The Development of a New Bi12ZnO20/AgI Heterosystem for the Degradation of Dye-Contaminated Water by Photocatalysis Under Solar Irradiation: Synthesis, Characterization and Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Semiconductors and Preparation of the Heterosystem
2.3. Characterization Techniques
2.4. Study of the Photodegradation of Basic Blue 41
3. Results and Discussion
3.1. Physicochemical Characterization of Nanomaterials
3.1.1. Evaluation of Thermal Properties
3.1.2. Identification of the Crystalline Phase
3.1.3. Surface Analysis
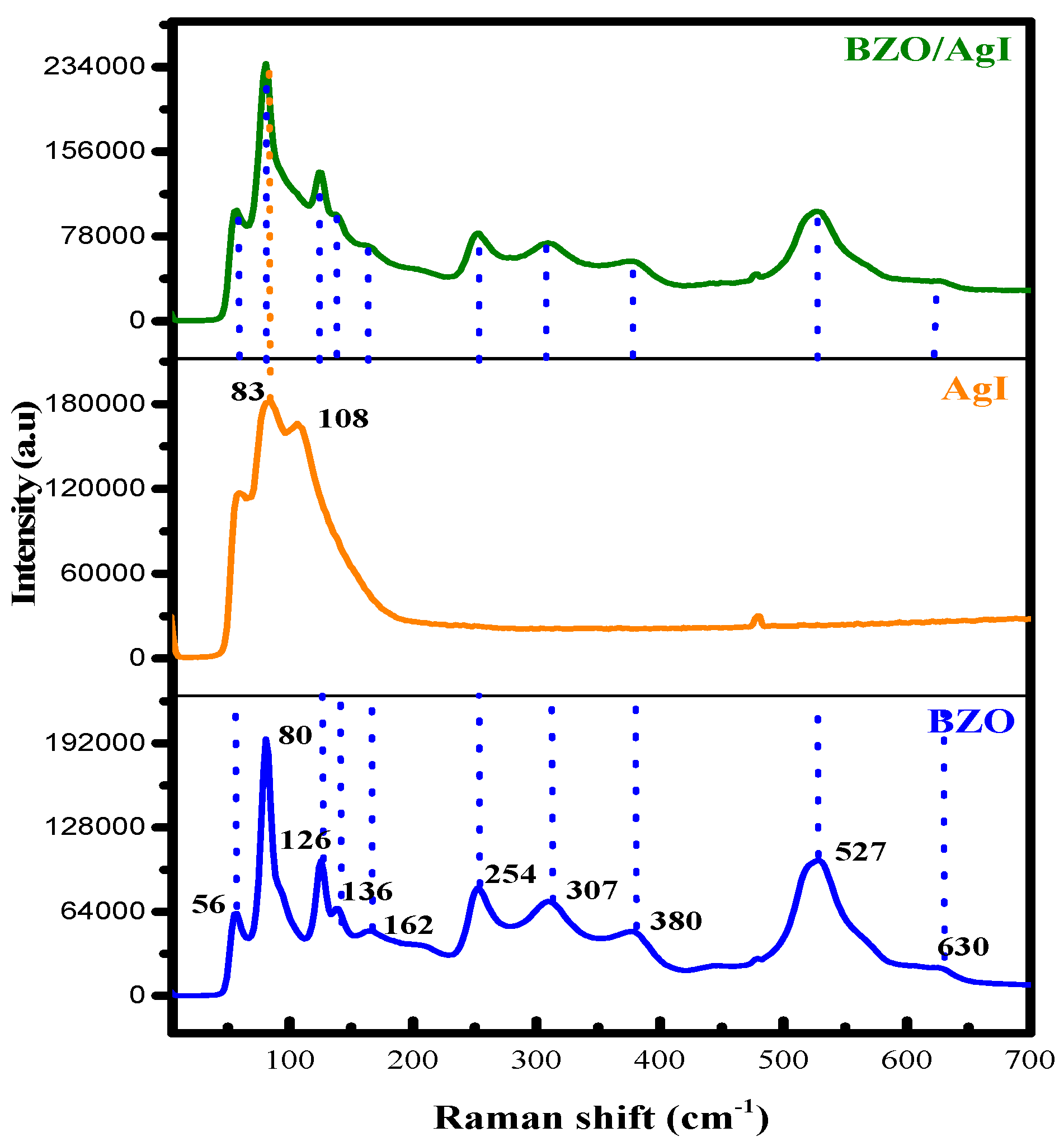
3.1.4. Raman Spectroscopy
3.1.5. BET Analysis
3.1.6. Optical Properties Analysis
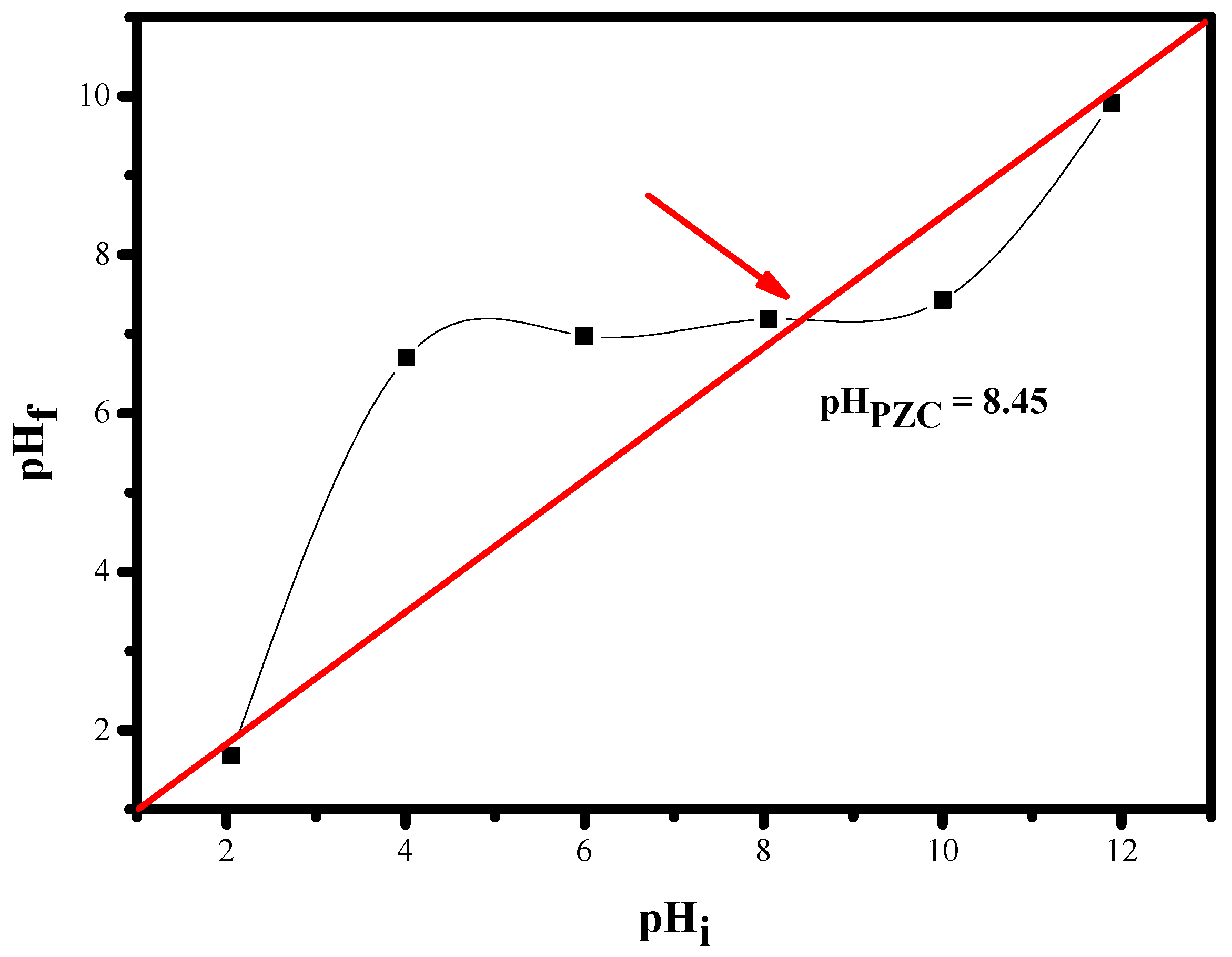
3.1.7. Determination of the Point of Zero Charge
3.2. Evaluation of the Adsorption Effect on the Degradation of Basic Blue 41
3.3. Study of the Photodegradation of Basic Blue 41
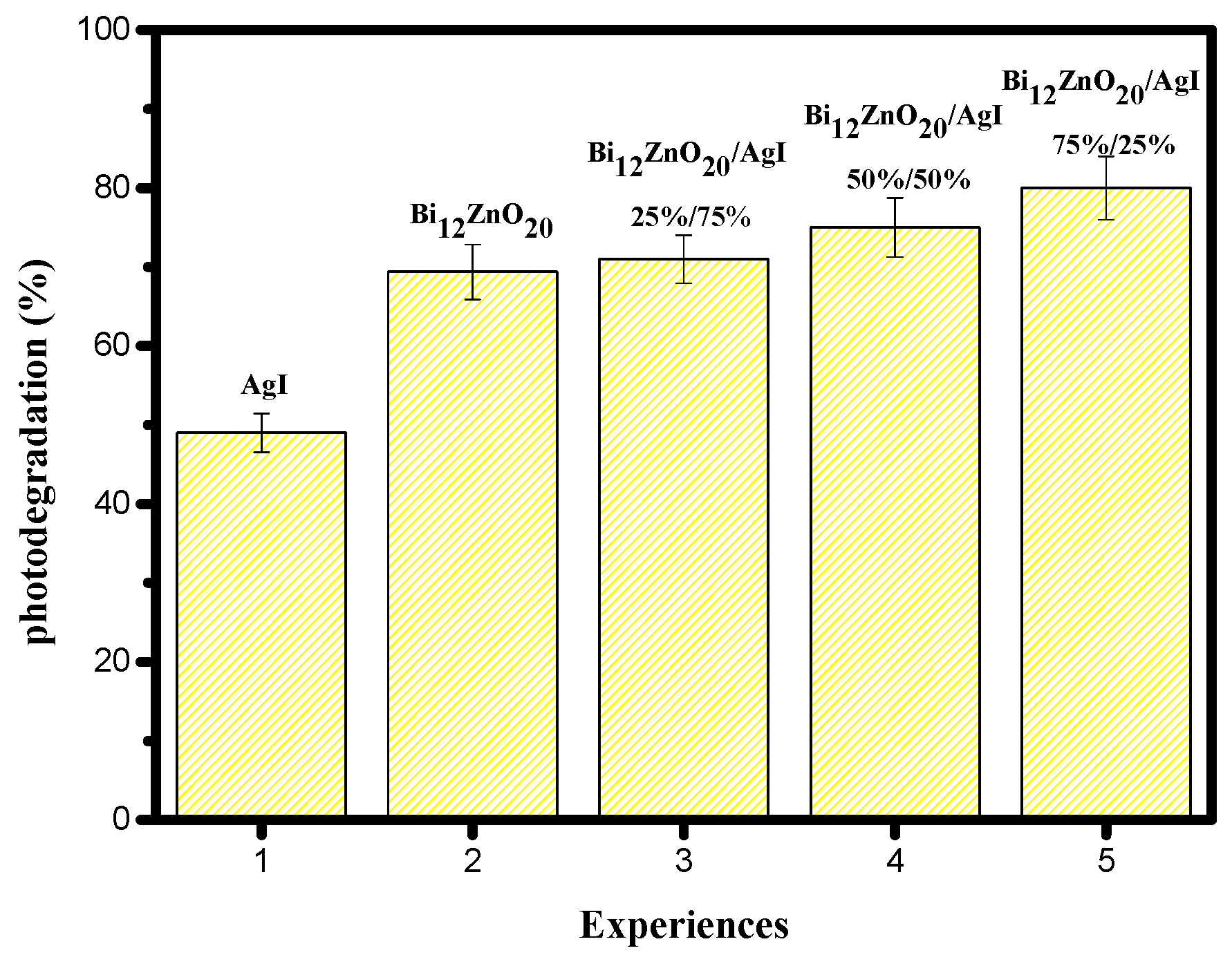
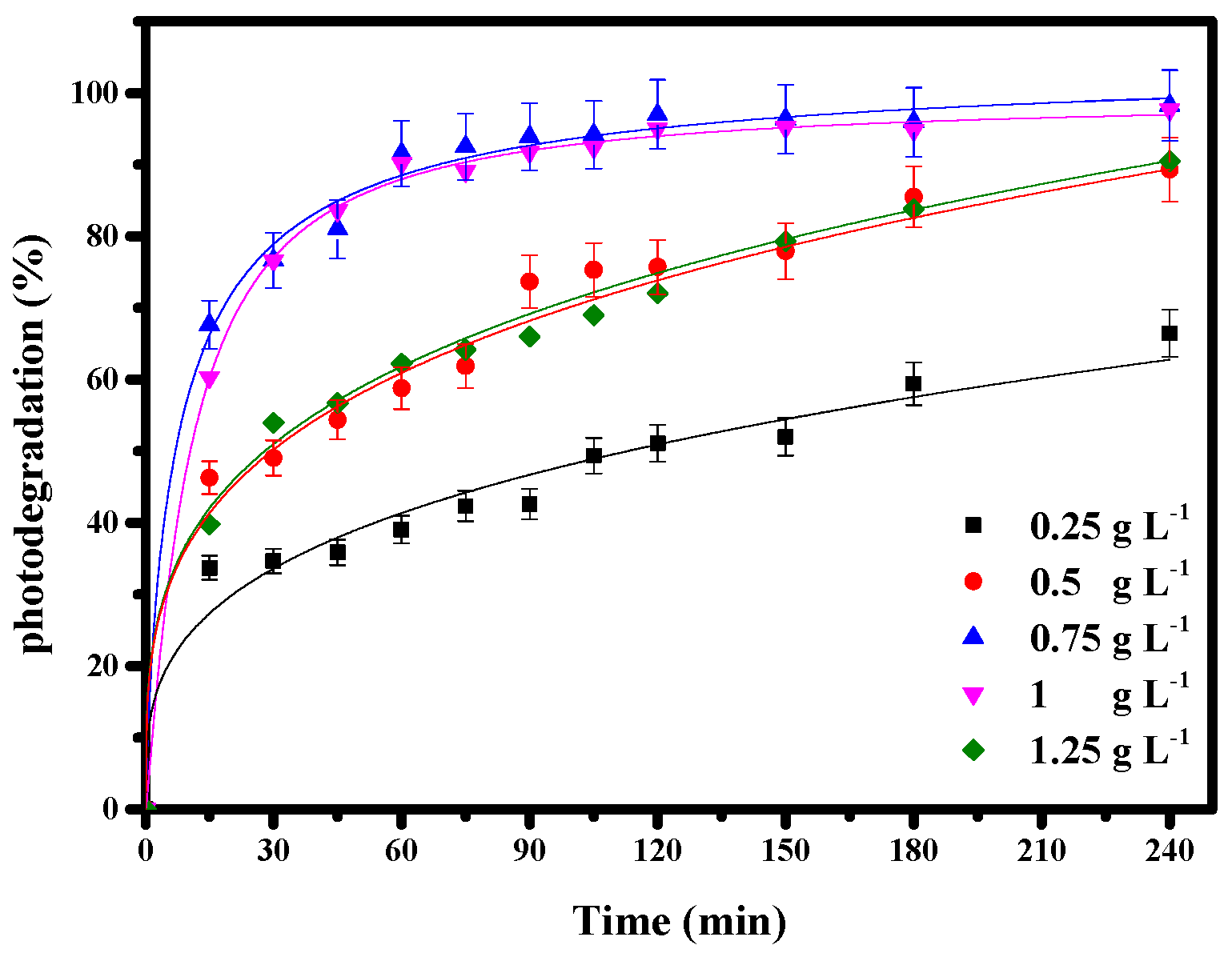
3.3.1. Influence of the Mass Ratio of the Bi12ZnO20/AgI Heterosystem
3.3.2. Influence of pH on the Photodegradation of Basic Blue 41
3.3.3. Influence of Bi12ZnO20/AgI Dose on Photodegradation of Basic Blue 41
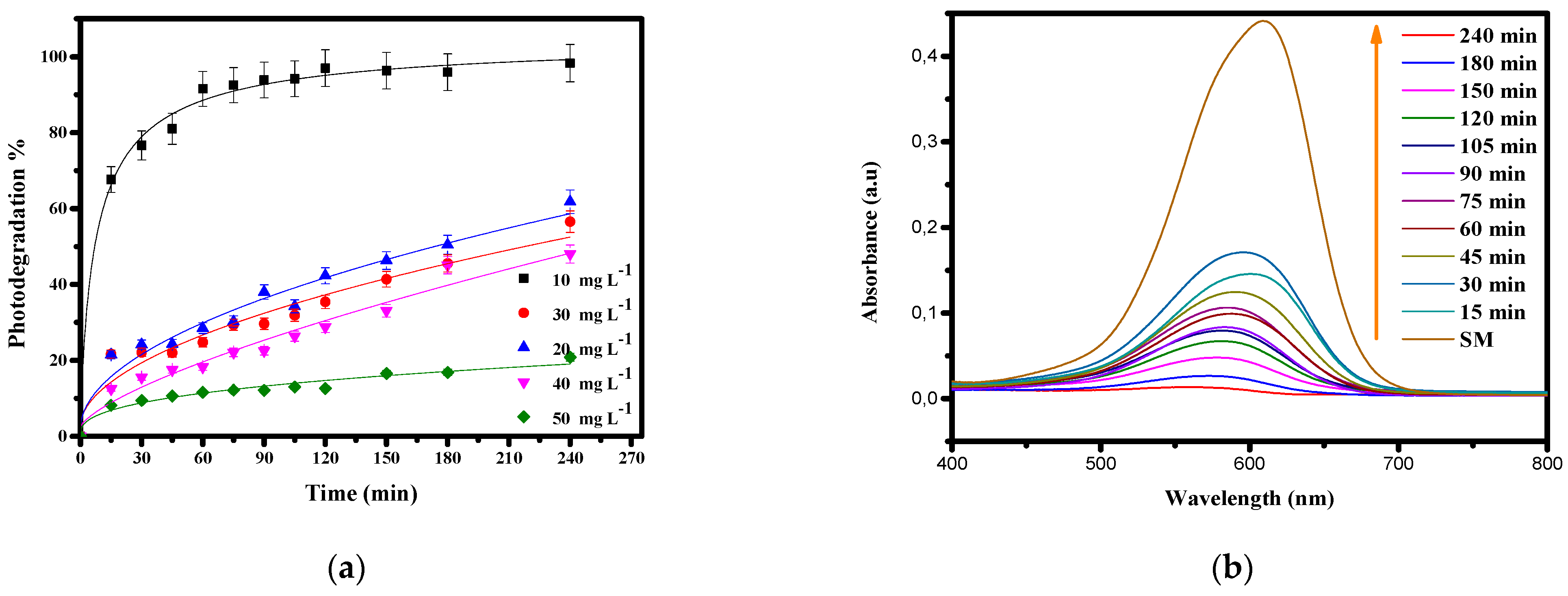
3.3.4. Influence of the Initial Concentration of Basic Blue 41
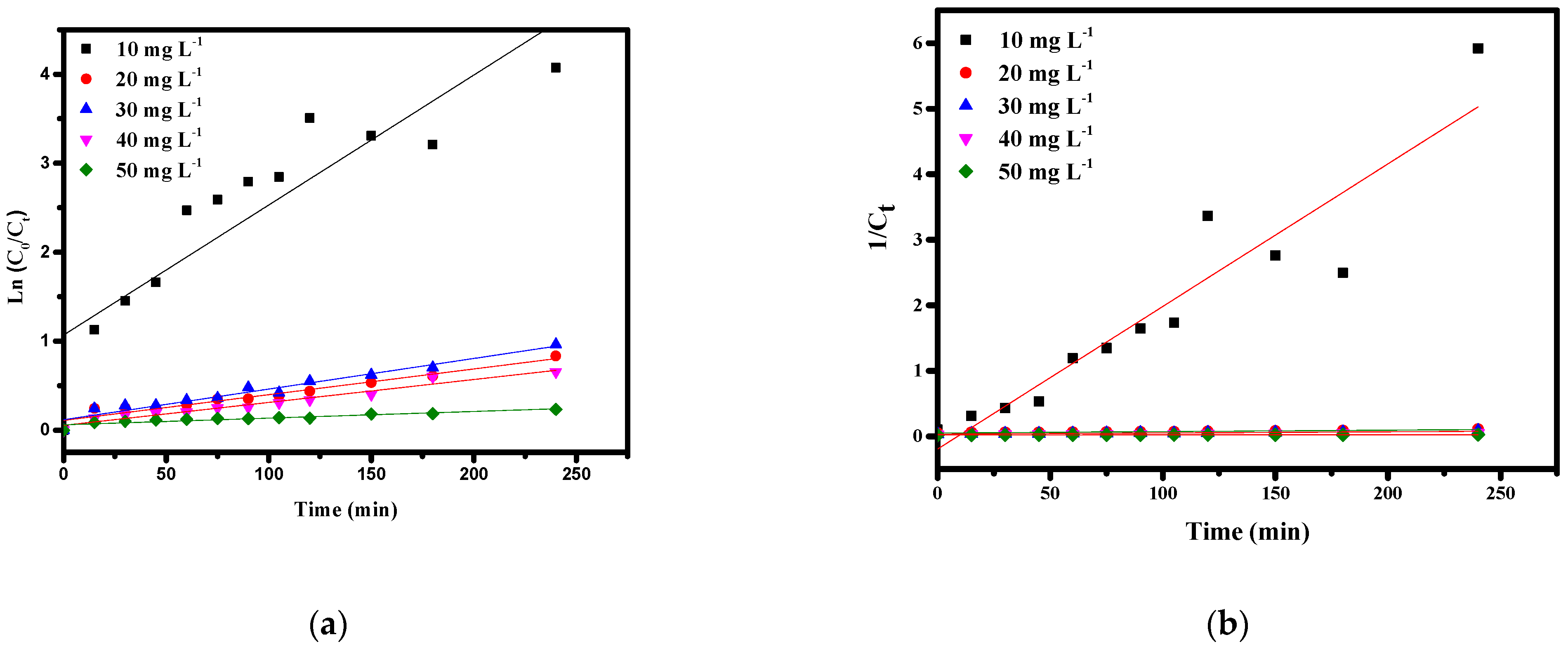
3.4. Photocatalytic Degradation Kinetics
3.5. Analysis of Dye Mineralization
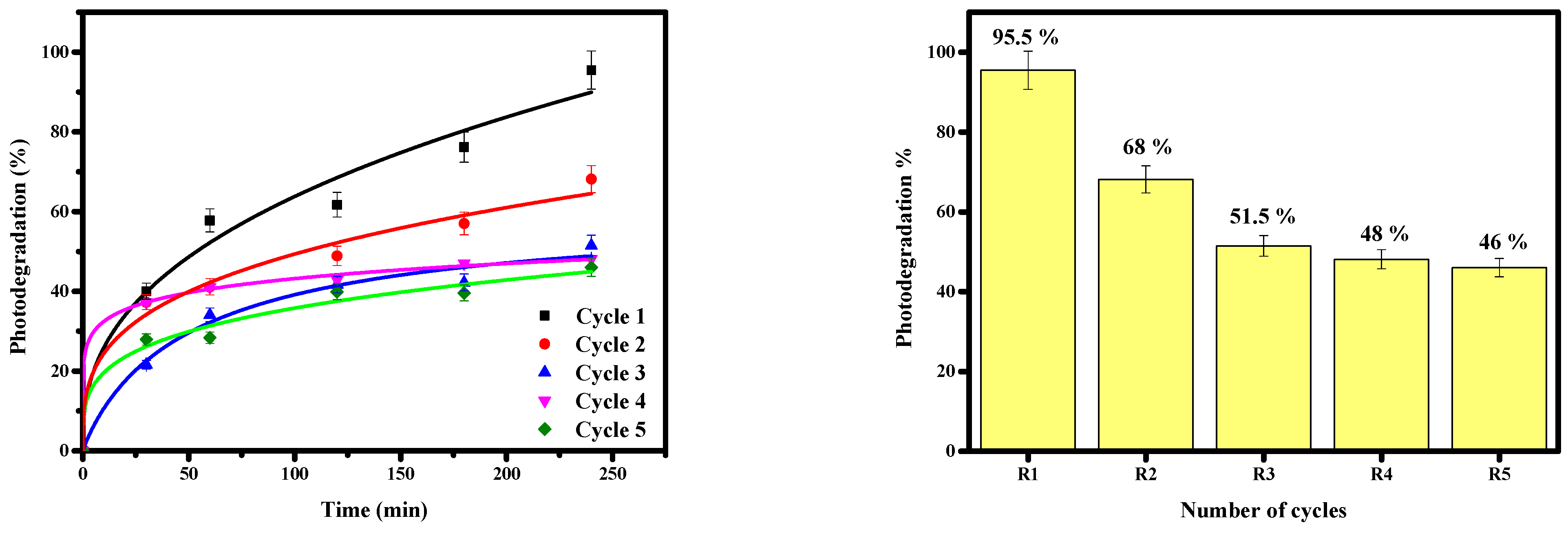
3.6. Study of the Regeneration of the Bi12ZnO20/AgI Heterosystem
3.7. Comparison of BB41 Photodegradation with Previous Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BB41 | Basic Blue 41 |
| BZO | Bi12ZnO20 |
| TOC | total organic carbon |
| XRD | X-ray diffraction |
| SEM | scanning electron microscopy |
| pHPZC | point of zero charge |
| Eg | optical band gap energy |
References
