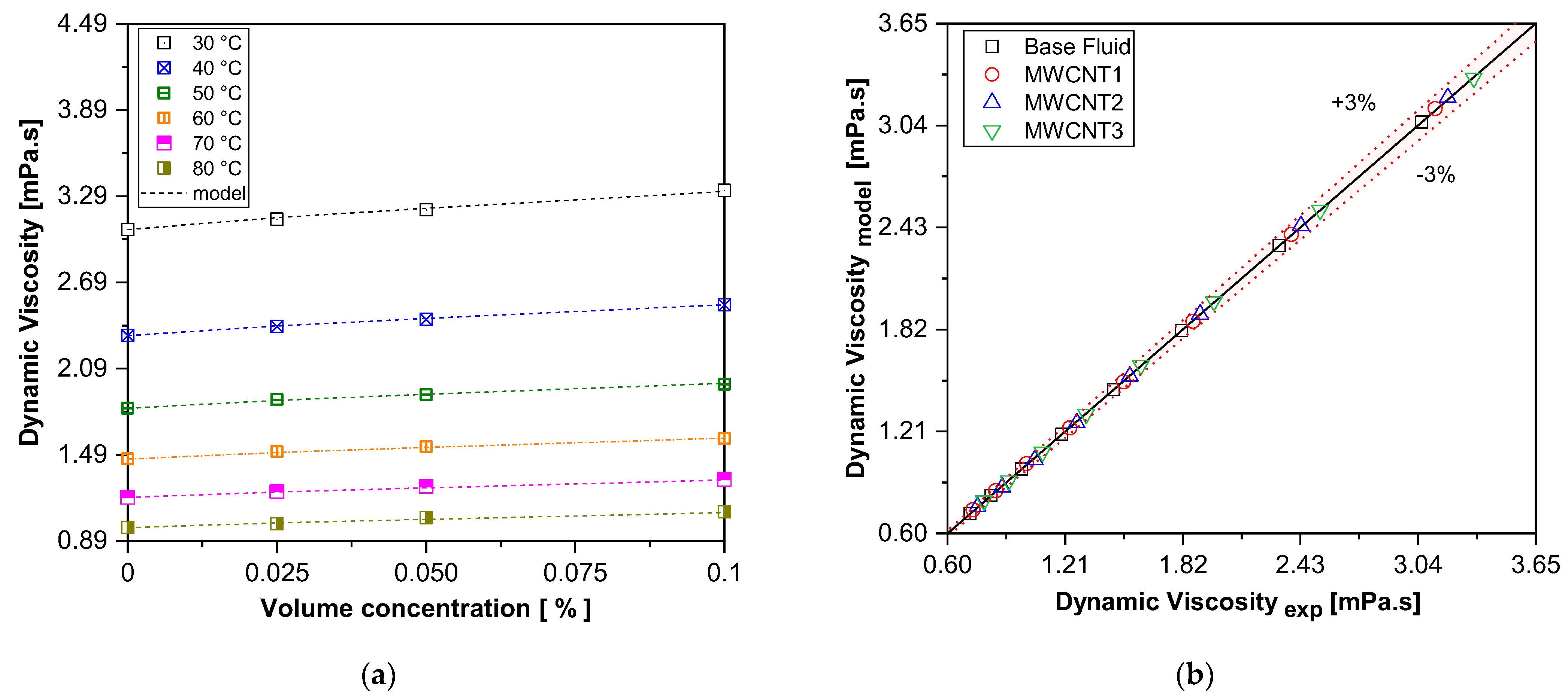
Based on the experimental results of the dynamic viscosity of carbon nanotube nanofluid samples, a mathematical model was proposed, as described in Equation (6), to estimate the dynamic viscosity. The adopted model is similar to the one used for predicting thermal conductivity and considers nanoparticle concentration, temperature, and the base fluid viscosity as key influencing factors.
where a = 14.74, b = 0.8412, and c = 0.1984
Figure 8 presents a comparison between the experimental values of the dynamic viscosity of the carbon nanotube nanofluid samples and the results obtained using the proposed correlation in Equation (6). The model shows good agreement with the experimental data, exhibiting a maximum deviation of only 3%.

Figure 8.
A comparison of the experimental results with those of the proposed correlation for dynamic viscosity. (a) Viscosity vs. volume concentration; (b) experimental vs. model.
