Study and Effect of Agitation on Kojic Acid Production by Aspergillus oryzae in Liquid Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Biological Material
2.2. Strain Culturing
2.3. Agitation Effect on Fermentation
2.4. Kojic Acid Determination
2.5. Kinetic Modeling
2.6. Statistical Analysis
3. Results
3.1. General Overview of KA Production Under Different Conditions
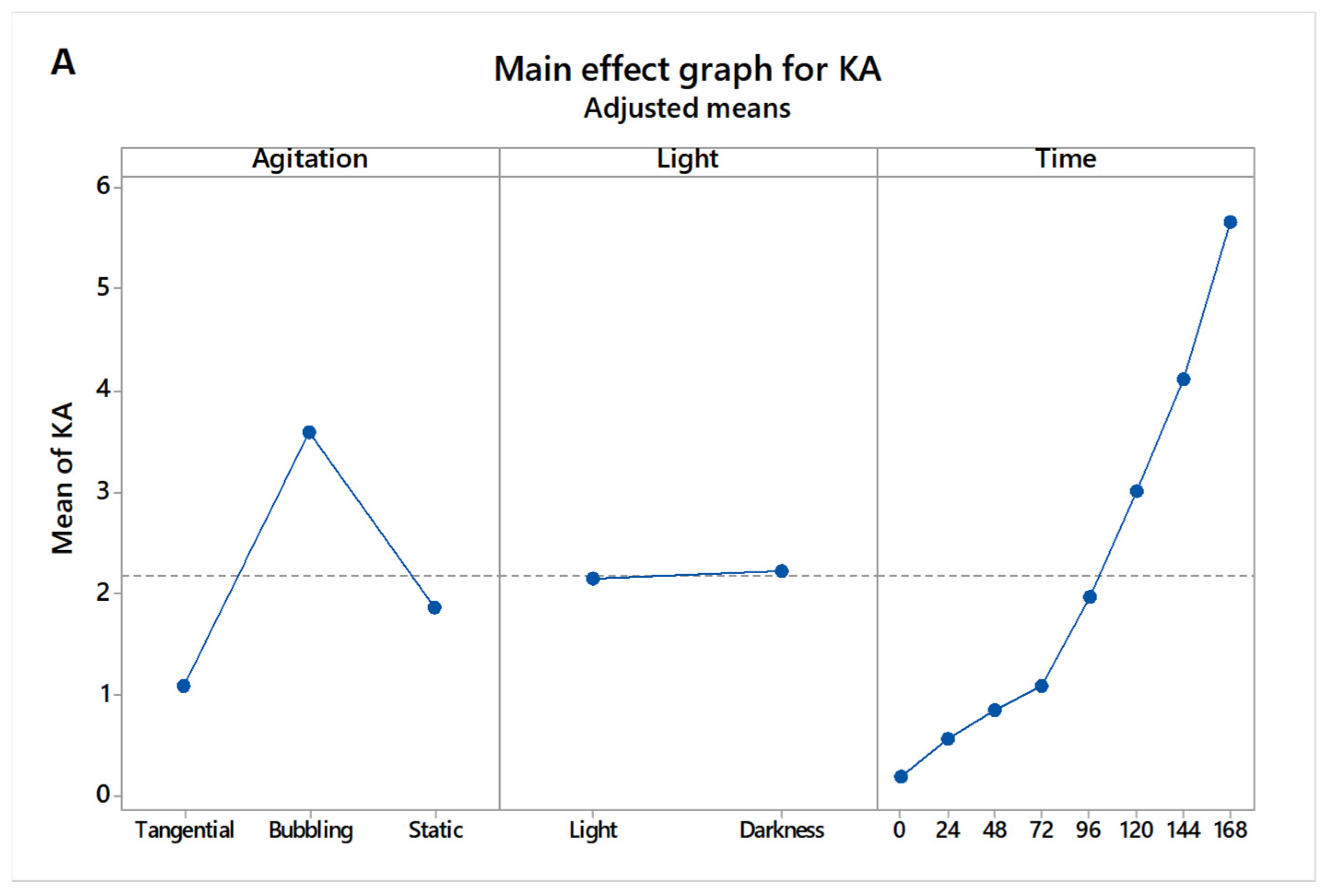
3.2. Statistical Analysis of Factor Effects
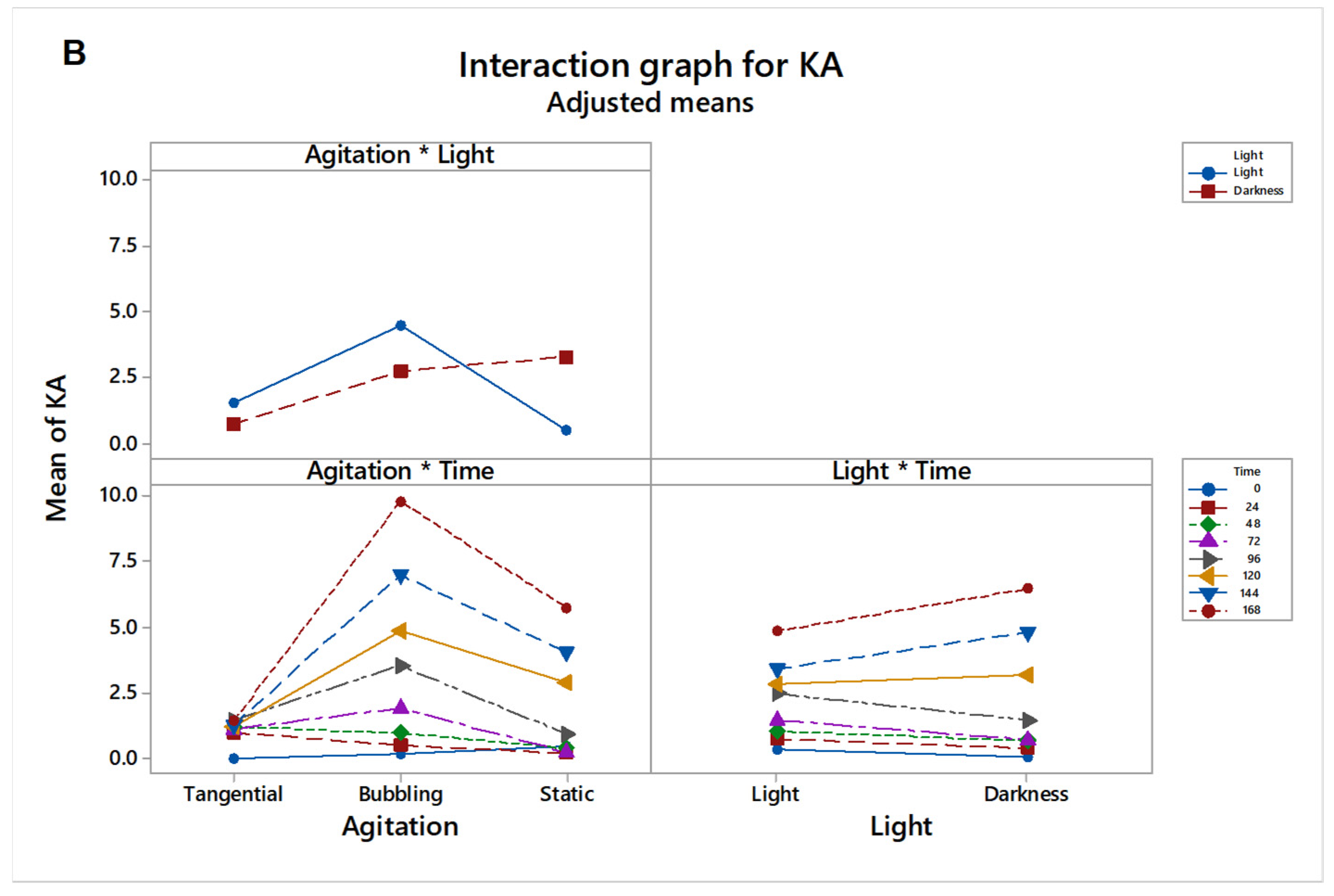
3.3. Main and Interaction Effects on Kojic Acid Production
3.4. Production Kinetics and Comparison Among Treatments
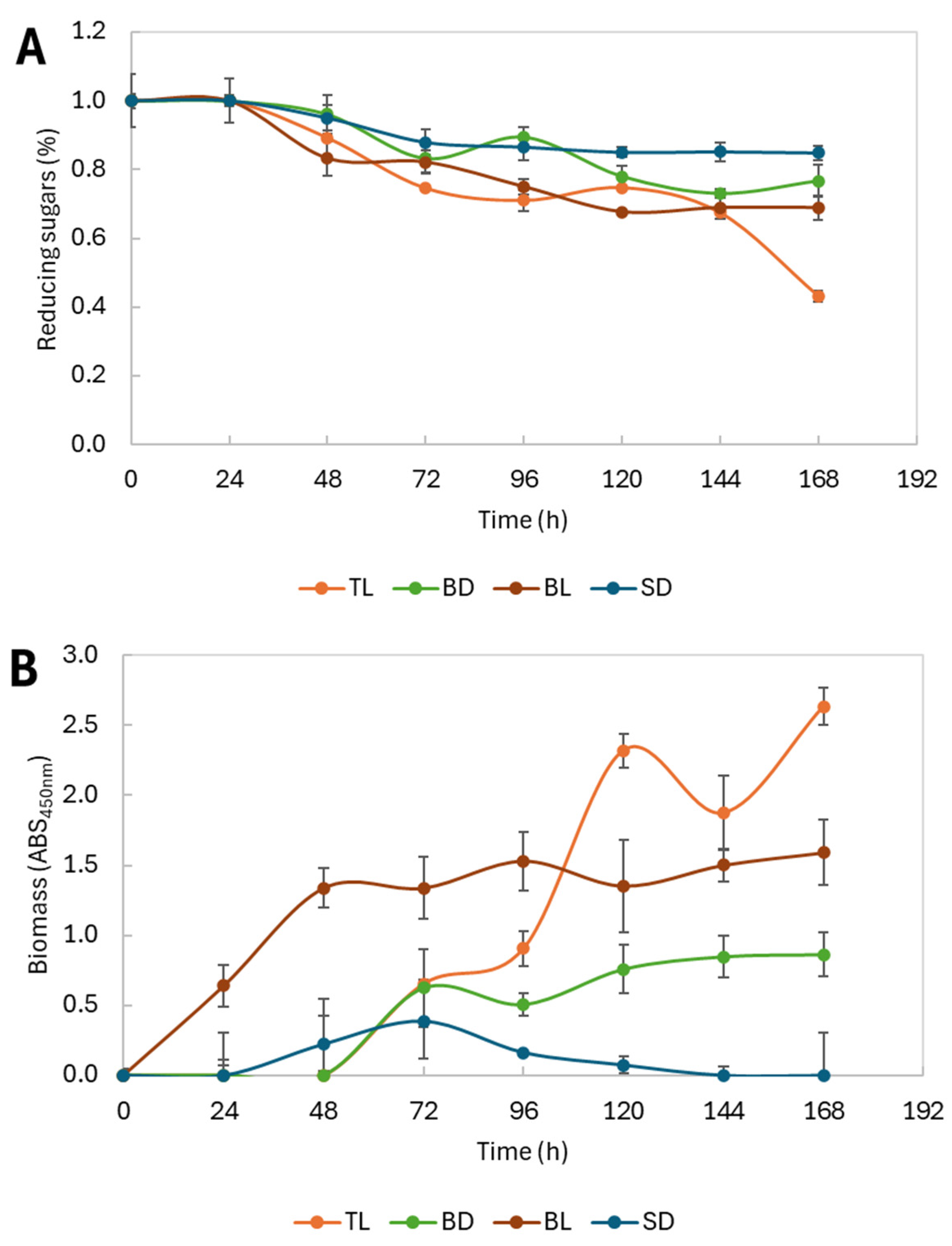
3.5. Reducing Sugar Consumption and Biomass Formation
3.6. Kinetic Parameter Estimation
4. Discussion
4.1. Strain Cultivation and Genetic Potencial
4.2. Effect of Agitation on Fermentation Efficiency
4.3. Influence of Light on Metabolite Biosynthesis
4.4. Carbon Source Utilization and Biomass Formation
4.5. Kinetic Parameters and Treatment Comparison
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanley, M.E.; Fenner, M.; Whibley, H.; Darvill, B. Early plant growth: Identifying the end point of the seedling phase. New Phytol. 2004, 163, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, S.; Yu, D. The Role of Light Quality in Regulating Early Seedling Development. Plants 2023, 12, 2746. [Google Scholar] [CrossRef] [PubMed]
- Brukhin, V.; Morozova, N. Plant growth and development—Basic knowledge and current views. Math. Model. Nat. Phenom. 2011, 6, 1–53. [Google Scholar] [CrossRef]
