Carbonisation of Quercus spp. Wood: Temperature, Yield and Energy Characteristics
Abstract
1. Introduction
2. Material and Methods
2.1. Wood Origin and Characterisation
2.2. Carbonisation Conditions
2.3. Charcoal Yields
2.4. Density of Charcoal
2.5. Proximate Analysis
2.6. Energy Properties of Charcoal
2.7. Statistical Analysis
3. Results
3.1. Charcoal Yield
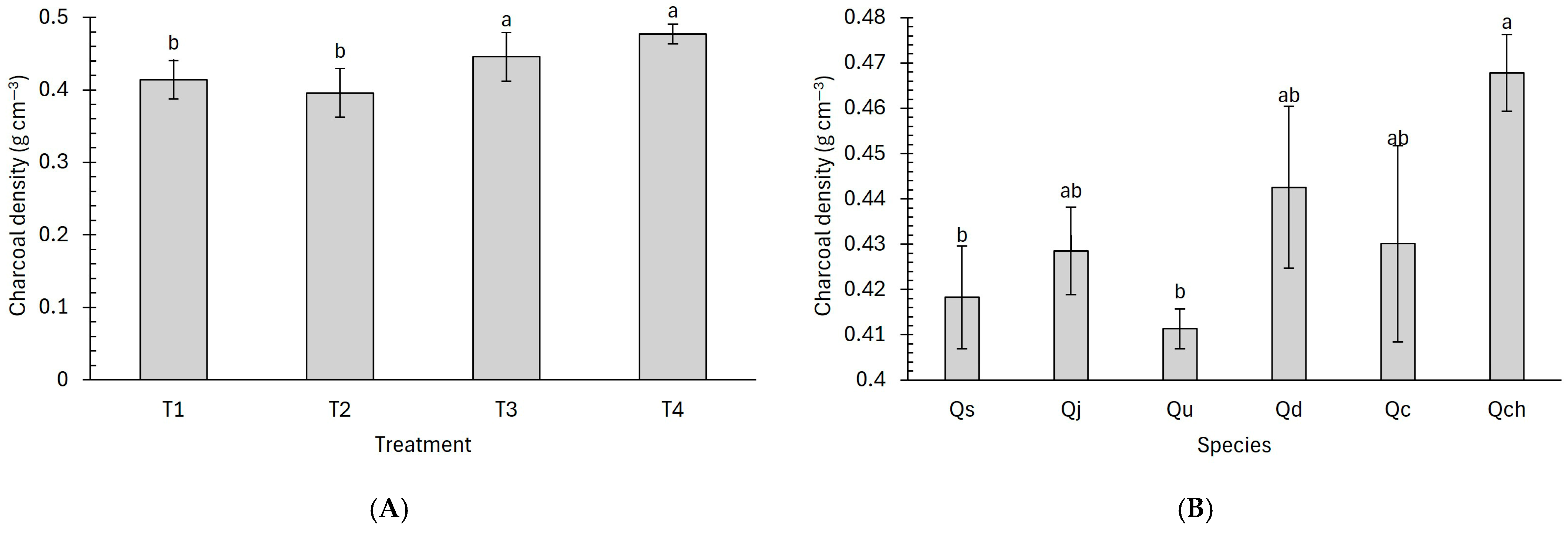
3.2. Density of Charcoal
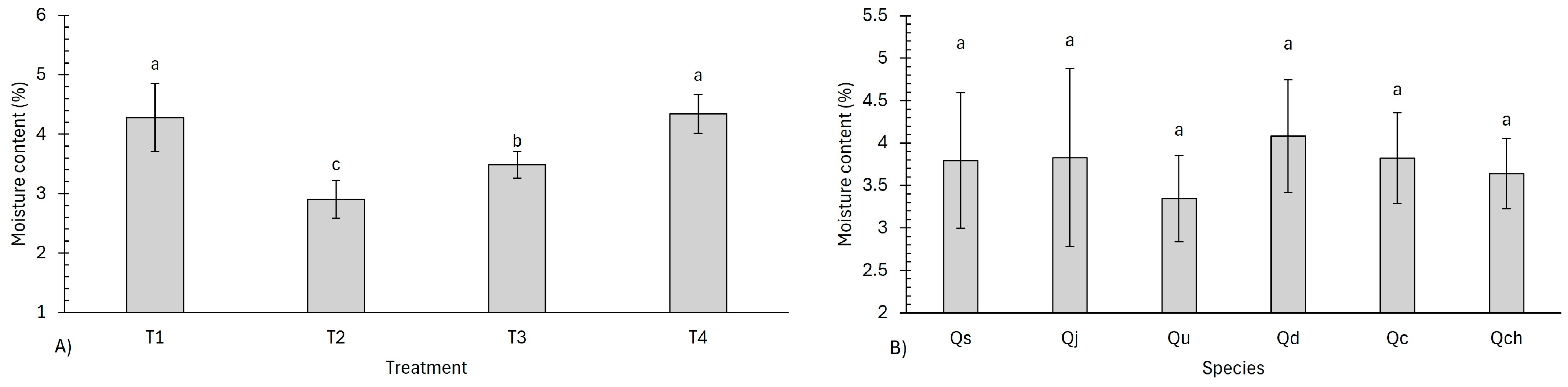
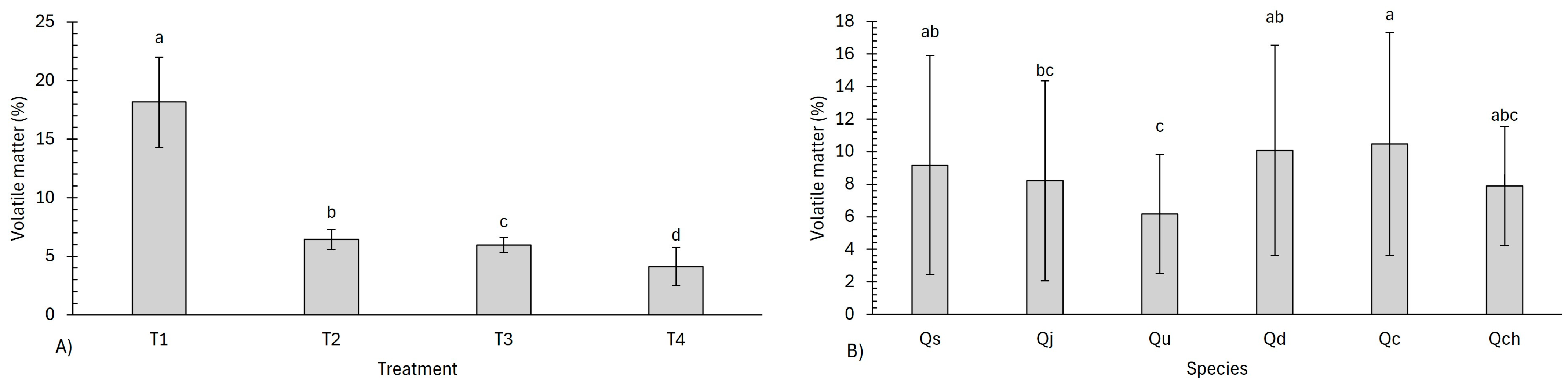
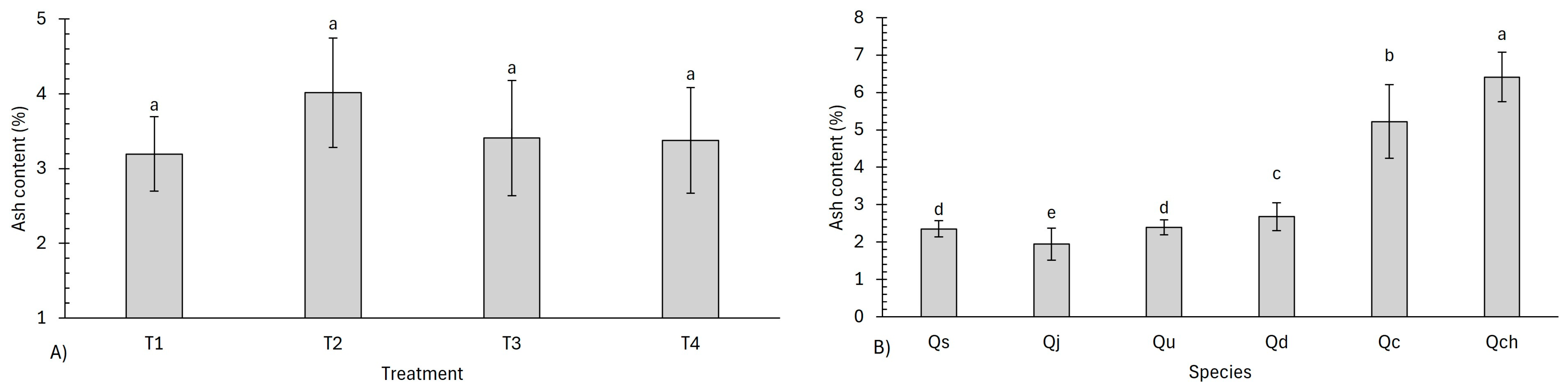
3.3. Proximate Analysis
3.4. Energy Properties of Charcoal
4. Discussion
4.1. Charcoal Yield
4.2. Density of Charcoal
4.3. Proximate Analysis
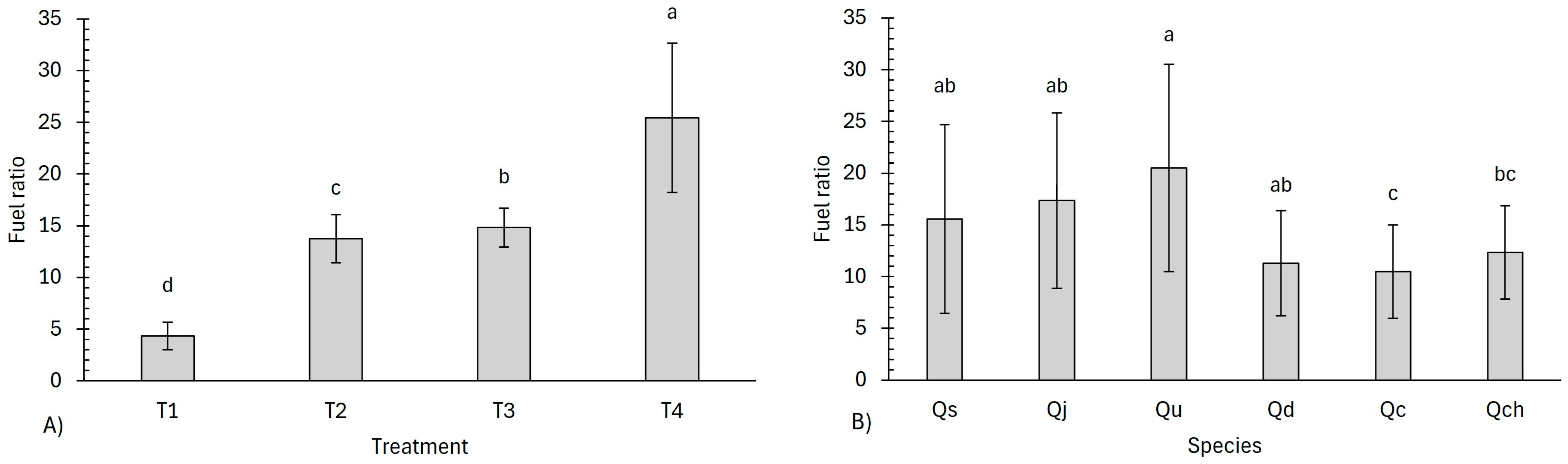
4.4. Energy Properties
5. Conclusions
- Carbonisation temperature significantly affects charcoal yield; lower temperatures (T1, 550 °C for 30 min) maximise mass yield, but at the expense of energy properties, while higher temperatures (T4, 300 °C for 2 h and then 1000 °C for 10 min) produce denser charcoal with better calorific value but a lower mass yield;
- Q. urbanii and Q. convallata showed the highest mass yields, making them more efficient for charcoal production;
- High density does not always relate to superior energetic properties, as seen in Q. chihuahuensis under T4 treatment;
- Volatile matter, moisture and ash content are sensitive to carbonisation conditions; higher temperatures reduce volatile matter, improving energy efficiency;
- For high energy efficiency and calorific value, high-temperature treatments like T4 are recommended, especially for species like Q. urbanii;
- For applications prioritising mass yield, Q. urbanii and Q. convallata at lower temperatures (T1) are preferable as they optimise yield without greatly reducing density.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Hou, F.; Le, H.P. The Impact of Natural Resources, Energy Consumption, and Population Growth on Environmental Quality: Fresh Evidence from the United States of America. Sci. Total Environ. 2021, 754, 142222. [Google Scholar] [CrossRef] [PubMed]
- Rehman, E.; Rehman, S. Modeling the Nexus between Carbon Emissions, Urbanization, Population Growth, Energy Consumption, and Economic Development in Asia: Evidence from Grey Relational Analysis. Energy Rep. 2022, 8, 5430–5442. [Google Scholar] [CrossRef]