4.4. Density Results
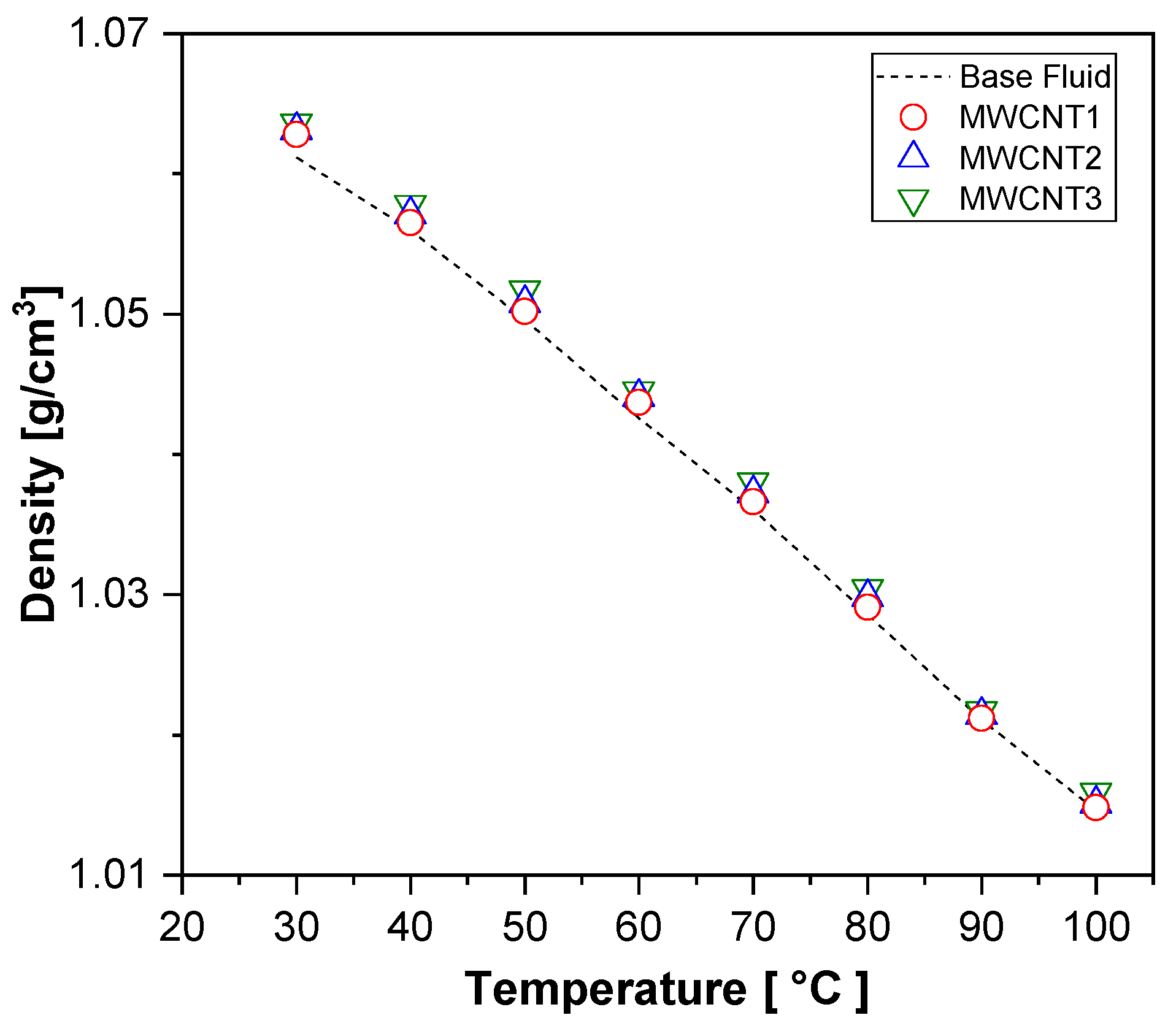
Figure 9 shows that the density of all the nanofluid samples decreased with increasing temperature, indicating an inversely proportional relationship. Moreover, the addition of nanoparticles resulted in a slight increase in the density of all the samples, with the maximum increment observed in the MWCNT samples, reaching 0.23%.
