Abstract
Cabbage is one of the most popular vegetables all over the world, with white cabbage generally being more popular than red cabbage. This study aimed at a comparison of the antioxidant properties of fresh and fermented white and red cabbage. Total phenolic content, the content of anthocyanins and carotenoids, and the Total Antioxidant Capacity (TAC) assayed by ABTS• scavenging, DPPH• scavenging, FRAP, and ORAC of fresh white and red cabbage, fermented white and red cabbage (sauerkraut), and sauerkraut juice were compared. The TAC of fresh and fermented red cabbage, and of red sauerkraut juice (110.3 ± 8.9, 47.4 ± 4.6 and 48.9 ± 5.7 mmol Trolox equivalents/kg, respectively) was significantly higher than the TAC of fresh and fermented white cabbage and white sauerkraut juice (5.1 ± 0.2, 7.9 ± 0.9 and 6.6 ± 0.9 mmol TE/kg, respectively, when assayed by ORAC). The TAC of white sauerkraut and white sauerkraut juice could be elevated by fermentation with 20% of black carrots (to 16.4 ± 1.2 and 10.5 ± 0.8 mmol TE/kg, respectively) but the TAC of red sauerkraut and red sauerkraut juice was diminished by a mixture of either orange or black carrots, which are of lower anthocyanin content than the red cabbage (41.8 ± 3.0 and 29.2 ± 3.1 mmol TE/kg, respectively). These results may justify the promotion of the broad consumption of red cabbage, both fresh and fermented, and encourage the usage of red cabbage as a promising material for functional foods.
1. Introduction
Anthocyanins—flavonoid pigments occurring in many flowers, fruits, and other plant organs—are excellent antioxidants [1,2] and are claimed to have numerous positive health effects related and unrelated to their antioxidant properties. They were found to ameliorate oxidative stress, dyslipidemia, and arterial stiffness, positively affect endothelium functions and glucose metabolism, prevent cognitive disorders, inhibit cyclooxygenases, have antiatherogenic, antihypertensive, antithrombotic, anti-inflammatory, antiglycative, anticarcinogenic, and antimicrobial activity [3,4,5,6,7] and induce favorable changes in the composition of gut microbiota [8,9].
The outstanding longevity of the Okinawa population has been attributed, at least in part, to the high consumption of food products rich in anthocyanins, such as purple potatoes, purple sweet potatoes, and purple and black carrots [8,10,11].
Globalization has made it possible to buy specific products like purple sweet potato globally and to produce transgenic plants overproducing anthocyanins, including tomato, rice, maize, and other species [12,13]. However, the transport of food products is costly and generates a carbon footprint, so the tendency to eat local products (zero-kilometer food strategy) is gaining popularity [14,15]. Moreover, there are numerous issues related to the acceptance of transgenic plants in societies and governments [16,17,18]. Therefore, locally cultured anthocyanin-rich crops need closer attention and evidence-based recommendations.
Cabbage (Brassica oleracea var. capitata), native to coastal regions of the Mediterranean, spread across northern Europe due to its ability to withstand cold climates, became an important staple in European cuisine [19,20], and is now cultivated and consumed all over the world [21]. This vegetable is consumed in the form of salads, coleslaw, sauerkraut, soup, and is also employed for other cooking purposes. Red (purple) cabbage (Brassica oleracea var. capitata rubra), rich in anthocyanins (≥10 g/kg dry mass), is a variety of cabbage originating from Southern Europe, but is nowadays produced and harvested worldwide [22], although it is generally less popular than white cabbage. This variety is plentiful year-round, but tastes the best when grown in cooler climates [21]. Interestingly, red cabbage is preferentially consumed in some regions (as Silesia in Poland) [23,24].