- Tshikovhi, A.; Motaung, T.E. Technologies and Innovations for Biomass Energy Production. Sustainability 2023, 15, 12121. [Google Scholar] [CrossRef]
- Kant Bhatia, S.; Palai, A.K.; Kumar, A.; Kant Bhatia, R.; Kumar Patel, A.; Kumar Thakur, V.; Yang, Y.H. Trends in Renewable Energy Production Employing Biomass-Based Biochar. Bioresour. Technol. 2021, 340, 125644. [Google Scholar] [CrossRef] [PubMed]
- Belousov, A.; Lushpeev, V.; Sokolov, A.; Sultanbekov, R.; Tyan, Y.; Ovchinnikov, E.; Shvets, A.; Bushuev, V.; Islamov, S. Experimental Research of the Possibility of Applying the Hartmann–Sprenger Effect to Regulate the Pressure of Natural Gas in Non-Stationary Conditions. Processes 2025, 13, 1189. [Google Scholar] [CrossRef]
- Bondarenko, A.V.; Islamov, S.R.; Ignatyev, K.V.; Mardashov, D.V. Laboratory Studies of Polymer Compositions for Well-Kill under Increased Fracturing. Perm. J. Pet. Min. Eng. 2020, 20, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Mi, B.; Liu, Z.; Hu, W.; Wei, P.; Jiang, Z.; Fei, B. Investigating Pyrolysis and Combustion Characteristics of Torrefied Bamboo, Torrefied Wood and Their Blends. Bioresour. Technol. 2016, 209, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass Applications in Iron and Steel Industry: An Overview of Challenges and Opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Akubuenyi, F.C.; Odokuma, L.O. Biogas Production from Domestic Waste and Its Purification with Charcoal. Pac. J. Sci. Technol. 2013, 14, 63–69. [Google Scholar]
- Shah, S.S.; Ahmad, I.; Ahmad, W.; Ishaq, M.; Khan, H. Deep Desulphurization Study of Liquid Fuels Using Acid Treated Activated Charcoal as Adsorbent. Energy Fuels 2017, 31, 7867–7873. [Google Scholar] [CrossRef]
- Budianto, A.; Kusdarini, E.; Effendi, S.S.W.; Aziz, M. The Production of Activated Carbon from Indonesian Mangrove Charcoal. IOP Conf. Ser. Mater. Sci. Eng. 2019, 462, 012006. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Dufourny, A.; Van De Steene, L.; Humbert, G.; Guibal, D.; Martin, L.; Blin, J. Influence of Pyrolysis Conditions and the Nature of the Wood on the Quality of Charcoal as a Reducing Agent. J. Anal. Appl. Pyrolysis 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Khaeso, K.; Sukhuna, B.; Katekaew, S.; Junsiri, C.; Monatrakul, W.; Srichat, A.; Senawong, K.; Laloon, K. Sustainable Conversion of Agricultural Waste into Solid Fuel (Charcoal) via Gasification and Pyrolysis Treatment. Energy Convers. Manag. X 2024, 24. [Google Scholar] [CrossRef]