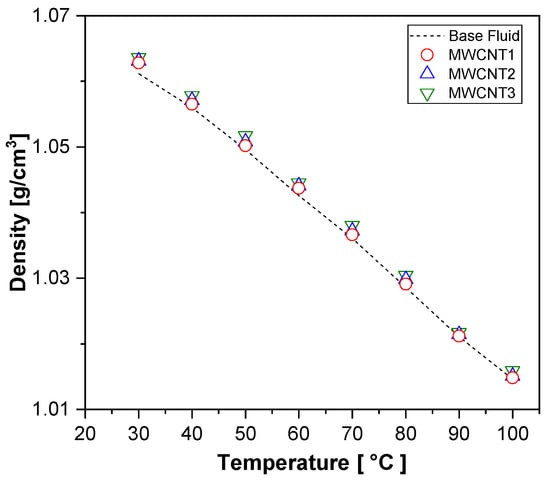
Figure 9.
Experimental results for density for MWCNT nanofluids and base fluid.
Table 7 presents the relative density increases of the nanofluids at 30 °C, comparing the experimental results with theoretical values obtained using the mixture rule (Equation (1)), as proposed by [50]. This model is widely adopted for estimating the density of nanofluids. The comparison revealed a maximum deviation of 0.94% and an average deviation of 0.88%, indicating good agreement between the experimental data and theoretical predictions.

Table 7.
A comparison between the experimental results and the mixture rule for the density of MWCNT nanofluids.
5. Conclusions
This study experimentally investigated the stability and thermophysical properties of MWCNT nanofluids in a water–ethylene glycol (50:50%) mixture. The main conclusions of this study can be summarized as follows:
- The stability of the prepared nanofluid was evaluated using UV-Vis spectrophotometry and DLS methods. These quantitative techniques provided an accurate assessment of the nanoparticle suspension behavior. Compared to the qualitative visual inspection method, they proved to be more effective, particularly for short-term comparisons. Over one month, the MWCNT2 sample showed a relative concentration decrease of up to 10%.
- Thermal conductivity increased with a higher nanoparticle concentration and temperature. Carbon nanotube nanofluids exhibited enhancements of up to 6.4% compared to the base fluid. In silver nanofluids, the surfactant concentration played a key role. While surfactants improved stability, excessive amounts limited the enhancement of thermal conductivity.
- Viscosity was influenced by the nanoparticle concentration, temperature, and surfactant content. A decrease in viscosity with increasing temperature was observed due to the weakening of intermolecular viscous forces between nanoparticles and base fluid molecules. The highest viscosity increase was 11% for the MWCNT3 sample.
- The theoretical model used to estimate density showed an average deviation of less than 1%. Specific heat was determined using a theoretical approach and validated against established parameters found in the literature.
- Correlations were proposed between thermal conductivity, viscosity, nanoparticle concentration, and temperature based on experimental data from carbon nanotube nanofluids. The average deviations for the predicted values were below 1.5% for thermal conductivity and 3% for dynamic viscosity.
Author Contributions
Conceptualization, E.M.C.C. and E.P.B.F.; methodology, E.M.C.C. and G.M.; formal analysis, E.M.C.C.; investigation, E.P.B.F.; resources, E.P.B.F.; data curation, E.M.C.C. and G.M.; writing—original draft preparation, E.M.C.C.; writing—review and editing, E.P.B.F. and G.M.; supervision, E.P.B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the support for this research given by FAPEMIG, CNPq, and CAPES.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ASHRAE | American Society of Heating, Refrigerating, and Air-Conditioning |
| DLS | dynamic light scattering |
| EG | ethylene glycol |
| MWCNT | multi-walled carbon nanotube |
| SDS | Sodium docecyl sulfate |
References
- Yadav, P.; Gupta, S.M.; Sharma, S.K. A Review on Stabilization of Carbon Nanotube Nanofluid. J. Therm. Anal. Calorim. 2022, 147, 6537–6561. [Google Scholar] [CrossRef]
- Tian, W.; Bao, Y.; Qin, G.; Liu, L.; Zheng, X. Influence Mechanism of Functionalization of CNTs on the Thermal Transport Property of Their Nanofluids. J. Mol. Liq. 2023, 392, 123433. [Google Scholar] [CrossRef]