- Madji, S.; Mekatel, E.; Belmedani, M.; Cosme, J.; Zouaoui, S.; Vieillard, J. Synthesis of an Original Bi12CoO20/ZnO Nanocomposites for Lead Adsorption. Mater. Sci. Eng. B 2025, 319, 118345. [Google Scholar] [CrossRef]
- Arif, A.; Malik, M.F.; Liaqat, S.; Asifa, A.; Mumtaz, K.; Afzal, A.; Ch, D.M.; Nisa, K.; Khurshid, F.; Arif, F.; et al. Water pollution and industries. Pure Appl. Biol. 2020, 9, 2214–2224. [Google Scholar] [CrossRef]
- Chang Chien, J.-R.; Joshiba Ganesan, J. Advancing Sustainable Approaches for the Removal and Recycling of Toxic Dyes from the Aquatic Environment. In Dye Chemistry-Exploring Colour from Nature to Lab; IntechOpen: London, UK, 2024. [Google Scholar]
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052001. [Google Scholar] [CrossRef]
- Herath, I.S.; Udayanga, D.; Jayasanka, D.J.; Hewawasam, C. Textile dye decolorization by white rot fungi—A review. Bioresour. Technol. Rep. 2024, 25, 101687. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of Synthetic Dyes—A Review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Saba, B.; Kjellerup, B.V.; Christy, A.D. Eco-friendly bio-electro-degradation of textile dyes wastewater. Bioresour. Technol. Rep. 2021, 15, 100734. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Anaerobic Membrane Bioreactor (AnMBR) for the Removal of Dyes from Water and Wastewater: Progress, Challenges, and Future Perspectives. Processes 2023, 11, 855. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Bao, J.; Wang, X.; Yu, W.; Shao, L. Degradation of Azo Dyes with Different Functional Groups in Simulated Wastewater by Electrocoagulation. Water 2022, 14, 123. [Google Scholar] [CrossRef]
- Lanfredi, S.; Nobre, M.A.L.; Poon, P.S.; Matos, J. Hybrid Material Based on an Amorphous-Carbon Matrix and ZnO/Zn for the Solar Photocatalytic Degradation of Basic Blue 41. Molecules 2019, 25, 96. [Google Scholar] [CrossRef] [PubMed]