- Yang, Z.; Qiao, Y.; Konakalla, N.C.; Strøbech, E.; Harris, P.; Peschel, G.; Agler-Rosenbaum, M.; Weber, T.; Andreasson, E.; Ding, L. Streptomyces alleviate abiotic stress in plant by producing pteridic acids. Nat. Commun. 2023, 14, 7398. [Google Scholar] [CrossRef]
- Gao, Q.; Yin, X.; Wang, F.; Zhang, C.; Xiao, F.; Wang, H.; Hu, S.; Liu, W.; Zhou, S.; Chen, L.; et al. Jacalin-related lectin 45 (OsJRL45) isolated from ‘sea rice 86’ enhances rice salt tolerance at the seedling and reproductive stages. BMC Plant Biol. 2023, 23, 533. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- arza-Alonso, C.A.; Olivares-Sáenz, E.; González-Morales, S.; Cabrera-De la Fuente, M.; Juárez-Maldonado, A.; González-Fuentes, J.A.; Tortella, G.; Valdés-Caballero, M.V.; Benavides-Mendoza, A. Strawberry Biostimulation: From Mechanisms of Action to Plant Growth and Fruit Quality. Plants 2022, 11, 3463. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.G. Secondary Metabolism and Antimicrobial Metabolites of Aspergillus; Elsevier B.V.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Kim, J.H.; Campbell, B.C.; Chan, K.L.; Mahoney, N.; Haff, R.P. Synergism of antifungal activity between mitochondrial respiration inhibitors and kojic acid. Molecules 2013, 18, 1564–1581. [Google Scholar] [CrossRef]
- Chen, J.S.; Wei, C.I.; Marshall, M.R. Inhibition mechanism of kojic acid on polyphenol oxidase. J. Agric. Food Chem. 1991, 39, 1897–1901. [Google Scholar] [CrossRef]
- Rousta, N.; Hellwig, C.; Wainaina, S.; Lukitawesa, L.; Agnihotri, S.; Rousta, K.; Taherzadeh, M.J. Filamentous fungus Aspergillus oryzae for food: From submerged cultivation to fungal burgers and their sensory evaluation—A pilot study. Foods 2021, 10, 2774. [Google Scholar] [CrossRef]