- Li, S.; Yan, J.; Zhang, Y.; Qin, Y.; Zhang, Y.; Du, S. Comparative Investigation of Carbon Nanotubes Dispersion Using Surfactants: A Molecular Dynamics Simulation and Experimental Study. J. Mol. Liq. 2023, 377, 121569. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Klemeš, J.J.; Tian, K.; Ma, T.; Sunden, B. A Review on Nanofluid Stability: Preparation and Application. Renew. Sustain. Energy Rev. 2023, 188, 113854. [Google Scholar] [CrossRef]
- Dosodia, A.; Vadapalli, S.; Jain, A.K.; Sanduru, B.; Mukkamala, S.B. Effect of Size of Multiwalled Carbon Nanotubes on Thermal Conductivity and Viscosity of Ethylene Glycol-Based Nanofluids for Solar Thermal Applications. Phys. Fluids 2023, 35, 092005. [Google Scholar] [CrossRef]
- Kanti, P.; Sharma, K.V.; Raja Sekhar, Y. Influence of Particle Size on Thermal Conductivity and Dynamic Viscosity of Water-based Indian Coal Fly Ash Nanofluid. Heat. Transf. 2022, 51, 413–433. [Google Scholar] [CrossRef]
- Tong, Y.; Ham, J.; Cho, H. Investigation of Thermo-Optical Properties and Photothermal Conversion Performance of MWCNT, Fe3O4, and ATO Nanofluid for Volumetric Absorption Solar Collector. Appl. Therm. Eng. 2024, 246, 123005. [Google Scholar] [CrossRef]
- Ali, N.; Bahman, A.M.; Aljuwayhel, N.F.; Ebrahim, S.A.; Mukherjee, S.; Alsayegh, A. Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review. Nanomaterials 2021, 11, 1628. [Google Scholar] [CrossRef]
- Kasaeian, A.; Daneshazarian, R.; Rezaei, R.; Pourfayaz, F.; Kasaeian, G. Experimental Investigation on the Thermal Behavior of Nanofluid Direct Absorption in a Trough Collector. J. Clean. Prod. 2017, 158, 276–284. [Google Scholar] [CrossRef]
- Sangeetha, M.; Manigandan, S.; Ashok, B.; Brindhadevi, K.; Pugazhendhi, A. Experimental Investigation of Nanofluid Based Photovoltaic Thermal (PV/T) System for Superior Electrical Efficiency and Hydrogen Production. Fuel 2021, 286, 119422. [Google Scholar] [CrossRef]
- Sundar, L.S.; Sintie, Y.T.; Said, Z.; Singh, M.K.; Punnaiah, V.; Sousa, A.C.M. Energy, Efficiency, Economic Impact, and Heat Transfer Aspects of Solar Flat Plate Collector with Al2O3 Nanofluids and Wire Coil with Core Rod Inserts. Sustain. Energy Technol. Assess. 2020, 40, 100772. [Google Scholar] [CrossRef]
- Mohammadi, M.; Taheri, A.; Passandideh-Fard, M.; Sardarabadi, M. Electronic Chipset Thermal Management Using a Nanofluid-Based Mini-Channel Heat Sink: An Experimental Study. Int. Commun. Heat. Mass. Transf. 2020, 118, 104836. [Google Scholar] [CrossRef]
- Wang, X.; Wen, Q.; Yang, J.; Shittu, S.; Wang, X.; Zhao, X.; Wang, Z. Heat Transfer and Flow Characteristic of a Flat Confined Loop Thermosyphon with Ternary Hybrid Nanofluids for Electronic Devices Cooling. Appl. Therm. Eng. 2023, 221, 119758. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Kumar Tiwari, A.; Rai Dixit, A.; Kumar Singh, R. Measurement of Machining Forces and Surface Roughness in Turning of AISI 304 Steel Using Alumina-MWCNT Hybrid Nanoparticles Enriched Cutting Fluid. Measurement 2020, 150, 107078. [Google Scholar] [CrossRef]
- Qasim, M.; Sajid Kamran, M.; Ammar, M.; Ali Jamal, M.; Yasar Javaid, M. Heat Transfer Enhancement of an Automobile Engine Radiator Using ZnO Water Base Nanofluids. J. Therm. Sci. 2020, 29, 1010–1024. [Google Scholar] [CrossRef]
- Cardenas Contreras, E.M.; Bandarra Filho, E.P. Heat Transfer Performance of an Automotive Radiator with MWCNT Nanofluid Cooling in a High Operating Temperature Range. Appl. Therm. Eng. 2022, 207, 118149. [Google Scholar] [CrossRef]