- Nouri, L.; Hemidouche, S.; Boudjemaa, A.; Kaouah, F.; Sadaoui, Z.; Bachari, K. Elaboration and characterization of photobiocomposite beads, based on titanium (IV) oxide and sodium alginate biopolymer, for basic blue 41 adsorption/photocatalytic degradation. Int. J. Biol. Macromol. 2020, 151, 66–84. [Google Scholar] [CrossRef]
- Ghosh, S.K. (Ed.) Waste Water Recycling and Management; Springer: Singapore, 2019. [Google Scholar]
- Aziz, F.F.A.; Jalil, A.A.; Triwahyono, S.; Mohamed, M. Controllable structure of fibrous SiO2–ZSM-5 support decorated with TiO2 catalysts for enhanced photodegradation of paracetamol. Appl. Surf. Sci. 2018, 455, 84–95. [Google Scholar] [CrossRef]
- Abdu, M.; Tibebu, S.; Babaee, S.; Worku, A.; Msagati, T.A.M.; Nure, J.F. Optimization of photocatalytic degradation of Eriochrome Black T from aqueous solution using TiO2-biochar composite. Results Eng. 2025, 25, 104036. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Boochakiat, S.; Inceesungvorn, B.; Nattestad, A.; Chen, J. Bismuth-Based Oxide Photocatalysts for Selective Oxidation Transformations of Organic Compounds. ChemNanoMat 2023, 9, e202300140. [Google Scholar] [CrossRef]
- Tho, N.T.M.; Khanh, D.N.N.; Thang, N.Q.; Lee, Y.-I.; Phuong, N.T.K. Novel reduced graphene oxide/ZnBi2O4 hybrid photocatalyst for visible light degradation of 2,4-dichlorophenoxyacetic acid. Environ. Sci. Pollut. Res. 2020, 27, 11127–11137. [Google Scholar] [CrossRef] [PubMed]
- Burkov, V.I.; Gorelik, V.S.; Egorysheva, A.V.; Kargin, Y.F. Laser Raman Spectroscopy of Crystals with the Structure of Sillenite. J. Russ. Laser Res. 2001, 22, 243–267. [Google Scholar] [CrossRef]
- Lima, A.F.; Lalic, M.V. First-principles study of the BiMO4 antisite defect in the Bi12MO20 (M=Si, Ge, Ti) sillenite compounds. J. Phys. Condens. Matter. 2013, 25, 495505. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, J.; Zhang, Y.; Tang, Y. Construction of a novel ZnCo2O4/Bi2O3 heterojunction photocatalyst with enhanced visible light photocatalytic activity. Chin. Chem. Lett. 2019, 30, 735–738. [Google Scholar] [CrossRef]