- Promsang, A.; Rungsardthong, V.; Thumthanaruk, B.; Puttanlek, C.; Uttapap, D.; Foophow, T.; Phalathanaporn, V.; Vatanyoopaisarn, S. Effect of culture conditions and medium compositions on kojic acid production by Aspergillus oryzae ATCC 10124. IOP Conf. Ser. Earth Environ. Sci. 2019, 346, 012047. [Google Scholar] [CrossRef]
- Foukrach, M.; Bouzit, M.; Ameur, H.; Kamla, Y. Effect of Agitator’s Types on the Hydrodynamic Flow in an Agitated Tank. Chin. J. Mech. Eng. (Engl. Ed.) 2020, 33, 37. [Google Scholar] [CrossRef]
- Yafetto, L. Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef] [PubMed]
- Palladino, F.; Marcelino, P.R.F.; Schlogl, A.E.; José, Á.H.M.; Rodrigues, R.D.C.L.B.; Fabrino, D.L.; Santos, I.J.B.; Rosa, C.A. Bioreactors: Applications and Innovations for a Sustainable and Healthy Future—A Critical Review. Appl. Sci. 2024, 14, 9346. [Google Scholar] [CrossRef]
- Kumar, A.; Udugama, I.A.; Gargalo, C.L.; Gernaey, K.V. Why is batch processing still dominating the biologics landscape? Towards an integrated continuous bioprocessing alternative. Processes 2020, 8, 1641. [Google Scholar] [CrossRef]
- El-Kady, I.A.; Zohri, A.N.A.; Hamed, S.R. Kojic Acid Production from Agro-Industrial By-Products Using Fungi. Biotechnol. Res. Int. 2014, 2014, 642385. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, X.; Lv, R.; Cao, W.; Gao, W.; Liu, J.; Xie, Z.; Liu, H. Effective production of kojic acid in engineered Aspergillus niger. Microb. Cell Fact. 2023, 22, 40. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.B. Improvement of Kojic Acid Production by a Mutant strain of Aspergillus flavus. J. Nat. Sci. Res. 2013, 3, 31–41. [Google Scholar]