- Savaş, A.; Şener, R.; Uslu, S.; Der, O. Experimental Study on Performance and Emission Optimization of MgO Nanoparticle-Enriched 2nd Generation Biodiesel: A Method for Employing Nanoparticles to Improve Cleaner Diesel Combustion. J. Energy Inst. 2025, 120, 102024. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D. A Review on Graphene Based Nanofluids: Preparation, Characterization and Applications. J. Mol. Liq. 2019, 279, 444–484. [Google Scholar] [CrossRef]
- Khairul, M.A.; Shah, K.; Doroodchi, E.; Azizian, R.; Moghtaderi, B. Effects of Surfactant on Stability and Thermo-Physical Properties of Metal Oxide Nanofluids. Int. J. Heat. Mass. Transf. 2016, 98, 778–787. [Google Scholar] [CrossRef]
- Akoh, H.; Tsukasaki, Y.; Yatsuya, S.; Tasaki, A. Magnetic Properties of Ferromagnetic Ultrafine Particles Prepared by Vacuum Evaporation on Running Oil Substrate. J. Cryst. Growth 1978, 45, 495–500. [Google Scholar] [CrossRef]
- Wagener, M.; Murty, B.S.; Günther, B. Preparation of Metal Nanosuspensions by High-Pressure DC-Sputtering on Running Liquids. MRS Proc. 1996, 457, 149. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously Increased Effective Thermal Conductivities of Ethylene Glycol-Based Nanofluids Containing Copper Nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Zhu, H.T.; Lin, Y.S.; Yin, Y.S. A Novel One-Step Chemical Method for Preparation of Copper Nanofluids. J. Colloid. Interface Sci. 2004, 277, 100–103. [Google Scholar] [CrossRef]
- Lo, C.H.; Tsung, T.T.; Chen, L.C.; Su, C.H.; Lin, H.M. Fabrication of Copper Oxide Nanofluid Using Submerged Arc Nanoparticle Synthesis System (SANSS). J. Nanoparticle Res. 2005, 7, 313–320. [Google Scholar] [CrossRef]
- Ali, A.R.I.; Salam, B. A Review on Nanofluid: Preparation, Stability, Thermophysical Properties, Heat Transfer Characteristics and Application. SN Appl. Sci. 2020, 2, 1636. [Google Scholar] [CrossRef]
- Sarsam, W.S.; Amiri, A.; Shanbedi, M.; Kazi, S.N.; Badarudin, A.; Yarmand, H.; Bashirnezhad, K.; Zaharinie, T. Synthesis, Stability, and Thermophysical Properties of Aqueous Colloidal Dispersions of Multi-Walled Carbon Nanotubes Treated with Beta-Alanine. Int. Commun. Heat. Mass. Transf. 2017, 89, 7–17. [Google Scholar] [CrossRef]
- Chen, Z.; Shahsavar, A.; Al-Rashed, A.A.; Afrand, M. The Impact of Sonication and Stirring Durations on the Thermal Conductivity of Alumina-Liquid Paraffin Nanofluid: An Experimental Assessment. Powder Technol. 2020, 360, 1134–1142. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of Nanofluid: A Review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion Stability of Thermal Nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Oliveira, L.R. Síntese e Caracterização de Nanofluidos Para Aplicação Em Sistemas Térmicos. Ph.D. Thesis, Universidade Federal de Uberlândia, Uberlândia, Brazil, 2018. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 435873. [Google Scholar] [CrossRef]
- Mehta, B.; Subhedar, D.; Panchal, H.; Said, Z. Synthesis, Stability, Thermophysical Properties and Heat Transfer Applications of Nanofluid—A Review. J. Mol. Liq. 2022, 364, 120034. [Google Scholar] [CrossRef]
- Chiam, H.W.; Azmi, W.H.; Usri, N.A.; Mamat, R.; Adam, N.M. Thermal Conductivity and Viscosity of Al2O3 Nanofluids for Different Based Ratio of Water and Ethylene Glycol Mixture. Exp. Therm. Fluid. Sci. 2017, 81, 420–429. [Google Scholar] [CrossRef]
- Sandhu, H.; Gangacharyulu, D. An Experimental Study on Stability and Some Thermophysical Properties of Multiwalled Carbon Nanotubes with Water–Ethylene Glycol Mixtures. Part. Sci. Technol. 2017, 35, 547–554. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Saedodin, S.; Mahian, O.; Wongwises, S. Efficiency of Ferromagnetic Nanoparticles Suspended in Ethylene Glycol for Applications in Energy Devices: Effects of Particle Size, Temperature, and Concentration. Int. Commun. Heat. Mass. Transf. 2014, 58, 138–146. [Google Scholar] [CrossRef]