- Jin, X.; Dasheng, G.; Shuang, C.; Xiaohua, W.; Ningning, L. Enhanced visible light photocatalytic activity of AgI/TiO2 composite fabricated by a grinding method. Water Qual. Res. J. 2019, 54, 257–264. [Google Scholar] [CrossRef]
- Madji, S.; Belmedani, M.; Mekatel, E.; Trari, M. Co-precipitation synthesis and characterization of the sillenite Bi12CoO20: Application to photoactivity for water treatment on the heterojunction with ZnO. J. Mater. Sci. Mater. Electron. 2023, 34, 2093. [Google Scholar] [CrossRef]
- Ferro-García, M.A.; Rivera-Utrilla, J.; Bautista-Toledo, I.; Moreno-Castilla, C. Adsorption of Humic Substances on Activated Carbon from Aqueous Solutions and Their Effect on the Removal of Cr(III) Ions. Langmuir 1998, 14, 1880–1886. [Google Scholar] [CrossRef]
- Nicholas, P. Cheremisinoff. 2—Thermal Analysis. In Polymer Characterization: Laboratory Techniques and Analysis; Noyes Publications: Park Ridge, NJ, USA, 1996; pp. 17–24. [Google Scholar] [CrossRef]
- Zhu, N.; Qian, F.; Xu, X.; Wang, M.; Teng, Q. Thermogravimetric Experiment of Urea at Constant Temperatures. Materials 2021, 14, 6190. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Kenfoud, H.; Algethami, F.; Modwi, A.; Guesmi, A.; Assadi, A.A.; Khezami, L. Application of Bi12ZnO20 Sillenite as an Efficient Photocatalyst for Wastewater Treatment: Removal of Both Organic and Inorganic Compounds. Materials 2021, 14, 5409. [Google Scholar] [CrossRef]
- Wang, X.-L.; Xiao, Y.; Lv, Z.-J.; Yu, H.; Yang, Y.; Dong, X.-T. A novel 2D nanosheets self-assembly camellia-like ordered mesoporous Bi12ZnO20 catalyst with excellent photocatalytic property. J. Alloys Compd. 2020, 835, 155409. [Google Scholar] [CrossRef]