- Trejo-Zamudio, D.; García-Trejo, F.; Antonio-Gutiérrez, C. Conversión de Residuos a Biocombustibles. Ciencia 2019, 70, 64–71. [Google Scholar]
- Ronsse, F.; Nachenius, R.W.; Prins, W. Carbonization of Biomass. In Recent Advances in Thermochemical Conversion of Biomass; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 293–324. ISBN 9780444632906. [Google Scholar]
- Chia, C.H.; Joseph, S.D.; Rawal, A.; Linser, R.; Hook, J.M.; Munroe, P. Microstructural Characterization of White Charcoal. J. Anal. Appl. Pyrolysis 2014, 109, 215–221. [Google Scholar] [CrossRef]
- Pereira, B.L.C.; Oliveira, A.C.; Carvalho, A.M.M.L.; de Carneiro, A.C.O.; Santos, L.C.; Vital, B.R. Quality of Wood and Charcoal from Eucalyptus Clones for Ironmaster Use. Int. J. For. Res. 2012, 2012, 523025. [Google Scholar] [CrossRef]
- Dias-Junior, A.F.; Esteves, R.P.; da Silva, Á.M.; Sousa-Júnior, A.D.; Oliveira, M.P.; Brito, J.O.; Napoli, A.; Braga, B.M. Investigating the Pyrolysis Temperature to Define the Use of Charcoal. Eur. J. Wood Wood Prod. 2020, 78, 193–204. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Kurniawan, E.W.; Amirta, R.; Budiarso, E.; Arung, E.T. Mixing of Acacia Bark and Palm Shells to Increase Caloric Value of Palm Shells White Charcoal Briquette. AIP Conf. Proc. 2017, 1854, 020021. [Google Scholar] [CrossRef]
- Pijarn, N.; Intaraprasert, J.; Ophap, S.; Uma, T.; Deekarnkol, S.; Bowornkietkaew, W. Microstructural Characterization of White Charcoal for Rapid Reduction of Chemical Oxygen Demand and Automatically Adjust PH to Neutral in Wastewater Treatment. J. Mater. Res. Technol. 2021, 13, 336–345. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Yang, J.; Gao, Q.; Ni, L.; Hou, Y.; He, Y.; Liu, Z. Combustion Behaviors of Molded Bamboo Charcoal: Influence of Pyrolysis Temperatures. Energy 2021, 226, 120253. [Google Scholar] [CrossRef]
- UNE-EN 14780; Biocombustibles Sólidos. Preparación de Muestras. AENOR: Madrid, Spain, 2012.
- UNE-EN 14774-3; Biocombustibles Sólidos. Métodos para la Determinación del Contenido de Humedad. Método de Secado en Estufa. Parte 3: Humedad en la Muestra de Análisis General. AENOR: Madrid, España, 2010.
- UNE-EN 15148; Biocombustibles Sólidos. Determinación del Contenido de Materias Volátiles. AENOR: Madrid, España, 2010.
- UNE-EN 14775; Biocombustibles Sólidos. Método para la Determinación del Contenido en Cenizas. AENOR: Madrid, España, 2010.