- Quiterio-Gutiérrez, T.; González-Morales, S.; González-Fuentes, J.A.; Benavides-Mendoza, A.; Fernández-Luqueño, F.; Medrano-Macías, J.; Robledo-Olivo, A. Production of Kojic Acid by Aspergillus niger M4 with Different Concentrations of Yeast Extract as a Nitrogen Source. Processes 2023, 11, 1724. [Google Scholar] [CrossRef]
- Bentley, R. Preparation and analysis of Kojic acid. Methods Enzymol. 1946, 25, 1946–1949. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Robledo, A.; Aguilera-Carbo, A.F.; Prado-Barragan, A.; Sepulveda-Torre, L.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Kinetics of Ellagic Acid Accumulation by Solid-State Fermentation. In Theoretical Models and Experimental Approaches in Physical Chemistry: Research Methodology and Practical Methods; Haghi, A.K., Thomas, S., Praveen, K.M., Pai, A.R., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2018. [Google Scholar]
- Yang, K.; Tian, J.; Keller, N.P. Post-translational modifications drive secondary metabolite biosynthesis in Aspergillus: A review. Environ. Microbiol. 2022, 24, 2857–2881. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, N.; Wolfe, K.H. Elusive origins of the extra genes in Aspergillus oryzae. PLoS ONE 2008, 3, e3036. [Google Scholar] [CrossRef]
- Jin, F.J.; Hu, S.; Wang, B.T.; Jin, L. Advances in Genetic Engineering Technology and Its Application in the Industrial Fungus Aspergillus oryzae. Front. Microbiol. 2021, 12, 644404. [Google Scholar] [CrossRef]