- Jagadeesh, C.; Sailaja, B.B.V. Photocatalytic Degradation of Rohdamine B Dye using CoBi2O4 Nanocatalyst and Effect of Various Operational Parameters. Cikitusi J. 2019, 6, 97–107. [Google Scholar]
- Baaloudj, O.; Nasrallah, N.; Bouallouche, R.; Kenfoud, H.; Khezami, L.; Assadi, A.A. High efficient Cefixime removal from water by the sillenite Bi12TiO20: Photocatalytic mechanism and degradation pathway. J. Clean. Prod. 2022, 330, 129934. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, G.; Zhang, A.; Tan, Z.; Wang, R.; Wang, H.; Mei, Y.; Zhang, X.; Ran, J. Enhanced photocatalytic activity of Bi24O31Br10 microsheets constructing heterojunction with AgI for Hg0 removal. Sep. Purif. Technol. 2021, 262, 118296. [Google Scholar] [CrossRef]
- Hemmi, A.; Belmedani, M.; Mekatel, E.; Djaballah, A.M.; Sadaoui, Z.; Brahimi, B.; Trari, M. Characterization of MgAl2O4 semiconductor prepared by co-precipitation route: Application as photocatalyst for tetracycline degradation. J. Mater. Sci. Mater. Electron. 2025, 36, 217. [Google Scholar] [CrossRef]
- Manzoor, Q.; Farrukh, M.A.; Sajid, A. Optimization of lead (II) and chromium (VI) adsorption using graphene oxide/ZnO/chitosan nanocomposite by response surface methodology. Appl. Surf. Sci. 2024, 655, 159544. [Google Scholar] [CrossRef]
- Gu, M.; Hao, L.; Wang, Y.; Li, X.; Chen, Y.; Li, W.; Jiang, L. The selective heavy metal ions adsorption of zinc oxide nanoparticles from dental wastewater. Chem. Phys. 2020, 534, 110750. [Google Scholar] [CrossRef]
- Singh, V.; Ahmed, G.; Vedika, S.; Kumar, P.; Chaturvedi, S.K.; Rai, S.N.; Vamanu, E.; Kumar, A. Toxic heavy metal ions contamination in water and their sustainable reduction by eco-friendly methods: Isotherms, thermodynamics and kinetics study. Sci. Rep. 2024, 14, 7595. [Google Scholar] [CrossRef] [PubMed]