Cabbage has been traditionally subjected to fermentation, mainly for the sake of preservation and as a source of vitamins and other phytochemicals in winter and spring, when fresh vegetables and fruits were not available, and also to obtain a product of a different taste. The effect of fermentation on the antioxidant properties of cabbage is therefore of considerable interest, but data from the literature concerning this question are somewhat contradictory. Fermentation for up to 14 days was reported to progressively increase the antioxidant activity of white cabbage juice assayed by DPPH• and ABTS• reduction [25]. Lactic fermentation increased the antioxidant capacity of aqueous extracts of Chinese cabbage (Brassica pekinensis) assayed by DPPH• decolorization [26]. An increase in TAC, a decrease in the content of ascorbic acid, and a profound increase in the ascorbigen content were noted upon fermentation of white cabbage cv. Megaton [27]. Fermentation of red cabbage was reported to increase the TAC estimated by DPPH• and ABTS• reduction [28]. However, it was also reported that fermentation of white and red cabbage decreased the phenolic content and the TAC estimated by DPPH• decolorization and CUPRAC assays of both white and red aqueous cabbage extracts [29]. Another study found a decrease in TAC estimated by ABTS• reduction and ORAC but saw no change in TAC assayed by DPPH• reduction after fermentation of red cabbage (cultivar Langedijker Polona) [30].
Another interesting question is the effect of an additive on the TAC of fermented cabbage. Various recipes propose the inclusion of additives for cabbage fermentation, chiefly to increase the sensory properties of the sauerkraut [31,32]. Such additives may also affect the antioxidant properties of the sauerkraut and the sauerkraut juice.
This study was aimed at: (i) re-investigation, in view of divergent results, of the effect of fermentation on the antioxidant capacity of white and red cabbage using several different methods of evaluation of TAC, and (ii) examination of the effects of addition of orange and black carrots on the antioxidant properties of sauerkraut and sauerkraut juice made of white and red cabbage.
2. Materials and Methods
2.1. Reagents and Chemicals
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; CAS no. 504-14-6; cat. no. 10102946001; purity ≥ 99%) was bought from Roche (Warsaw, Poland). 2,2-Diphenyl-1-picrylhydrazyl (DPPH•; CAS no. 1898-66-4; cat. no. HY-112053, purity ≥ 99.13%), and iron(III) chloride (FeCl3; CAS no. 7705-08-0; cat. no. 451649; purity ≥ 99.99%) were provided by MedChemExpress (Monmouth Junction, NJ, USA). 2,4,6-2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ; CAS no. 3682-35-7; cat. no. T1253), fluorescein sodium salt; CAS no. 518-47-8; cat. no. 103887), 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH; CAS no. 2997-92-4; cat. no. 440914; purity ≥ 97), gallic acid monohydrate (CAS no. 5995-86-8; cat. no. 398225), potassium persulfate (CAS no. 7727-21-1, cat. no. 216224), Trolox (CAS no. 53188-07-1, cat. no. 648471), the Folin–Ciocalteu phenol reagent (cat. no. 4764) and dimethyl sulfoxide (DMSO; CAS no. 67-68-5; cat. no. D2438) were purchased from Merck (Poznan, Poland). Sodium dihydrogen phosphate (CAS no. 10049-21-5; cat. no. PM306.500, purity 98–103%), and sodium hydrogen phosphate (CAS no. 7782-85-6; cat. no. SPD579.1, purity 98–102%) produced by BioShop Canada Inc. (Burlington, ON, Canada) were obtained from Lab Empire (Rzeszow, Poland). Methanol (CAS no. 67-56-1; cat. no. 6219900110, purity ≥ 99.9%), ethanol (CAS no. 64-17-5, cat. no. 396420113, 96%), glacial acetic acid CAS no. 64-19-7; cat. no. JT9522-2) and sodium acetate anhydrous (CAS no. 127-09-3; cat. no. BN60/6191; purity ≥ 99%) were provided by Avantor Performance Materials (Gliwice, Poland).
Water was purified by a Milli-Q system (Millipore, Bedford, MA, USA). Transparent flat-bottom 96-well plates (cat. no. 655101) as well as black-flat-bottom 96-well plates (cat. no. 655209) (Greiner, Kremsmünster, Austria) were used. Absorbance and fluorescence were measured in a Spark microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
2.2. Plant Material
White and red cabbage (Brassica oleracea var. capitata f. alba cv. Amazon F1 and f. rubra cv. Lodero F1), grown in the continental agroclimatic zone, were bought in a supermarket in Rzeszów.
2.3. Preparation of Extracts
External leaves of the cabbage head were removed, the cabbage was washed and chopped into about 1 × 1 × 1 cm fragments, frozen and thawed, and homogenized with a 9-fold excess of deionized water, shaken for 30 min, again frozen and thawed, homogenized again, and centrifuged. The supernatant was aliquoted and stored frozen at −80 °C until analysis, for no more than 1 month.