- Schrader, M.; Schrinner, K.; Polomsky, L.; Ivanov, D.; Kampen, I.; Schilde, C.; Krull, R.; Kwade, A. Quantification and modeling of macroparticle-induced mechanical stress for varying shake flask cultivation conditions. Front. Bioeng. Biotechnol. 2023, 11, 1254136. [Google Scholar] [CrossRef] [PubMed]
- MBöl; Schrinner, K.; Tesche, S.; Krull, R. Challenges of influencing cellular morphology by morphology engineering techniques and mechanical induced stress on filamentous pellet systems—A critical review. Eng. Life Sci. 2021, 21, 51–67. [Google Scholar] [CrossRef]
- Buffo, M.M.; Corrêa, L.J.; Esperança, M.N.; Cruz, A.J.G.; Farinas, C.S.; Badino, A.C. Influence of dual-impeller type and configuration on oxygen transfer, power consumption, and shear rate in a stirred tank bioreactor. Biochem. Eng. J. 2016, 114, 130–139. [Google Scholar] [CrossRef]
- Ibrahim, D. Effect of agitation speed on the morphology of Aspergillus niger HFD5A-1 hyphae and its pectinase production in submerged fermentation. World J. Biol. Chem. 2015, 6, 265. [Google Scholar] [CrossRef]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.A.; Lenaers, G.; et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018, 9, 287. [Google Scholar] [CrossRef]
- Jia, X.; Song, J.; Wu, Y.; Feng, S.; Sun, Z.; Hu, Y.; Yu, M.; Han, R.; Zeng, B. Strategies for the Enhancement of Secondary Metabolite Production via Biosynthesis Gene Cluster Regulation in Aspergillus oryzae. J. Fungi 2024, 10, 312. [Google Scholar] [CrossRef]