- Yin, C.-Y. Prediction of Higher Heating Values of Biomass from proximate and Ultimate Analyses. Fuel 2011, 90, 1128–1132. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- Cordero, T.; Marquez, F.; Rodriguez-Mirasol, J.; Rodriguez, J.J. Predicting Heating Values of Lignocellulosics and Carbonaceous Materials from Proximate Analysis. Fuel 2001, 80, 1567–1571. [Google Scholar] [CrossRef]
- Canal, W.D.; Carvalho, A.M.M.; Figueiró, C.G.; de Cássia Oliveira Carneiro, A.; de Freitas Fialho, L.; Donato, D.B. Impact of Wood Moisture in Charcoal Production and Quality. Floresta Ambiente 2020, 27, e20170999. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of Solid Fuel Biochar from Waste Biomass by Low Temperature Pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Charvet, F.; Silva, F.; Ruivo, L.; Tarelho, L.; Matos, A.; da Silva, J.F.; Neves, D. Pyrolysis Characteristics of Undervalued Wood Varieties in the Portuguese Charcoal Sector. Energies 2021, 14, 2537. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and Characterization of Slow Pyrolysis Biochar: Influence of Feedstock Type and Pyrolysis Conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Massuque, J.; De Assis, M.R.; Loureiro, B.A.; Matavel, C.E.; Trugilho, P.F. Influence of Lignin on Wood Carbonization and Charcoal Properties of Miombo Woodland Native Species. Eur. J. Wood Wood Prod. 2021, 79, 527–535. [Google Scholar] [CrossRef]
- Elyounssi, K.; Collard, F.X.; Mateke, J.A.N.; Blin, J. Improvement of Charcoal Yield by Two-Step Pyrolysis on Eucalyptus Wood: A Thermogravimetric Study. Fuel 2012, 96, 161–167. [Google Scholar] [CrossRef]
- Wang, L.; Skreiberg, Ø.; Van Wesenbeeck, S.; Grønli, M.; Antal, M.J. Experimental Study on Charcoal Production from Woody Biomass. Energy Fuels 2016, 30, 7994–8008. [Google Scholar] [CrossRef]
- García-Quezada, J.; Musule-Lagunes, R.; Prieto-Ruíz, J.A.; Vega-Nieva, D.J.; Carrillo-Parra, A. Evaluation of Four Types of Kilns Used to Produce Charcoal from Several Tree Species in Mexico. Energies 2023, 16, 333. [Google Scholar] [CrossRef]
- Bustamante-García, V.; Carrillo-Parra, A.; González-Rodríguez, H.; Ramírez-Lozano, R.G.; Corral-Rivas, J.J.; Garza-Ocañas, F. Evaluation of a Charcoal Production Process from Forest Residues of Quercus Sideroxyla Humb., & Bonpl. in a Brazilian Beehive Kiln. Ind. Crops Prod. 2013, 42, 169–174. [Google Scholar] [CrossRef]
- Somerville, M.; Jahanshahi, S. The Effect of Temperature and Compression during Pyrolysis on the Density of Charcoal Made from Australian Eucalypt Wood. Renew. Energy 2015, 80, 471–478. [Google Scholar] [CrossRef]
- Lima, M.D.R.; Massuque, J.; Bufalino, L.; Trugilho, P.F.; Ramalho, F.M.G.; de Protásio, T.P.; Hein, P.R.G. Clarifying the Carbonization Temperature Effects on the Production and Apparent Density of Charcoal Derived from Amazonia Wood Wastes. J. Anal. Appl. Pyrolysis 2022, 166. [Google Scholar] [CrossRef]
- de Abreu Neto, R.; de Assis, A.A.; Ballarin, A.W.; Hein, P.R.G. Effect of Final Temperature on Charcoal Stiffness and Its Correlation with Wood Density and Hardness. SN Appl. Sci. 2020, 2, 1020. [Google Scholar] [CrossRef]
- Trugilho, P.F.; da Silva, D.A. Influence of final carbonization temperature in the physical and chemical characteristics of the jatobá (Himenea Courbaril L.) charcoal. Sci. Agrar. 2001, 2, 45–53. [Google Scholar] [CrossRef]
- Miki, M.; Kikuchi, T.; Nakamura, M.; Hatakeyama, K.; Takada, J. Electromagnetic Wave Absorption Characteristics of Bincho-Charcoal and Bamboo Charcoal. Trans. Mater. Res. Soc. Jpn. 2003, 28, 1053–1057. [Google Scholar]
- Kwon, G.J.; Kim, A.R.; Lee, H.S.; Lee, S.H.; Hidayat, W.; Febrianto, F.; Kim, N.H. Characteristics of White Charcoal Produced from the Charcoal Kiln for Thermotherapy. J. Korean Wood Sci. Technol. 2018, 46, 527–540. [Google Scholar] [CrossRef]
- Kim, N.H.; Hanna, R.B. Morphological Characteristics of Quercus Variabilis Charcoal Prepared at Different Temperatures. Wood Sci. Technol. 2006, 40, 392–401. [Google Scholar] [CrossRef]
- Couto, A.M.; Trugilho, P.F.; Napoli, A.; Lima, J.T.; Da Silva, J.R.M.; Protásio, T.D.P. Quality of Charcoal from Corymbia and Eucalyptus Produced at Different Final Carbonization Temperatures. Sci. For. 2015, 43, 817–831. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; Gatto, D.A.; De Lima, E.A. Thermal Analysis of Charcoal from Fast-Growing Eucalypt Wood: Influence of Raw Material Moisture Content. J. Wood Chem. Technol. 2014, 34, 191–201. [Google Scholar] [CrossRef]
- Kwon, S.-M.; Kwon, G.-J.; Jang, J.-H.; Kim, N.-H. Characteristics of Charcoal in Different Carbonization Temperatures. J. For. Environ. Sci. 2012, 28, 263–267. [Google Scholar] [CrossRef][Green Version]
- Manabe, T.; Ohata, M.; Yoshizawa, S.; Nakajima, D.; Goto, S.; Uchida, K.; Yajima, H. Effect of Carbonization Temperature on the Physicochemical Structure of Wood Charcoal. Trans. Mater. Res. Soc. Jpn. 2007, 32, 1035–1038. [Google Scholar] [CrossRef]
- Hu, E.; Zeng, X.; Ma, D.; Wang, F.; Yi, X.; Li, Y.; Fu, X. Effect of the Moisture Content in Coal on the Pyrolysis Behavior in an Indirectly Heated Fixed-Bed Reactor with Internals. Energy Fuels 2017, 31, 1347–1354. [Google Scholar] [CrossRef]
- Zanuncio, A.J.V.; Carvalho, A.G.; Trugilho, P.F.; Monteiro, T.C. Extractive and Energetic Properties of Wood and Charcoal. Rev. Árvore 2014, 38, 369–374. [Google Scholar] [CrossRef]
- Patlán, E.E.S.; Aquino, V.M.; Prado, C.O.; López, M.E.F.; Ortiz, A.O. Análisis de Calidad Del Carbón de Mezquite (Prosopis sp.), Encino (Quercus sp.) y Eucalipto (Eucalyptus sp.). Braz. J. Anim. Environ. Res. 2024, 7, e75544. [Google Scholar] [CrossRef]
- Briseño-Uribe, K.C.; Carrillo-Parra, A.; Bustamante-García, V.; González-Rodríguez, H.; Foroughbachk, R. Firewood Production, Yield and Quality of Charcoal from Eucalyptus Camaldulensis and E. Microtheca Planted in the Semiarid Land of Northeast Mexico. Int. J. Green. Energy 2015, 12, 961–969. [Google Scholar] [CrossRef]
- Carrillo-Parra, A.; Foroughbakhch-Pournavab, R.; Bustamante-García, V. Calidad del carbón de Prosopis Laevigata (Humb. & Bonpl. Ex Willd.) M.C. Johnst. y Ebenopsis Ebano (Berland.) Barneby & J.W. Grimes elaborado en horno tipo fosa. Rev. Mex. Cienc. For. 2013, 4, 62–71. [Google Scholar]
- DIN 51749; Grill Charcoal and Grill Charcoal Briquettes–Requirements, Tests. DIN: Berlin, Germany, 1989.