2.4. Cabbage Fermentation
External leaves were removed from medium-sized cabbage heads, which were then washed, chopped, and divided into three sets of portions, each set containing either 400 g (control) or 320 g of cabbage; the latter portions were supplemented with 80 g of orange or black carrots (20% by weight); all were added with 8 g of table salt. The components were thoroughly mixed, kneaded with a wooden spoon, and left for 30 min. This treatment was repeated three times. Then, the cabbage was placed in 500 mL jars and pressed to obtain a juice layer covering the cabbage. The jars were covered with a paper towel and left at 20 °C for 3 days. Then, the released gas was expelled with a sterile spoon, and the jars were closed tightly and left at ambient temperature for the next 12 days. Every 3 days, the level of juice was controlled, and the cabbage was pressed to be fully covered by the juice.
In the fermented cabbage, both the juice and cabbage fragments were analyzed. The latter were dried with a paper towel and extracted as described above.
2.5. Estimation of the Polyphenol Content
The polyphenol concentration in the extracts was determined using the Folin–Ciocalteu reagent [33]. In brief, 25 µL of a 10-fold diluted extract was pipetted to 125 µL of 1 M Folin–Ciocalteu reagent in wells of a microplate, mixed, and incubated for 4 min. Then, saturated sodium carbonate solution (100 µL) was added to the wells, the plate was incubated for 1 h at room temperature (21 ± 1 °C), and the absorbance of the samples was measured at 750 nm. The polyphenol concentration in the extracts was calculated based on a standard curve prepared with gallic acid and expressed in gallic acid equivalents (GAE).
2.6. Estimation of the Anthocyanin Content
The content of anthocyanin was estimated according to a slightly modified method of Lee et al. [34]. Briefly, 125 µL aliquots of the extract were added with 875 µL of 0.1 M acetate buffer, pH 4.5 or 1.5 M HCl, and the absorbance of both samples was measured at the absorption maximum of anthocyanins (at about 520 nm) and at 700 nm.
- The anthocyanin concentration, c, was calculated as:c [mg/L] = A × MW × dilution × 103)/(ε × l), whereA = (Amaximum − A700 nm)pH 1 − (Amaximum − A700 nm)pH 4.5,
MW, molecular weight; ε, molar absorption coefficient [M−1 cm−1]; l, length of the optical path. The following values were assumed: MW for cyanidin-3-glucoside of 484.8 g mol−1, ε = 26,900 M−1 cm−1.
2.7. Estimation of the Carotenoid Content
The content of carotenoids was estimated in hexane extracts of the white and red cabbage by measurement of absorbance of the extracts at 446 nm. Total carotenoid content TCC was calculated as:
where A, absorbance at 446 nm; V, volume of hexane [mL], m, mass of a sample [g] [35].
2.8. Estimation of Total Antioxidant Capacity
2.8.1. ABTS• Scavenging Assay
The ABTS• decolorization assay [36] was used in a modification described previously [37]. In brief, a stock solution of ABTS•, prepared by the oxidation of ABTS with 2.45 mM potassium persulfate for 16 h, was diluted with phosphate-buffered saline (145 mM NaCl in 10 mM sodium phosphate, pH 7,4; PBS) to obtain a solution providing absorbance 1.0 at a wavelength of 734 nm when 200 µL of such solution was measured in a well of a 96-well microplate. Wells containing such an ABTS• solution were added with various volumes (1–10 µL) of solutions of a cabbage extract or juice. The decrease in absorbance after 30 min incubation at ambient temperature (21 ± 1 °C) in the dark, was measured and corrected for the drop of absorbance of a blank sample, containing ABTS• solution and no additive.
The antioxidant capacity was calculated as a ratio of the slope of the dependence of absorbance change on the volume of a studied extract to the slope of the dependence of absorbance change in standard samples containing Trolox on the concentration of Trolox, as explained in [38]. Total Antioxidant Capacity (TAC) is expressed in mmoles of Trolox equivalents (TE)/kg of cabbage or cabbage juice. The same procedure was applied to the results of DPPH• scavenging and FRAP assays. TAC is expressed in mmoles of Trolox equivalents (TE)/kg of cabbage or cabbage juice.