- Dos Reis, T.F.; Nitsche, B.M.; De Lima, P.B.A.; De Assis, L.J.; Mellado, L.; Harris, S.D.; Meyer, V.; Dos Santos, R.A.C.; Riaño-Pachón, D.M.; Ries, L.N.A.; et al. The low affinity glucose transporter HxtB is also involved in glucose signalling and metabolism in Aspergillus nidulans. Sci. Rep. 2017, 7, 45073. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, L.R.; He, H.W.; Sang, B.; Yu, D.L.; Feng, J.T.; Zhang, X. Effects of agitation, aeration and temperature on production of a novel glycoprotein gp-1 by Streptomyces kanasenisi zx01 and scale-up based on volumetric oxygen transfer coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.A.M.; Ezzat, S.M.; Houseny, A.M. Improved production of kojic acid by mutagenesis of Aspergillus flavus HAk1 and Aspergillus oryzae HAk2 and their potential antioxidant activity. 3 Biotech. 2017, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, Y.; Long, S.; Feng, S.; Jia, X.; Hu, Y.; Ma, M.; Liu, J.; Zeng, B. Aspergillus oryzae as a Cell Factory: Research and Applications in Industrial Production. J. Fungi 2024, 10, 248. [Google Scholar] [CrossRef]
- Charles-Rodríguez, V.; Guerrero-Mata, A.; Martínez-Vázquez, G.; Cruz-Hernández, M.A.; Belmares-Cerda, R.E.; Robledo, A. Bioreactor Analysis for the Corn-Cob Valorization in the Xylanase Production. Waste Biomass Valorization 2018, 9, 995–1001. [Google Scholar] [CrossRef]
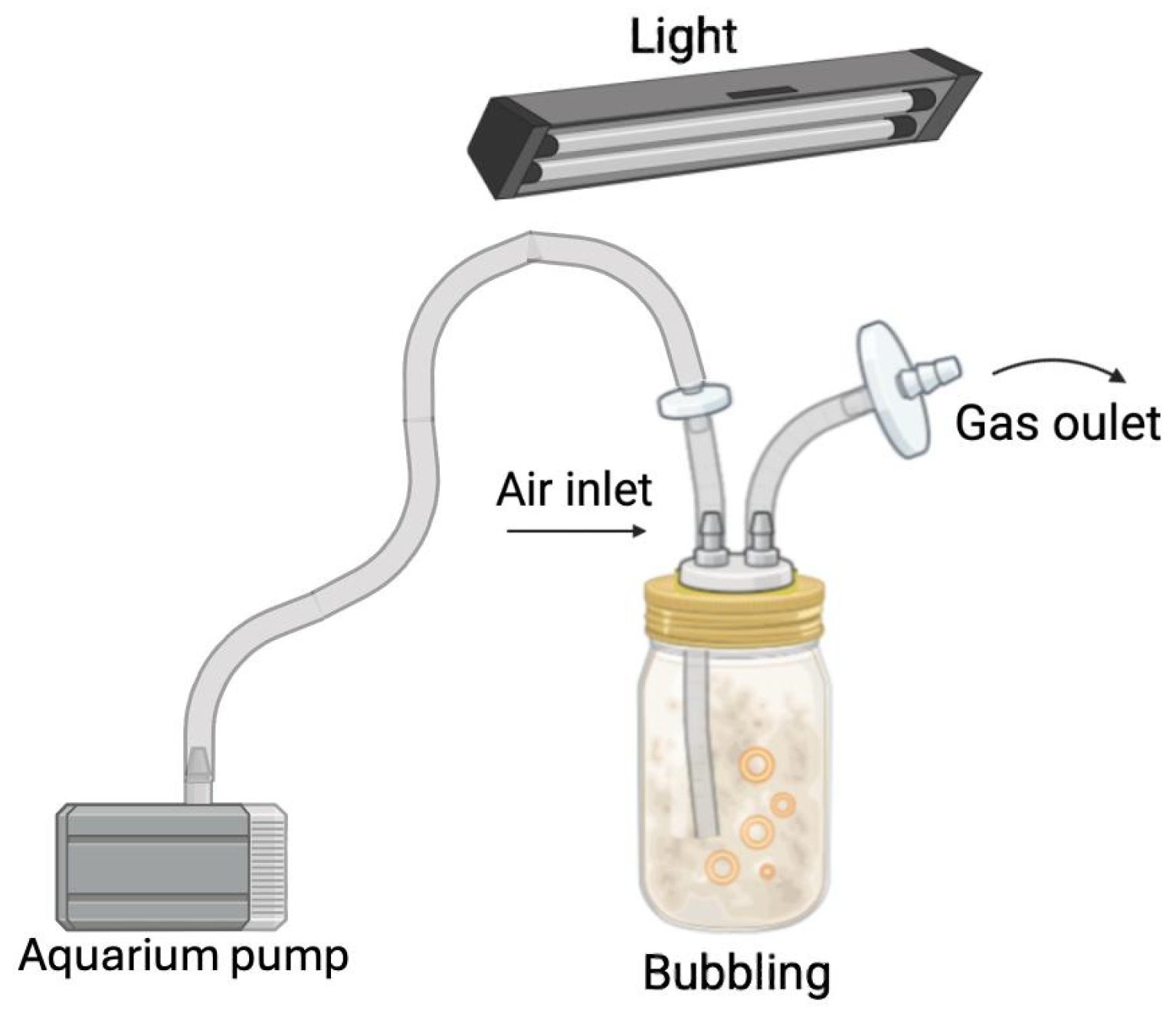

| Treatment | Description | Abbreviation |
|---|---|---|
| 1 | Bubbling in light | BL |
| 2 | Bubbling in darkness | BD |
| 3 | Tangential in light | TL |
| 4 | Tangential in darkness | TD |
| 5 | Static in light | SL |
| 6 | Static in darkness | SD |
| Source | DF | SS Adjust | MS Adjust | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 47 | 1135.56 | 24.1608 | 6.73 | 0.000 |
| Lineal | 10 | 564.69 | 56.4687 | 15.72 | 0.000 |