- De la Cruz-Montelongo, C.; Herrera Gamboa, J.; Ortiz Sánchez, I.A.; Ríos Saucedo, J.C.; Rosales Serna, R.; Carrillo-Parra, A. Caracterización Energética Del Carbón Vegetal Producido En El Norte-Centro de México. Madera Y Bosques 2020, 26, e2621971. [Google Scholar] [CrossRef]
- Ruiz-Aquino, F.; Ruiz-Ángel, S.; Santiago-García, W.; Fuente-Carrasco, M.E.; Sotomayor-Castellanos, J.R.; Carrillo-Parra, A. Energy Characteristics of Wood and Charcoal of Selected Tree Species in Mexico. Wood Res. 2019, 1, 71–82. [Google Scholar]
- EN 1860-2; Appliances, Solid Fuels and Firelighters for Barbecueing—Part 2: Barbecue Charcoal and Barbecue Charcoal Briquettes. Requirements and Test Methods. CEN: Brussels, Belgium, 2005.
- Contreras-Trejo, J.C.; Vega-Nieva, D.J.; Heya, M.N.; Prieto-Ruíz, J.A.; Nava-Berúmen, C.A.; Carrillo-Parra, A. Sintering and Fusibility Risks of Pellet Ash from Different Sources at Different Combustion Temperatures. Energies 2022, 15, 5026. [Google Scholar] [CrossRef]
- Kleinhans, U.; Wieland, C.; Frandsen, F.J.; Spliethoff, H. Ash Formation and Deposition in Coal and Biomass Fired Combustion Systems: Progress and Challenges in the Field of Ash Particle Sticking and Rebound Behavior. Prog. Energy Combust. Sci. 2018, 68, 65–168. [Google Scholar] [CrossRef]
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An Overview of Slagging and Fouling Indicators and Their Applicability to Biomass Fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zhao, Y.; Wei, Z.; Li, Y.; Hao, W.; Zhang, Y. The Characteristics of Mineralogy, Morphology and Sintering during Co-Combustion of Zhundong Coal and Oil Shale. RSC Adv. 2017, 7, 51036–51045. [Google Scholar] [CrossRef]
- Wang, L.; Skreiberg, Ø.; Gronli, M.; Specht, G.P.; Antal, M.J. Is Elevated Pressure Required to Achieve a High Fixed-Carbon Yield of Charcoal from Biomass? Part 2: The Importance of Particle Size. Energy Fuels 2013, 27, 2146–2156. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Lam, K.L.; Hui, C.W. Charcoal Production via Multistage Pyrolysis. Chin. J. Chem. Eng. 2012, 20, 455–460. [Google Scholar] [CrossRef]
- NBN-MII-001; Charcoal and Charcoal Briquettes for Domestic Use—Designation, Specifications, Tests. NBN: Brussels, Belgium, 1984.
- Cheng, Z.; Yang, J.; Zhou, L.; Liu, Y.; Wang, Q. Characteristics of Charcoal Combustion and Its Effects on Iron-Ore Sintering Performance. Appl. Energy 2016, 161, 364–374. [Google Scholar] [CrossRef]
- Oke, M.A.; Sonibare, J.A.; Odunlami, O.A.; Elehinafe, F.B.; Onakpohor, A.; Akeredolu, A.F. Proximate Analysis of Some Common Charcoal in Southwestern Nigeria. Results Eng. 2022, 15, 100454. [Google Scholar] [CrossRef]
- Ngangyo-Heya, M.; Pournavab, F.R.; Carrillo-Parra, A.; Serafin, C.U. Bioenergy Potential of Shrub from Native Species of Northeastern Mexico. Int. J. Agric. Policy Res. 2014, 2, 475–483. [Google Scholar] [CrossRef]
- Mencarelli, A.; Cavalli, R.; Greco, R. Variability on the Energy Properties of Charcoal and Charcoal Briquettes for Barbecue. Heliyon 2022, 8, e10052. [Google Scholar] [CrossRef] [PubMed]
- Seboka, Y. Bio-Carbon Opportunities in Eastern & Southern Africa. In Charcoal Production: Opportunities and Barriers for Improving Efficiency and Sustainability; United Nations Development Programme: New York, NY, USA, 2009; Volume 102. [Google Scholar]
- Dias Júnior, A.F.; Andrade, C.R.; Lana, A.Q.; da Silva, Á.M.; Brito, J.O.; Milan, M. Tips on the Variability of BBQ Charcoal Characteristics to Assist Consumers in Product Choice. Eur. J. Wood Wood Prod. 2021, 79, 1017–1026. [Google Scholar] [CrossRef]
- García-Quezada, J.; Musule-Lagunes, R.; Wehenkel, C.; Prieto-Ruíz, J.A.; Núñez-Retana, V.; Carrillo-Parra, A. Effect of Firewood Moisture Content on Quality, Yield, and Economic Gain during Charcoal Production in a Modified Half-Orange Kiln. Fuels 2023, 5, 1–16. [Google Scholar] [CrossRef]