2.8.2. DPPH• Scavenging Assay
A procedure described previously [38] was employed. In brief, increasing volumes of the extract/juice supplemented with water to a final volume of 20 μL were added to Eppendorf tubes containing 300 µL of 0.3 mM DPPH• solution in methanol and incubated for 30 min at ambient temperature, in the dark. The samples were centrifuged, and 200-μL aliquots of the supernatants were pipetted into wells of a 96-well plate. Their absorbance at 517 nm was then read. The absorbance decrease, with respect to samples containing DPPH• solution added with water only, was a measure of the antioxidant activity.
2.8.3. Ferric Reducing Antioxidant Power (FRAP) Assay
A slight modification of the method described by Benzie and Strain [39] was used. Briefly, increasing volumes of diluted extract/juice were pipetted to wells of a 96-well microplate containing 200 μL of the working solution (0.3 M acetate buffer, pH 3.6/10 mM TPTZ in 40 mM HCl/20 mM FeCl3; 10/1/1, v/v/v). The samples were incubated for 30 min at ambient temperature, and their absorbance was measured at 593 nm.
2.8.4. Oxygen Radical Absorbing Capacity (ORAC) Assay
The method of Ou et al. [40] was employed in a slight modification. In brief, increasing volumes (1–10 μL) of the extract/juice were added to wells of a 96-well microplate containing fluorescein (0.2 μM), AAPH (50 mM), and sodium phosphate buffer (50 mM; final concentrations) in a total volume of 200 μL (including the extract volume). The fluorescence was measured at the excitation/emission wavelengths of 478 nm and 520 nm, respectively, every 90 s for 180 min at a temperature of 37 °C. The sum of fluorescence intensities for all measurements was calculated for each sample. The per cent protection of the fluorescein fluorescence was calculated according to the formula:
where Ssample is the sum of fluorescence intensities for a studied sample; SAAPH is the sum of fluorescence intensities of a sample containing fluorescein, AAPH and no antioxidant additives; Sblank is the sum of fluorescence intensities of a sample containing fluorescein and no AAPH or antioxidant additives.
% protection = 100% [(Ssample − SAAPH)/(Sblank − SAAPH)]
The antioxidant capacity was calculated as the ratio of the slope of the dependence of the percentage protection of fluorescence on the volume of the studied extract to the slope of the dependence of the protection of fluorescence of standard samples containing Trolox on the concentration of Trolox. Thus, the determined TAC is expressed in mmoles of Trolox equivalents (TE)/kg of cabbage or cabbage juice.
2.9. Statistical Analysis
The results are presented as means ± SD of at least three replicates. The statistical significance of differences between antioxidants within individual assay methods was estimated using one-way ANOVA with the post hoc Tukey’s test, for p = 0.05, using XLSTAT, version 27.1.3.0 (Lumivero, Denver, CO, USA). PCA analysis was performed using STATISTICA, version 13.3 (TIBCO, Palo Alto, CA, USA).
3. Results
Comparison of the antioxidant capacity of fresh white and red cabbage estimated by various methods is shown in Table 1. Significant differences were seen between white and red cabbage. The latter, apart from the presence of anthocyanins, had higher polyphenol content (due, i.a., to anthocyanins), and higher TAC values estimated by all assays employed.

Table 1.
Antioxidant properties of fresh white and red cabbage.