- Soltanimehr, M.; Afrand, M. Thermal Conductivity Enhancement of COOH-Functionalized MWCNTs/Ethylene Glycol–Water Nanofluid for Application in Heating and Cooling Systems. Appl. Therm. Eng. 2016, 105, 716–723. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; Xi, T.; Liu, Y. Thermal Conductivity of Suspensions Containing Nanosized SiC Particles. Int. J. Thermophys. 2002, 23, 571–580. [Google Scholar] [CrossRef]
- Huminic, A.; Huminic, G.; Fleaca, C.; Dumitrache, F.; Morjan, I. Thermal Conductivity, Viscosity and Surface Tension of Nanofluids Based on FeC Nanoparticles. Powder Technol. 2015, 284, 78–84. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Cardenas Contreras, E.M.; Bandarra Filho, E.P. Experimental Study of Thermophysical Properties of MWCNT and Graphene Coolant Nanofluids for Automotive Application. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 140. [Google Scholar] [CrossRef]
- Arzani, H.K.; Amiri, A.; Kazi, S.N.; Chew, B.T.; Badarudin, A. Experimental and Numerical Investigation of Thermophysical Properties, Heat Transfer and Pressure Drop of Covalent and Noncovalent Functionalized Graphene Nanoplatelet-Based Water Nanofluids in an Annular Heat Exchanger. Int. Commun. Heat. Mass. Transf. 2015, 68, 267–275. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Behera, D.K.; Pal, S.K.; Chakraborty, S. Experimental Investigation on the Effect of Dispersant Addition on Thermal and Rheological Characteristics of TiO2 Nanofluid. Powder Technol. 2017, 307, 10–24. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, H.; Liu, R.; Zhai, Y. Effects of Surfactant on the Stability and Thermal Conductivity of Al2O3/Deionized Water Nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Iyahraja, S.; Rajadurai, J.S. Study of Thermal Conductivity Enhancement of Aqueous Suspensions Containing Silver Nanoparticles. AIP Adv. 2015, 5, 057103. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, S.; Angue Mintsa, H. Temperature and Particle-Size Dependent Viscosity Data for Water-Based Nanofluids–Hysteresis Phenomenon. Int. J. Heat. Fluid. Flow. 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Khodadadi, H.; Toghraie, D.; Karimipour, A. Effects of Nanoparticles to Present a Statistical Model for the Viscosity of MgO-Water Nanofluid. Powder Technol. 2019, 342, 166–180. [Google Scholar] [CrossRef]
- Ijam, A.; Saidur, R.; Ganesan, P.; Moradi Golsheikh, A. Stability, Thermo-Physical Properties, and Electrical Conductivity of Graphene Oxide-Deionized Water/Ethylene Glycol Based Nanofluid. Int. J. Heat. Mass. Transf. 2015, 87, 92–103. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, V.; Kumar, R.; Said, Z. A Review on Thermophysical Properties of Nanofluids and Heat Transfer Applications. Renew. Sustain. Energy Rev. 2017, 74, 638–670. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle Shape Effects on Thermophysical Properties of Alumina Nanofluids. J. Appl. Phys. 2009, 106, 014304. [Google Scholar] [CrossRef]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A.; Sharifpur, M.; Meyer, J.P. Effect of Various Surfactants on the Viscosity, Thermal and Electrical Conductivity of Graphene Nanoplatelets Nanofluid. Int. J. Thermophys. 2021, 42, 158. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and Heat Transfer Study of Dispersed Fluids with Submicron Metallic Oxide Particles. Exp. Heat. Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Chaichan, M.T.; Sopian, K.; Kazem, H.A. Influence of the Base Fluid on the Thermo-Physical Properties of PV/T Nanofluids with Surfactant. Case Stud. Therm. Eng. 2019, 13, 100340. [Google Scholar] [CrossRef]