- Ge, S.; Foong, S.Y.; Ma, N.L.; Liew, R.K.; Wan Mahari, W.A.; Xia, C.; Yek, P.N.Y.; Peng, W.; Nam, W.L.; Lim, X.Y.; et al. Vacuum Pyrolysis Incorporating Microwave Heating and Base Mixture Modification: An Integrated Approach to Transform Biowaste into Eco-Friendly Bioenergy Products. Renew. Sustain. Energy Rev. 2020, 127, 109871. [Google Scholar] [CrossRef]



| Code | Species | Proximate Analysis (%) | Compositional Analysis (%) | Basic Density (g cm−3) | Calorific Value (MJ kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | VM | AC | FC | Cel | Hem | Lig | Ext | BD | HHV | ||
| Qs | Q. sideroxyla | 5.46 | 76.89 | 1.10 | 16.55 | 62.28 | 17.64 | 13.24 | 5.74 | 0.63 | 18.82 |
| Qj | Q. jonesii | 5.81 | 77.43 | 0.92 | 15.83 | 57.66 | 18.27 | 13.44 | 9.71 | 0.66 | 18.74 |
| Qu | Q. urbanii | 5.39 | 77.21 | 0.79 | 16.62 | 58.54 | 17.97 | 12.46 | 10.24 | 0.70 | 18.89 |
| Qd | Q. durifolia | 5.02 | 77.33 | 1.02 | 16.62 | 63.61 | 17.72 | 14.94 | 2.71 | 0.63 | 18.92 |
| Qc | Q. convallata | 5.72 | 76.40 | 1.60 | 16.27 | 56.04 | 18.94 | 11.87 | 11.55 | 0.64 | 18.65 |
| Qch | Q. chihuahuensis | 6.29 | 75.59 | 1.95 | 16.17 | 58.41 | 18.66 | 12.80 | 8.18 | 0.70 | 18.47 |
| Treatment | Temperature | Time |
|---|---|---|
| T1 | 550 °C | 30 min |
| T2 | 700 °C | 30 min |
| T3 | 550 °C → 700 °C | 30 min → 30 min |
| T4 | 300 °C → 1000 °C | 2 h → 10 min |
| Variable | Formula | Description |
|---|---|---|
| Mass yield | MY = mass yield (%) | |
| Dc = dry mass of charcoal (g) | ||
| Dw = dry mass of wood (g) | ||
| Volumetric yield | VY = volumetric yield (m3 t−1) | |
| Vw = volume of wood used (m3) | ||
| Mc = mass of charcoal produced (t) |
| Variable | Formula | Description |
|---|---|---|
| Moisture content | MC = moisture content (%) | |
| A = grams of air-dry sample used | ||
| Volatile matter | B = grams of sample after drying at 105 °C | |
| VM = volatile matter (%) | ||
| Ash content | C = grams of sample after drying at 950 °C | |
| AC = ash content (%) | ||
| Fixed carbon | D = grams of residue | |
| FC = fixed carbon (%) |
| Variable | Formula | Description |
|---|---|---|
| Higher heating value | HHV = higher heating value (MJ kg−1) | |
| VM = Volatile matter from charcoal (%) | ||
| Energy yield | FC = fixed carbon from charcoal (%) | |
| EY = energy yield (%) | ||
| Fuel ratio | MY = mass yield (%) | |
| FR = fuel ratio |
| Mass Yield (MY) | Shapiro–Wilk Test | Kruskal–Wallis Test | ||
|---|---|---|---|---|
| Statistic | p-Value | Chi-Squared | p-Value | |
| Treatments | 43.568 | 1.86 × 10−9 | ||
| Species | 0.917 | 1.58 × 10−4 | 11.658 | 0.03 |
| Treatment–species interaction | 64.962 | 7.10 × 10−6 | ||
| Volumetric Yield (VY) | ||||
| Treatments | 24.587 | 1.88 × 10−5 | ||
| Species | 0.953 | 9.52 × 10−3 | 25.77 | 9.88 × 10−5 |
| Treatment–species interaction | 57.888 | 7.67 × 10−5 | ||
| Code | Treatment | Mass Yield (%) | Volumetric Yield (m3 t−1) |
|---|---|---|---|
| Qs | T1 | 28.11 ± 1.34 ab | 5.77 ± 0.22 def |
| Qj | T1 | 28.03 ± 0.64 ab | 5.51 ± 0.24 fg |
| Qu | T1 | 29.22 ± 1.76 a | 4.81 ± 0.59 g |
| Qd | T1 | 28.69 ± 0.68 ab | 5.44 ± 0.12 fg |
| Qc | T1 | 30.10 ± 0.95 a | 5.17 ± 0.18 g |
| Qch | T1 | 26.86 ± 0.47 bc | 5.32 ± 0.19 fg |
| Qs | T2 | 23.69 ± 0.13 hi | 6.80 ± 0.39 ab |
| Qj | T2 | 23.39 ± 0.50 hi | 6.41 ± 0.17 abc |
| Qu | T2 | 25.90 ± 1.57 cde | 5.56 ± 0.75 fg |
| Qd | T2 | 22.66 ± 0.11 i | 6.88 ± 0.04 a |
| Qc | T2 | 23.57 ± 0.59 hi | 6.43 ± 0.06 abc |
| Qch | T2 | 24.22 ± 0.42 fg | 5.71 ± 0.11def |
| Qs | T3 | 23.94 ± 0.35 gh | 6.47 ± 0.25 abc |
| Qj | T3 | 23.22 ± 0.09 hi | 6.37 ± 0.12 bc |
| Qu | T3 | 26.78 ± 0.91 bc | 5.31 ± 0.20 fg |
| Qd | T3 | 24.49 ± 0.21 ef | 6.58 ± 0.31 abc |
| Qc | T3 | 25.29 ± 0.32 cde | 6.27 ± 0.23 cd |
| Qch | T3 | 24.97 ± 0.32 de | 5.73 ± 0.34 ef |
| Qs | T4 | 24.48 ± 0.09 fg | 6.23 ± 0.11 cde |
| Qj | T4 | 23.92 ± 0.35 gh | 6.41 ± 0.12 abc |