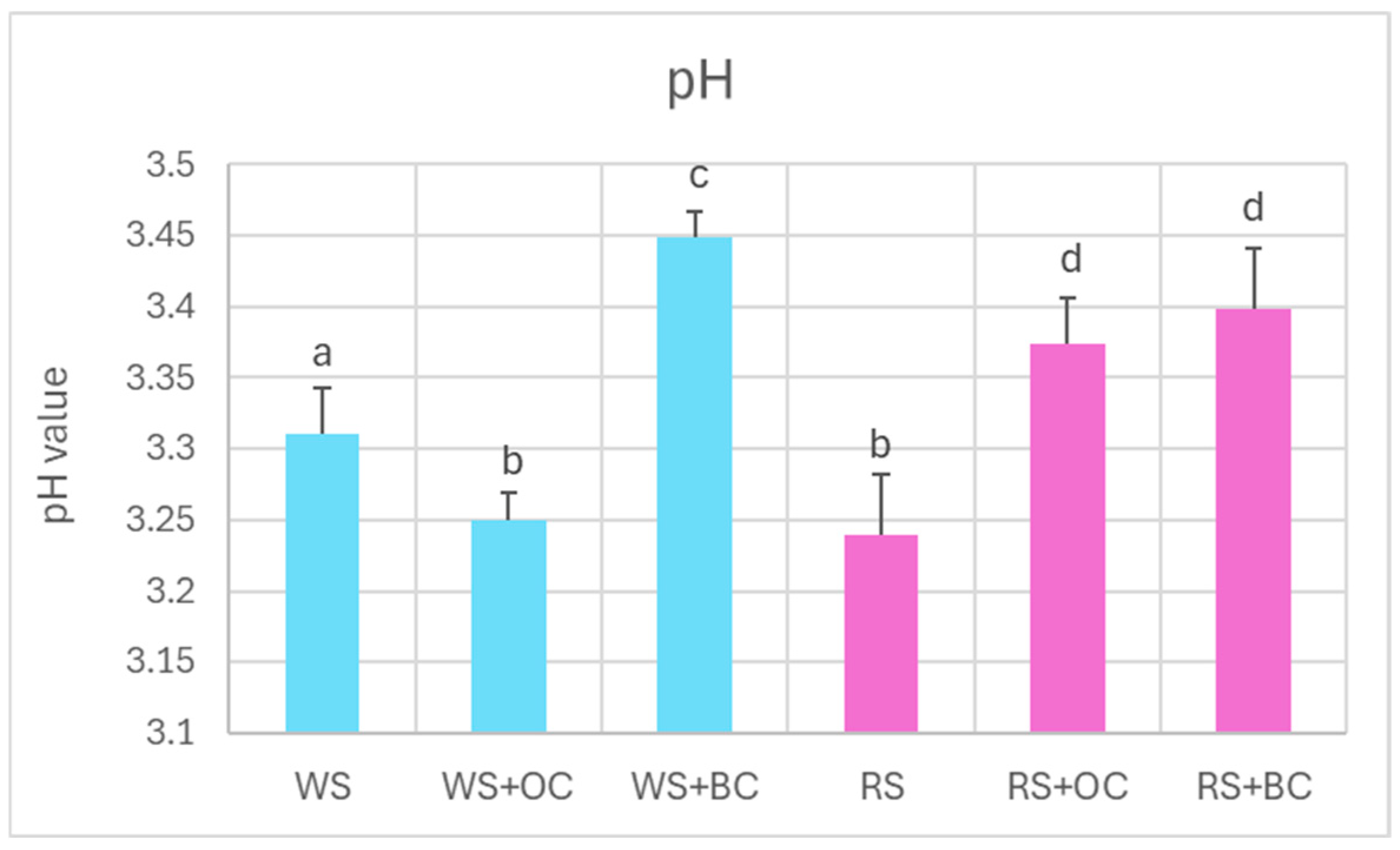
Fermentation of cabbage caused a decrease in pH to values in the range of 3.2–3.5, with no significant differences between white and red sauerkraut. The presence of black carrots during the fermentation led to a slight elevation in the pH of both white and red sauerkraut (from 3.31 ± 0.03 to 3.45 ± 0.02 and from 3.24 ± 0.04 to 3.40 ± 0.04; mean ± SD, respectively); in the red sauerkraut, such an effect was also caused by the addition of orange carrots (Figure 1).
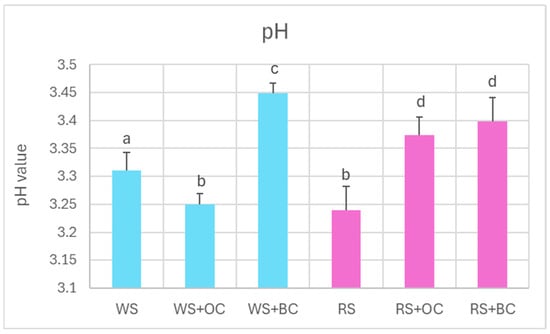
Figure 1.
pH of white and red sauerkraut. WS, white sauerkraut; WS+OC, white sauerkraut with orange carrots; WS+BC, white sauerkraut with black carrots; RS, red sauerkraut; RS+OC, red sauerkraut with orange carrots; RS+BC, red sauerkraut with black carrots. Pale blue, white cabbage; purple, red cabbage. Different letters indicate statistically significant differences (p < 0.05). One-way ANOVA with post hoc Tukey test.