- Chavan, D.; Pise, A. Experimental Investigation of Effective Viscosity and Density of Nanofluids. Mater. Today Proc. 2019, 16, 504–515. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.N.; Bagheri, S.; Abdelrazek, A.H.; Ahmadi, G.; Zubir, N.; Ahmad, R.; Abidin, N.I.Z. A Facile, Bio-Based, Novel Approach for Synthesis of Covalently Functionalized Graphene Nanoplatelet Nano-Coolants toward Improved Thermo-Physical and Heat Transfer Properties. J. Colloid. Interface Sci. 2018, 509, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, V.; Velraj, R. Experimental Investigation of the Thermo-Physical Properties of Water-Ethylene Glycol Mixture Based CNT Nanofluids. Thermochim. Acta 2012, 545, 180–186. [Google Scholar] [CrossRef]
- Xuan, Y.; Roetzel, W. Conceptions for Heat Transfer Correlation of Nanofluids. Int. J. Heat. Mass. Transf. 2000, 43, 3701–3707. [Google Scholar] [CrossRef]
- Adun, H.; Wole-Osho, I.; Okonkwo, E.C.; Kavaz, D.; Dagbasi, M. A Critical Review of Specific Heat Capacity of Hybrid Nanofluids for Thermal Energy Applications. J. Mol. Liq. 2021, 340, 116890. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Reinert, L.; Zeiger, M.; Suárez, S.; Presser, V.; Mücklich, F. Dispersion Analysis of Carbon Nanotubes, Carbon Onions, and Nanodiamonds for Their Application as Reinforcement Phase in Nickel Metal Matrix Composites. RSC Adv. 2015, 5, 95149–95159. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Sun, J. Production of Aqueous Colloidal Dispersions of Carbon Nanotubes. J. Colloid. Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Swinehart, F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333–335. [Google Scholar] [CrossRef]
- Taylor, B.N.; Kuyatt, C.E. Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement; National Institute for Standards and Technology (NIST): Gaithersburg, MD, USA, 1994. Available online: http://physics.nist.gov/TN1297 (accessed on 15 April 2025).
- Choi, T.J.; Jang, S.P.; Kedzierski, M.A. Effect of Surfactants on the Stability and Solar Thermal Absorption Characteristics of Water-Based Nanofluids with Multi-Walled Carbon Nanotubes. Int. J. Heat. Mass. Transf. 2018, 122, 483–490. [Google Scholar] [CrossRef]
- Kumar, P.G.; Kumaresan, V.; Velraj, R. Stability, Viscosity, Thermal Conductivity, and Electrical Conductivity Enhancement of Multi-Walled Carbon Nanotube Nanofluid Using Gum Arabic. Fuller. Nanotub. Carbon. Nanostructures 2017, 25, 230–240. [Google Scholar] [CrossRef]
- ASHRAE Standard. ASHRAE Handbook: Fundamentals; ASHRAE: Atlanta, GA, USA, 2001. [Google Scholar]
- Yu-Hua, L.; Wei, Q.; Jian-Chao, F. Temperature Dependence of Thermal Conductivity of Nanofluids. Chin. Phys. Lett. 2008, 25, 3319–3322. [Google Scholar] [CrossRef]
- Adhami Dehkordi, R.; Hemmat Esfe, M.; Afrand, M. Effects of Functionalized Single Walled Carbon Nanotubes on Thermal Performance of Antifreeze: An Experimental Study on Thermal Conductivity. Appl. Therm. Eng. 2017, 120, 358–366. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Mishra, P.C.; Mukherjee, S.; Nayak, S.K.; Panda, A. A Brief Review on Viscosity of Nanofluids. Int. Nano Lett. 2014, 4, 109–120. [Google Scholar] [CrossRef]
- Meyer, J.P.; Adio, S.A.; Sharifpur, M.; Nwosu, P.N. The Viscosity of Nanofluids: A Review of the Theoretical, Empirical, and Numerical Models. Heat. Transf. Eng. 2016, 37, 387–421. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).