| Agitation | 2 | 155.80 | 77.8992 | 21.69 | 0.000 |
| Light | 1 | 0.18 | 0.1812 | 0.05 | 0.823 |
| Time | 7 | 412.91 | 58.9875 | 16.42 | 0.000 |
| 2-term interactions | 23 | 371.71 | 16.1612 | 4.50 | 0.000 |
| Agitation × Light | 2 | 110.95 | 55.4740 | 15.45 | 0.000 |
| Agitation × Time | 14 | 224.83 | 16.0595 | 4.47 | 0.000 |
| Light × Time | 7 | 27.17 | 3.8810 | 1.08 | 0.383 |
| 3-term interactions | 14 | 120.44 | 8.6032 | 2.40 | 0.007 |
| Agitation × Light × Time | 14 | 120.44 | 8.6032 | 2.40 | 0.007 |
| Error | 86 | 308.86 | 3.5915 | ||
| Total | 133 | 1444.42 |
| Treatment | Time (h) | Kojic Acid (g/L) | Treatment | Time (h) | Kojic Acid (g/L) | Treatment | Time (h) | Kojic Acid (g/L) |
|---|---|---|---|---|---|---|---|---|
| BL | 24 | 0.70 ± 0.57 | TL | 24 | 1.20 ± 0.08 | SL | 24 | 0.30 + 0.19 |
| BL | 48 | 1.16 ± 0.44 | TL | 48 | 1.63 ± 0.15 | SL | 48 | 0.33 + 0.03 |
| BL | 72 | 2.79 ± 0.37 | TL | 72 | 1.46 ± 0.14 | SL | 72 | 0.18 + 0.15 |
| BL | 96 | 5.02 ± 0.98 | TL | 96 | 1.47 ± 0.09 | SL | 96 | 0.32 + 0.29 |
| BL | 120 | 6.56 ± 1.82 | TL | 120 | 1.72 ± 0.35 | SL | 120 | 0.27 + 0.11 |
| BL | 144 | 7.86 ± 2.21 | TL | 144 | 1.77 ± 0.15 | SL | 144 | 0.61 + 0.24 |
| BL | 168 | 7.61 ± 2.36 | TL | 168 | 2.07 ± 0.47 | SL | 168 | 1.02 + 0.39 |
| BD | 24 | 0.42 ± 0.00 | TD | 24 | 0.76 ± 0.01 | SD | 24 | 0.06 + 0.13 |
| BD | 48 | 0.77 ± 0.21 | TD | 48 | 0.78 ± 0.01 | SD | 48 | 1.25 + 0.00 |
| BD | 72 | 1.06 ± 0.32 | TD | 72 | 0.77 ± 0.00 | SD | 72 | 0.33 + 0.00 |
| BD | 96 | 2.68 ± 1.97 | TD | 96 | 0.78 + 0.01 | SD | 96 | 1.56 + 0.78 |
| BD | 120 | 3.17 ± 0.33 | TD | 120 | 0.76 + 0.01 | SD | 120 | 5.54 + 4.81 |