- Benrighi, Y.; Nasrallah, N.; Chaabane, T.; Sivasankar, V.; Darchen, A.; Baaloudj, O. Characterization of CoCr2O4 semiconductor: A prominent photocatalyst in the degradation of basic blue 41 from wastewater. Opt. Mater. 2021, 122, 111819. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J. Nanoporous metal-organic framework (MOF-199): Synthesis, characterization and photocatalytic degradation of Basic Blue 41. Microchem. J. 2019, 144, 436–442. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Keshavarzi, S.; Ghezelbash, M. Synthesis of nanoparticle and modelling of its photocatalytic dye degradation ability from colored wastewater. J. Environ. Chem. Eng. 2017, 5, 3684–3689. [Google Scholar] [CrossRef]
- Kalpaklı, Y. The effect of in-situ Pr6O11 phase formation on photocatalytic Performance: Mono azo dye degradation. Inorg. Chem. Commun. 2024, 159, 111788. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Alinia Asli, M.; Vossoughi, M. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B.; Gomaa, E.; Helal, M.H.; Abdelhameed, R.M. Recyclable photocatalyst composites based on Ag3VO4 and Ag2WO4 @MOF@cotton for effective discoloration of dye in visible light. Cellulose 2020, 27, 7139–7155. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Mahajan, S.; Sharma, R.; Stathatos, E. Novel development of nanocrystalline kesterite Cu2ZnSnS4 thin film with high photocatalytic activity under visible light illumination. J. Phys. Chem. Solids 2018, 112, 37–42. [Google Scholar] [CrossRef]
- Kafian, S.; Sadeghzadeh-Attar, A. Photocatalytic degradation of Basic Blue 41 dye under visible light over SrTiO3/Ag3PO4 hetero-nanostructures. Int. J. Appl. Ceram. Technol. 2022, 19, 3347–3357. [Google Scholar] [CrossRef]
- Abhayasimha, K.C.; Rao, C.S.; Nair, V. Combination of ensemble machine learning models in photocatalytic studies using nano TiO2-Ligninbased biochar. Chemosphere 2024, 352, 141326. [Google Scholar] [CrossRef]
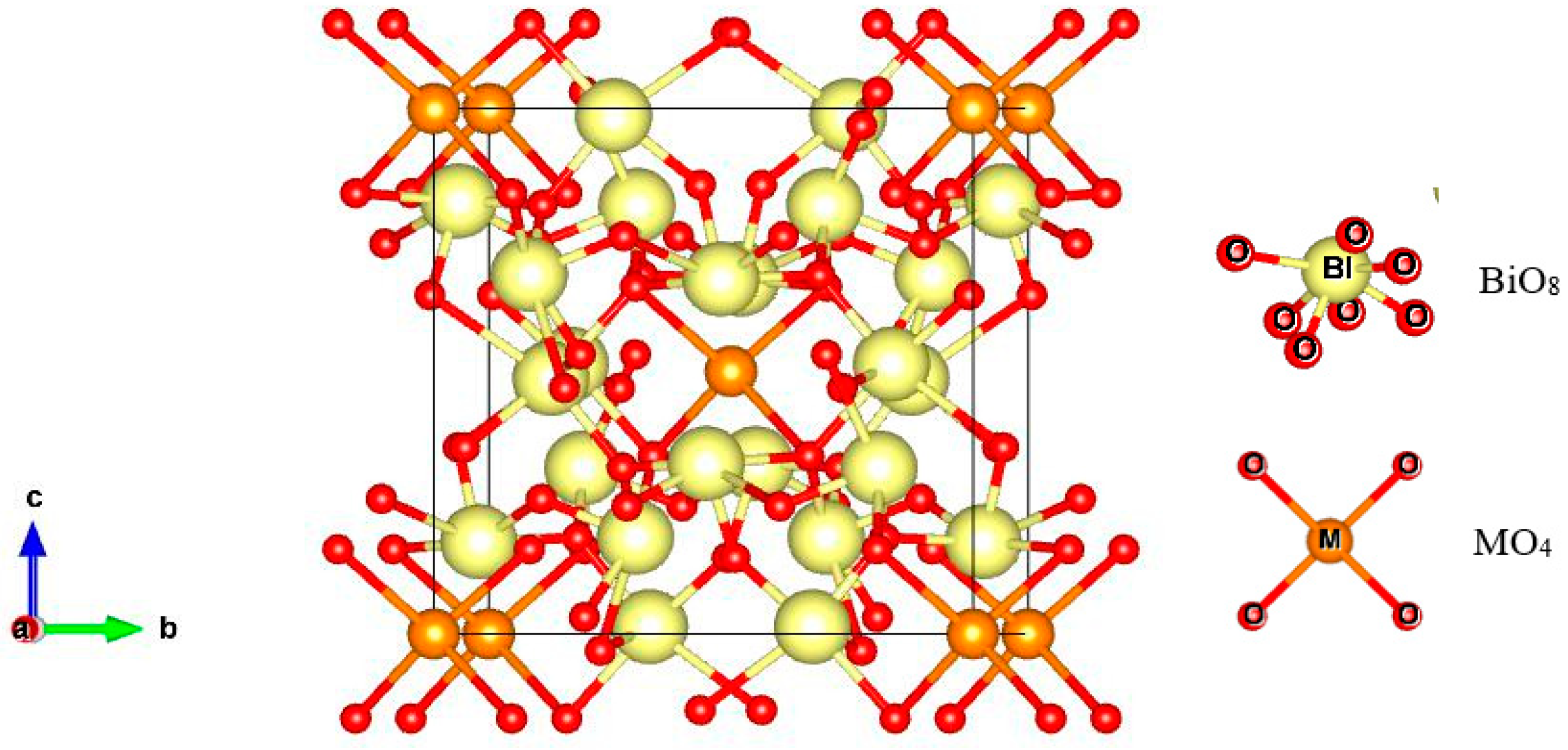