| Qu | T4 | 25.47 ± 1.40 def | 5.76 ± 0.51def |
| Qd | T4 | 24.24 ± 0.46 fg | 6.47 ± 0.18 abc |
| Qc | T4 | 24.67 ± 0.21 ef | 6.31 ± 0.28 cd |
| Qch | T4 | 26.14 ± 0.43 cd | 5.34 ± 0.04 fg |
| Source of Variation | Shapiro–Wilk Test | Kruskal–Wallis Test | ||
|---|---|---|---|---|
| Statistic | p-Value | Chi-Squared | p-Value | |
| Treatment | 28.447 | 2.92 × 10−6 | ||
| Species | 0.957 | 0.015 | 7.60 | 0.179 |
| Treatment–species | 56.269 | 1.29 × 10−4 | ||
| Code | Treatment | Charcoal Density (g cm−3) |
|---|---|---|
| Qs | T1 | 0.39 ± 0.02 ijkl |
| Qj | T1 | 0.39 ± 0.06 jkl |
| Qu | T1 | 0.40 ± 0.03 ijkl |
| Qd | T1 | 0.41 ± 0.02 fghi |
| Qc | T1 | 0.43 ± 0.01 defg |
| Qch | T1 | 0.44 ± 0.02 bcde |
| Qs | T2 | 0.40 ± 0.01 ijkl |
| Qj | T2 | 0.41 ± 0.03 fghi |
| Qu | T2 | 0.34 ± 0.08 l |
| Qd | T2 | 0.41 ± 0.02 ghij |
| Qc | T2 | 0.42 ± 0.06 efgh |
| Qch | T2 | 0.36 ± 0.03 kl |
| Qs | T3 | 0.43 ± 0.08 defg |
| Qj | T3 | 0.42 ± 0.01 efgh |
| Qu | T3 | 0.40 ± 0.01 ijkl |
| Qd | T3 | 0.46 ± 0.02 abcd |
| Qc | T3 | 0.45 ± 0.02 bcde |
| Qch | T3 | 0.49 ± 0.01 ab |
| Qs | T4 | 0.43 ± 0.04 cdef |
| Qj | T4 | 0.48 ± 0.03 abc |
| Qu | T4 | 0.48 ± 0.02 abc |
| Qd | T4 | 0.48 ± 0.01 abcd |
| Qc | T4 | 0.41 ± 0.02 ghij |
| Qch | T4 | 0.56 ± 0.02 a |
| Moisture Content (MC) | Shapiro–Wilk Test | Kruskal–Wallis Test | ANOVA Test | |||
|---|---|---|---|---|---|---|
| Statistic | p-Value | Chi-Squared | p-Value | F Value | p-Value | |
| Treatments | 58.457 | 2.2 × 10−16 | ||||
| Species | 0.968 | 0.07 | 1.48 | 0.205 | ||
| Treatment–species | 54.693 | 2.2 × 10−16 | ||||
| Volatile matter (VM) | ||||||
| Treatments | 51.85 | 3.22 × 10−11 | ||||
| Species | 0.764 | 2.11 × 10−9 | 11.772 | 0.038 | ||
| Treatment–species | 69.626 | 1.38 × 10−6 | ||||
| Ash content (AC) | ||||||
| Treatments | 5.536 | 0.136 | ||||
| Species | 0.824 | 8.23 × 10−8 | 56.062 | 7.89 × 10−11 | ||
| Treatment–species | 67.309 | 3.14 × 10−6 | ||||
| Fixed carbon (FC) | ||||||
| Treatments | 41.821 | 4.37 × 10−9 | ||||
| Species | 0.864 | 1.52 × 10−6 | 24.639 | 6.13 × 10−4 | ||
| Treatment–species | 70.451 | 1.03 × 10−6 | ||||
| Code | Treatment | Proximate Analysis (%) | |||
|---|---|---|---|---|---|
| MC | VM | AC | FC | ||
| Qs | T1 | 4.61 ± 0.20 ab | 20.48 ± 0.20 bc | 2.02 ± 0.05 kl | 72.86 ± 0.16 mn |
| Qj | T1 | 4.82 ± 0.38 a | 18.61 ± 0.31 cd | 2.15 ± 0.10 jk | 74.40 ± 0.50 lm |
| Qu | T1 | 3.66 ± 0.24 def | 12.18 ± 0.25 ef | 2.39 ± 0.30 hi | 81.75 ± 0.22 jk |
| Qd | T1 | 4.80 ± 0.09 a | 21.17 ± 0.39 ab | 2.16 ± 0.08 jk | 71.85 ± 0.33 no |
| Qc | T1 | 4.26 ± 0.05 bc | 22.30 ± 0.25 a | 4.80 ± 0.09 cd | 68.62 ± 0.22 o |
| Qch | T1 | 3.50 ± 0.09 defg | 14.13 ± 0.15 de | 5.62 ± 0.21 bc | 76.73 ± 0.08 kl |
| Qs | T2 | 2.78 ± 0.01 hij | 6.75 ± 0.18 h | 2.58 ± 0.06 fg | 87.88 ± 0.19 e |
| Qj | T2 | 2.46 ± 0.18 j | 5.71 ± 0.12 mn | 2.36 ± 0.39 hij | 89.45 ± 0.44 cd |
| Qu | T2 | 2.63 ± 0.22 ij | 5.05 ± 0.19 p | 2.58 ± 0.09 fg | 89.72 ± 0.11 c |
| Qd | T2 | 3.19 ± 0.01 fgh | 7.43 ± 0.07 fg | 2.74 ± 0.16 ef | 86.62 ± 0.26 f |
| Qc | T2 | 3.11 ± 0.03 ghi | 6.82 ± 0.20 gh | 6.57 ± 0.43 ab | 83.48 ± 0.37 hi |
| Qch | T2 | 3.23 ± 0.10 fgh | 6.81 ± 0.45 hi | 7.24 ± 0.26 a | 82.70 ± 0.31 ij |
| Qs | T3 | 3.35 ± 0.06 defg | 6.65 ± 0.13 hi | 2.43 ± 0.03 gh | 87.56 ± 0.18 e |
| Qj | T3 | 3.31 ± 0.06 defg | 5.86 ± 0.32 lm | 1.54 ± 0.46 l | 89.27 ± 0.26 d |
| Qu | T3 | 3.27 ± 0.25 efgh | 5.12 ± 0.46 op | 2.23 ± 0.15 ijk | 89.35 ± 0.30 d |
| Qd | T3 | 3.79 ± 0.10 cde | 6.35 ± 0.03 jk | 2.76 ± 0.31 ef | 87.08 ± 0.20 f |
| Qc | T3 | 3.57 ± 0.13 defg | 6.52 ± 0.01 ij | 5.28 ± 0.45 bc | 84.61 ± 0.37 g |
| Qch | T3 | 3.58 ± 0.12 defg | 5.20 ± 0.26 op | 6.18 ± 0.11 ab | 85.02 ± 0.22 g |
| Qs | T4 | 4.43 ± 0.15 ab | 2.80 ± 0.31 q | 2.36 ± 0.03 hij | 90.39 ± 0.43 b |
| Qj | T4 | 4.71 ± 0.17 ab | 2.64 ± 0.11 q | 1.70 ± 0.08 l | 90.93 ± 0.22 ab |