| BD | 144 | 4.99 ± 1.03 | TD | 144 | 0.78 + 0.01 | SD | 144 | 7.50 + 3.82 |
| BD | 168 | 6.55 ± 0.27 | TD | 168 | 0.77 + 0.00 | SD | 168 | 7.60 + 5.66 |
| µmax (h−1) | YP/X (g KA/g X) | qP (g KA/g X·h) | |
|---|---|---|---|
| TL | 0.007 | 0.79 | 0.005 |
| BD | 0.019 | 7.59 | 0.147 |
| BL | 0.029 | 4.78 | 0.138 |
| SD | 0.121 | 0.85 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soberón-Nakasima-Cerda, J.F.; Robledo-Olivo, A.; Charles-Rodríguez, A.V.; Ruiz, H.A.; González-Morales, S.; Benavides-Mendoza, A. Study and Effect of Agitation on Kojic Acid Production by Aspergillus oryzae in Liquid Fermentation. Processes 2025, 13, 1341. https://doi.org/10.3390/pr13051341
Soberón-Nakasima-Cerda JF, Robledo-Olivo A, Charles-Rodríguez AV, Ruiz HA, González-Morales S, Benavides-Mendoza A. Study and Effect of Agitation on Kojic Acid Production by Aspergillus oryzae in Liquid Fermentation. Processes. 2025; 13(5):1341. https://doi.org/10.3390/pr13051341
Chicago/Turabian StyleSoberón-Nakasima-Cerda, Juan Fernando, Armando Robledo-Olivo, Ana Verónica Charles-Rodríguez, Héctor A. Ruiz, Susana González-Morales, and Adalberto Benavides-Mendoza. 2025. "Study and Effect of Agitation on Kojic Acid Production by Aspergillus oryzae in Liquid Fermentation" Processes 13, no. 5: 1341. https://doi.org/10.3390/pr13051341
APA StyleSoberón-Nakasima-Cerda, J. F., Robledo-Olivo, A., Charles-Rodríguez, A. V., Ruiz, H. A., González-Morales, S., & Benavides-Mendoza, A. (2025). Study and Effect of Agitation on Kojic Acid Production by Aspergillus oryzae in Liquid Fermentation. Processes, 13(5), 1341. https://doi.org/10.3390/pr13051341