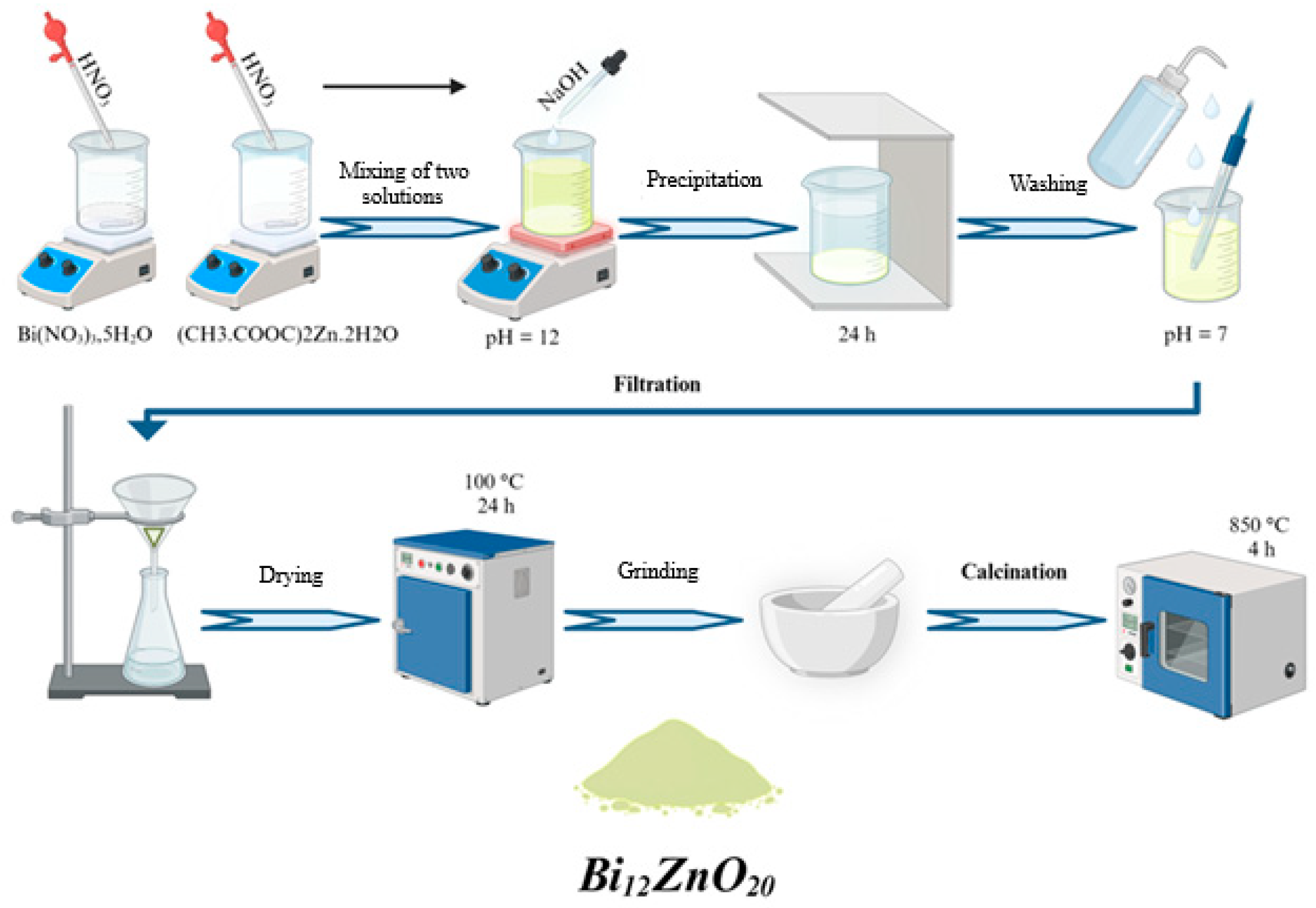
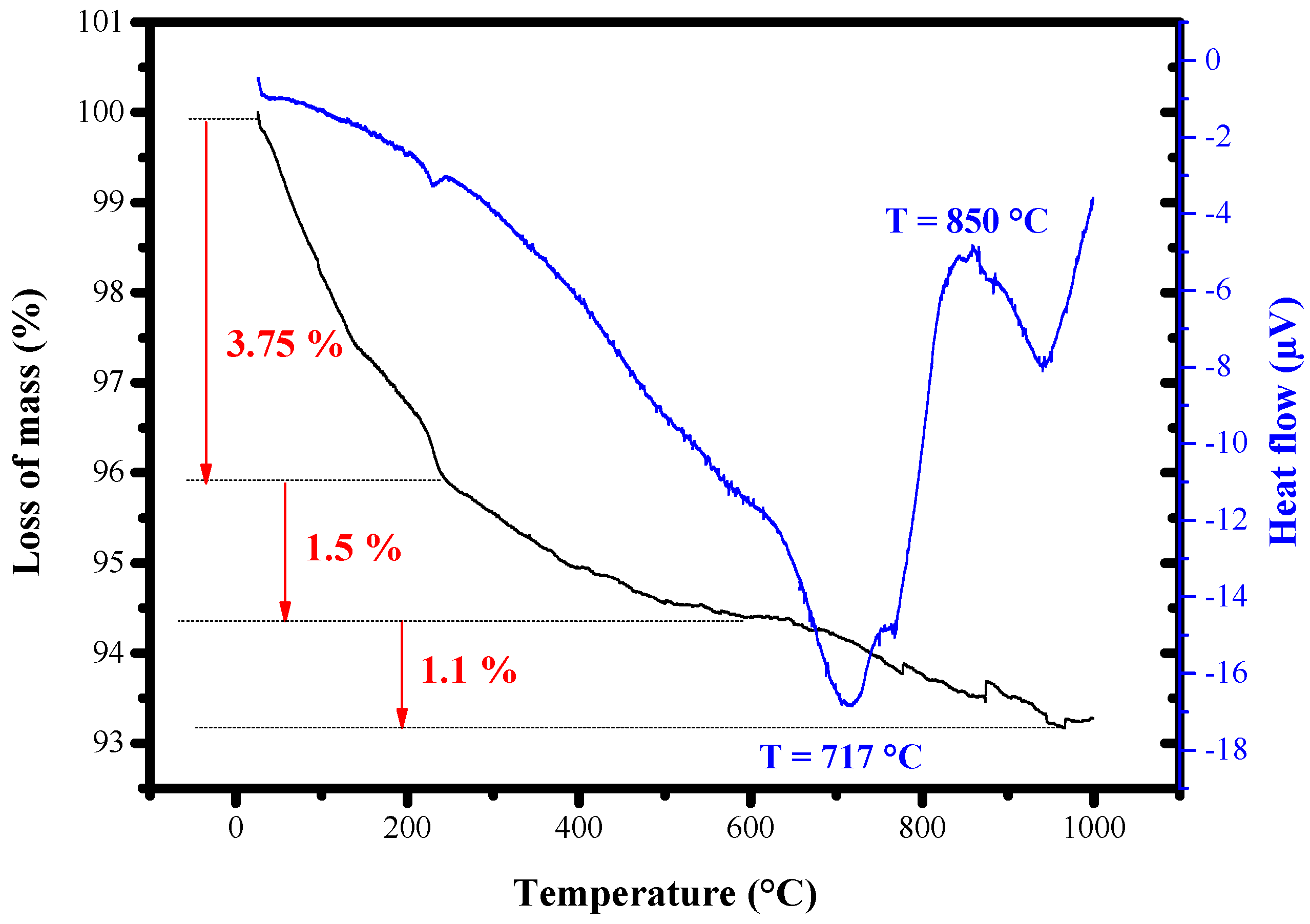
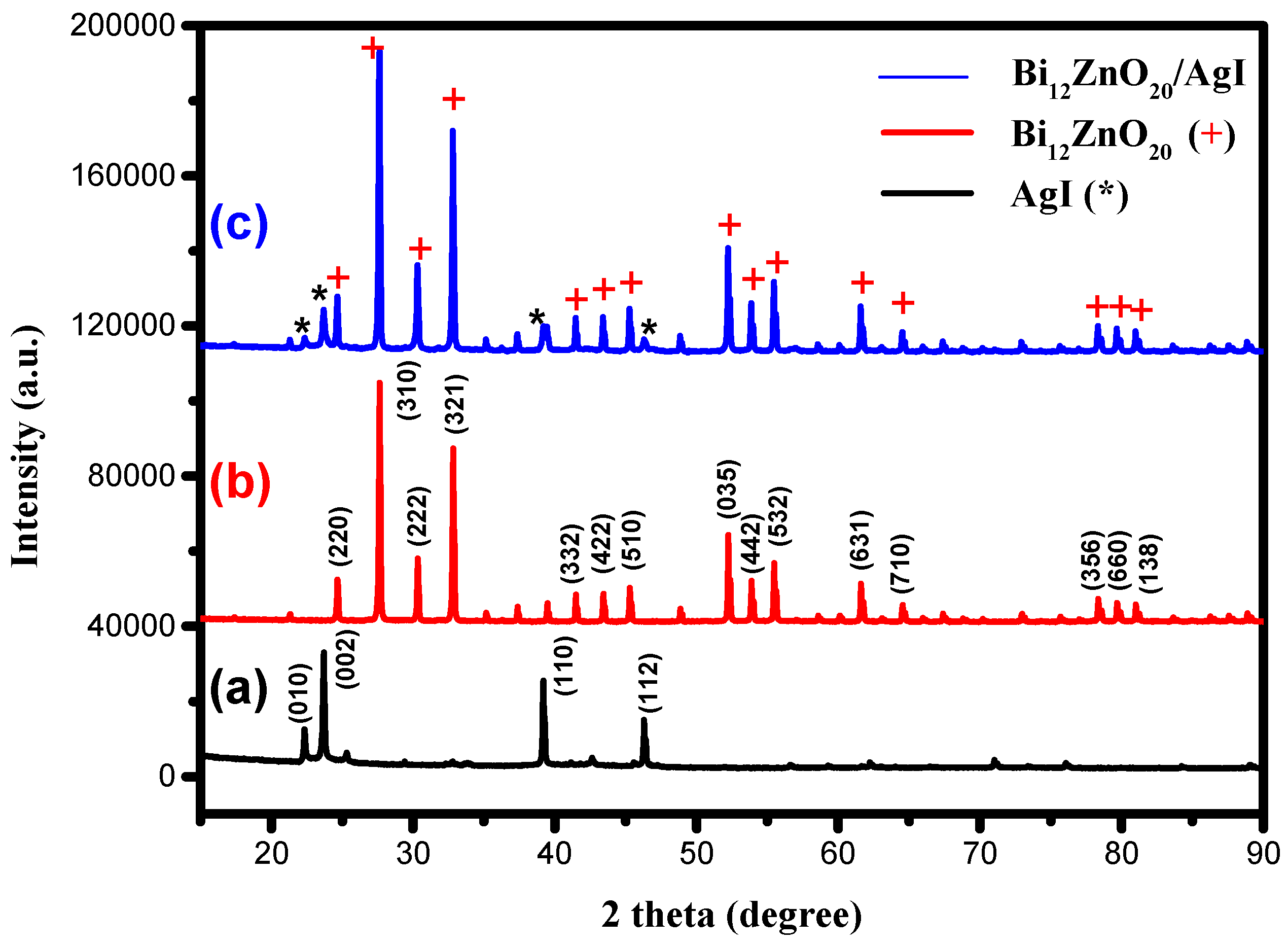
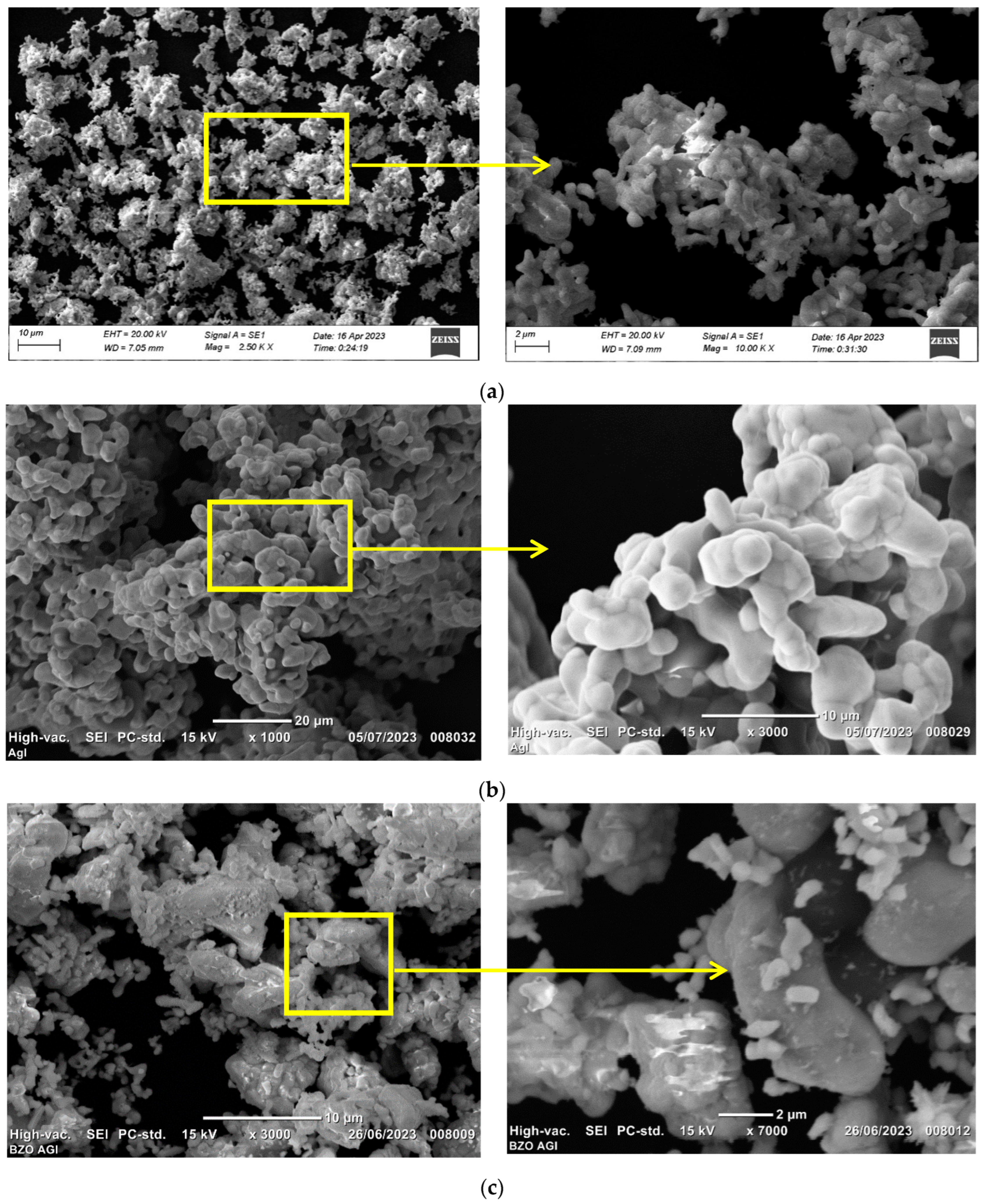





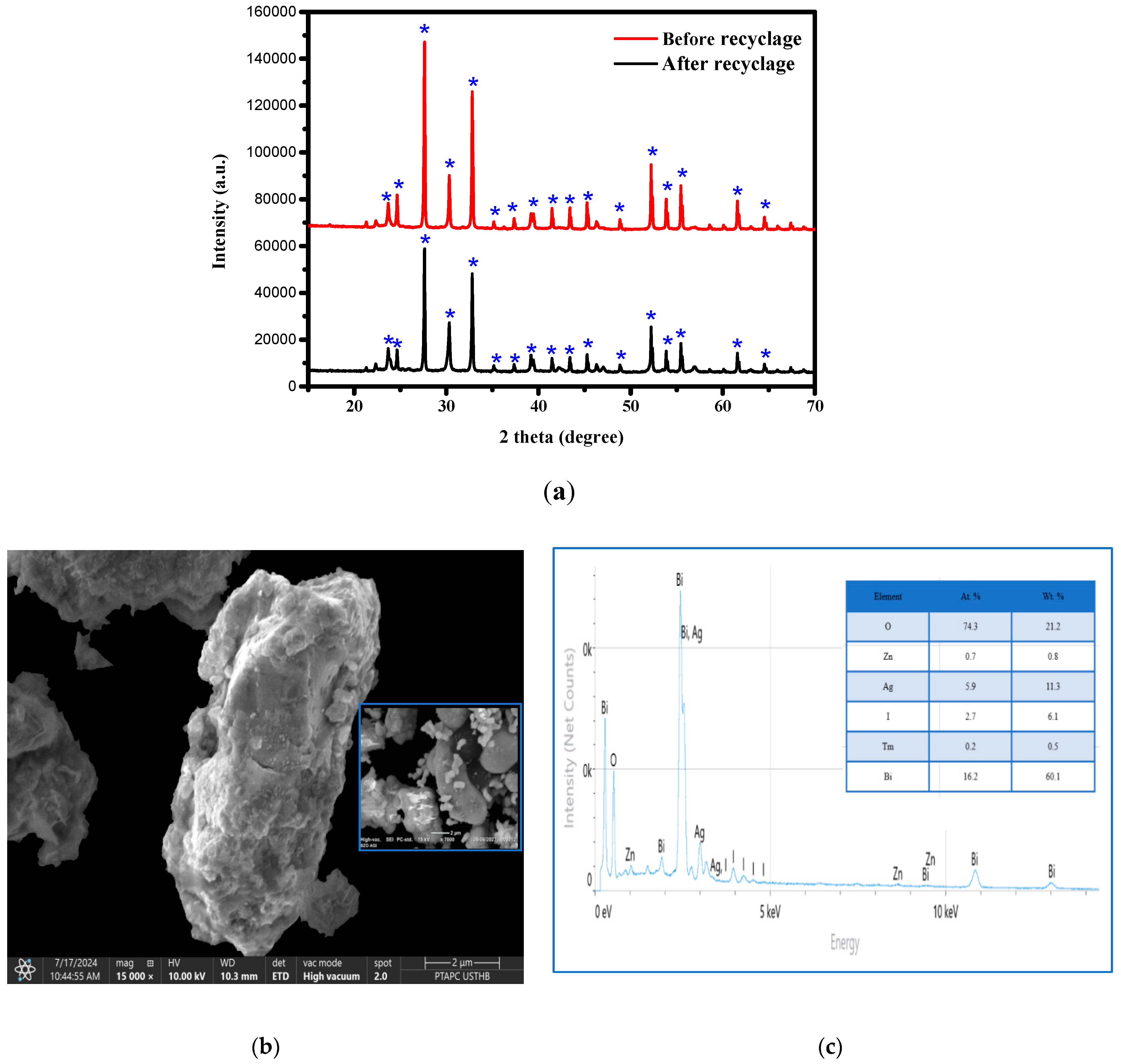
| Peak Position (°2th) | FWHM (°2th) | Crystal Size (nm) | Average Crystal Size (nm) | Density (g cm−3) |
|---|---|---|---|---|
| 24.676 | 0.133 | 61.157 | 53 | 9.03 |
| 27.645 | 0.138 | 59.431 | ||
| 30.344 | 0.141 | 58.559 | ||
| 32.842 | 0.148 | 55.776 | ||
| 52.272 | 0.194 | 45.729 | ||
| 53.907 | 0.194 | 46.011 | ||
| 55.509 | 0.203 | 44.142 |
| Bi12ZnO20 Raman Peaks Observed in This Study | Raman Peaks of Zinc sillenite Bi12.66Zn0.33O19.33 (Ref. [31]) | Raman Peaks of Sil-Lenite Bi12ZnO20 ([26]) | Raman Peaks of BZnO Sillenite ([18]) |
|---|---|---|---|
| 52 | - | 56 | |
| 80 | 78 | 80 | 83 |
| - | 91 | 92 | - |
| 126 | 122 | 123 | 127 |
| 136 | 136 | 137 | 141 |
| 162 | 162 | 162 | 166 |
| - | 208 | - | 209 |
| - | - | 181 | - |
| - | - | 207 | - |
| 254 | 250 | 251 | 257 |
| 307 | 310 | 305 | 311 |
| 380 | 380 | 372 | 377 |
| - | 446 | 444 | 434 |
| 527 | 527 | 527 | 526 |
| 630 | 623 | 666 | 622 |
| C0 (mg L−1) | Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|---|
| R2 | k1(min−1) | R2 | k2 (L mg−1 min−1) | |
| 10 | 0.8408 | 0.0123 | 0.8788 | 0.0218 |
| 20 | 0.9671 | 0.0027 | 0.9443 | 0.0002 |
| 30 | 0.9733 | 0.0032 | 0.9532 | 0.0002 |
| 40 | 0.9554 | 0.0025 | 0.9458 | 0.00009 |
| 50 | 0.9647 | 0.0006 | 0.8765 | 0.00002 |
| C0 (mg L−1) | R2 | kapp (min−1) | t1/2 (min) | r0 (mg L−1 min−1) |
|---|---|---|---|---|
| 10 | 0.8408 | 0.0123 | 56.353 | 0.123 |
| 20 | 0.9671 | 0.0027 | 256.721 | 0.054 |
| 30 | 0.9733 | 0.0032 | 216.608 | 0.096 |
| 40 | 0.9554 | 0.0025 | 277.259 | 0.1 |
| 50 | 0.9647 | 0.0006 | 1155.245 | 0.03 |
| Photocatalyst | Light Source | [BB41] | pH | Dose | Degradation Rate (%) | Reference |
|---|---|---|---|---|---|---|