| Qu | T4 | 3.80 ± 0.08 cd | 2.32 ± 0.39 q | 2.34 ± 0.08 hij | 91.51 ± 0.42 a |
| Qd | T4 | 4.53 ± 011 ab | 5.32 ± 0.21 o | 3.03 ± 0.20 de | 87.10 ± 0.40 f |
| Qc | T4 | 4.33 ± 0.10 ab | 6.24 ± 0.12 kl | 4.22 ± 0.66 cd | 85.19 ± 0.70 g |
| Qch | T4 | 4.23 ± 0.27 bc | 5.44 ± 0.25 no | 6.59 ± 0.42 ab | 83.71 ± 0.27 h |
| Charcoal Calorific Value (CCV) | Shapiro–Wilk Test | Kruskal–Wallis Test | ANOVA Test | |||
|---|---|---|---|---|---|---|
| Statistic | p-Value | Chi-Squared | p-Value | F Value | p-Value | |
| Treatments | 34.213 | 1.78 × 10−7 | ||||
| Species | 0.906 | 5.41 × 10−5 | 28.589 | 2.78 × 10−5 | ||
| Treatment–species | 69.83 | 1.29 × 10−6 | ||||
| Energy yield (EY) | ||||||
| Treatments | 30.778 | 9.46 × 10−7 | ||||
| Species | 0.953 | 0.009 | 19.791 | 0.001 | ||
| Treatment–species | 62.079 | 1.90 × 10−5 | ||||
| Fuel ratio (FR) | ||||||
| Treatments | 50.445 | 6.42 × 10−11 | ||||
| Species | 0.838 | 2.17 × 10−7 | 13.063 | 0.022 | ||
| Treatment–species | 69.714 | 1.34 × 10−6 | ||||
| Code | Treatment | Higher Heating Value (MJ kg−1) | Energy Yield (%) | Fuel Ratio |
|---|---|---|---|---|
| Qs | T1 | 29.31 ± 0.06 lm | 43.79 ± 2.05 bcde | 3.55 ± 0.03 mn |
| Qj | T1 | 29.54 ± 0.13 kl | 44.18 ± 0.92 abc | 3.99 ± 0.09 lm |
| Qu | T1 | 31.04 ± 0.11 i | 48.02 ± 2.93 a | 6.71 ± 0.12 jk |
| Qd | T1 | 29.07 ± 0.06 mn | 44.08 ± 0.98 abcd | 3.39 ± 0.07 no |
| Qc | T1 | 28.12 ± 0.05 n | 45.38 ± 1.41 abc | 3.07 ± 0.04 o |
| Qch | T1 | 29.60 ± 0.02 kl | 43.03 ± 0.73 cdef | 5.42 ± 0.06 kl |
| Qs | T2 | 32.29 ± 0.04 e | 40.64 ± 0.27 ijk | 13.01 ± 0.39 h |
| Qj | T2 | 32.66 ± 0.17 b | 40.78 ± 0.94 hij | 15.64 ± 0.27 e |
| Qu | T2 | 32.65 ± 0.04 b | 44.76 ± 2.75 abcd | 17.76 ± 0.66 b |
| Qd | T2 | 31.96 ± 0.07 fg | 38.27 ± 0.19 k | 11.65 ± 0.30 ij |
| Qc | T2 | 30.74 ± 0.14 j | 38.84 ± 1.07 k | 12.24 ± 0.37 i |
| Qch | T2 | 30.46 ± 0.04 jk | 39.94 ± 0.70 jk | 12.16 ± 0.83 i |
| Qs | T3 | 32.15 ± 0.04 ef | 40.91 ± 0.55 hij | 13.17 ± 0.29 gh |
| Qj | T3 | 32.63 ± 0.12 bc | 40.44 ± 0.20 ijk | 15.25 ± 0.86 e |
| Qu | T3 | 32.53 ± 0.03 cd | 46.12 ± 1.71 ab | 17.51 ± 1.59 bc |
| Qd | T3 | 31.93 ± 0.07 fg | 41.33 ± 0.34 ghi | 13.69 ± 0.06 f |
| Qc | T3 | 31.09 ± 0.13 i | 42.15 ± 0.62 efg | 12.96 ± 0.07 h |
| Qch | T3 | 31.01 ± 0.06 i | 41.92 ± 0.64 fg | 16.36 ± 0.82 cde |
| Qs | T4 | 32.50 ± 0.09 d | 42.28 ± 0.24 defg | 32.52 ± 4.05 a |
| Qj | T4 | 32.66 ± 0.66 b | 41.70 ± 0.81 gh | 34.49 ± 1.59 a |
| Qu | T4 | 32.82 ± 0.09 a | 44.25 ± 2.43 bcde | 40.10 ± 6.65 a |
| Qd | T4 | 31.77 ± 0.11 fg | 40.70 ± 0.88 hij | 16.37 ± 0.73 cd |
| Qc | T4 | 31.25 ± 0.22 hi | 41.33 ± 0.16 ghi | 13.63 ± 0.38 fg |
| Qch | T4 | 30.59 ± 0.08 j | 43.28 ± 0.84 cdef | 15.39 ± 0.72 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Trejo, J.C.; Carrillo-Parra, A.; Ngangyo-Heya, M.; Rutiaga-Quiñones, J.G.; Chávez-Simental, J.A.; Goche-Télles, J.R. Carbonisation of Quercus spp. Wood: Temperature, Yield and Energy Characteristics. Processes 2025, 13, 2302. https://doi.org/10.3390/pr13072302
Contreras-Trejo JC, Carrillo-Parra A, Ngangyo-Heya M, Rutiaga-Quiñones JG, Chávez-Simental JA, Goche-Télles JR. Carbonisation of Quercus spp. Wood: Temperature, Yield and Energy Characteristics. Processes. 2025; 13(7):2302. https://doi.org/10.3390/pr13072302
Chicago/Turabian StyleContreras-Trejo, Juan Carlos, Artemio Carrillo-Parra, Maginot Ngangyo-Heya, José Guadalupe Rutiaga-Quiñones, Jorge Armando Chávez-Simental, and José Rodolfo Goche-Télles. 2025. "Carbonisation of Quercus spp. Wood: Temperature, Yield and Energy Characteristics" Processes 13, no. 7: 2302. https://doi.org/10.3390/pr13072302
APA StyleContreras-Trejo, J. C., Carrillo-Parra, A., Ngangyo-Heya, M., Rutiaga-Quiñones, J. G., Chávez-Simental, J. A., & Goche-Télles, J. R. (2025). Carbonisation of Quercus spp. Wood: Temperature, Yield and Energy Characteristics. Processes, 13(7), 2302. https://doi.org/10.3390/pr13072302