| (mg L−1) | (g L−1) | |||||
| BZO/AgI | Solar irradiation | 10 | 8 | 0.75 | 98 | This study |
| Cu2ZnSnS4 | 4 fluorescentlamps (4 W) | 10 | free | - | 97.5 | [41] |
| SrTiO3/Ag3PO4 | Irradiation visible | 20 | free | 5 | 99 | [42] |
| C/ZnO/Zn | Lumière UV | 12.5 | free | 0.1 | 96 | [10] |
| TiO2- biochar based on lignin | Lumière UV | 40 | 6.1 | 0.75 | 96.72 | [43] |
| (8 W) | ||||||
| TiO2/CaAlg | Solar irradiation | 30 | 7 | 1 | 96 | [11] |
| CoCr2O4 | Tungsten lamp (200 W) | 10 | 5 | 0.5 | 99 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madji, S.; Belmedani, M.; Mekatel, E.; Zouaoui, S.; Lebouachera, S.E.I. The Development of a New Bi12ZnO20/AgI Heterosystem for the Degradation of Dye-Contaminated Water by Photocatalysis Under Solar Irradiation: Synthesis, Characterization and Kinetics. Processes 2025, 13, 1342. https://doi.org/10.3390/pr13051342
Madji S, Belmedani M, Mekatel E, Zouaoui S, Lebouachera SEI. The Development of a New Bi12ZnO20/AgI Heterosystem for the Degradation of Dye-Contaminated Water by Photocatalysis Under Solar Irradiation: Synthesis, Characterization and Kinetics. Processes. 2025; 13(5):1342. https://doi.org/10.3390/pr13051342
Chicago/Turabian StyleMadji, Serine, Mohamed Belmedani, Elhadj Mekatel, Sarra Zouaoui, and Seif El Islam Lebouachera. 2025. "The Development of a New Bi12ZnO20/AgI Heterosystem for the Degradation of Dye-Contaminated Water by Photocatalysis Under Solar Irradiation: Synthesis, Characterization and Kinetics" Processes 13, no. 5: 1342. https://doi.org/10.3390/pr13051342
APA StyleMadji, S., Belmedani, M., Mekatel, E., Zouaoui, S., & Lebouachera, S. E. I. (2025). The Development of a New Bi12ZnO20/AgI Heterosystem for the Degradation of Dye-Contaminated Water by Photocatalysis Under Solar Irradiation: Synthesis, Characterization and Kinetics. Processes, 13(5), 1342. https://doi.org/10.3390/pr13051342