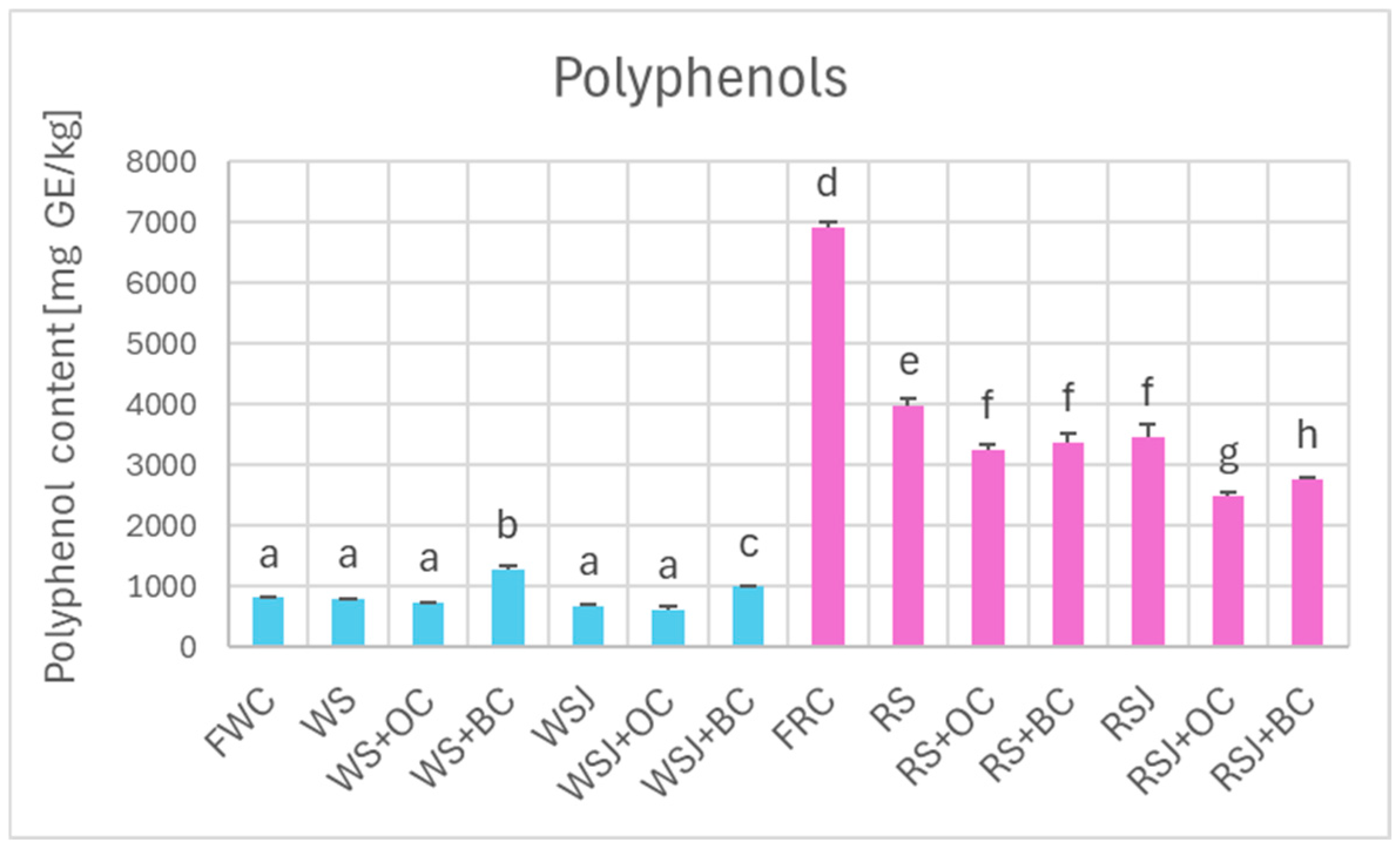
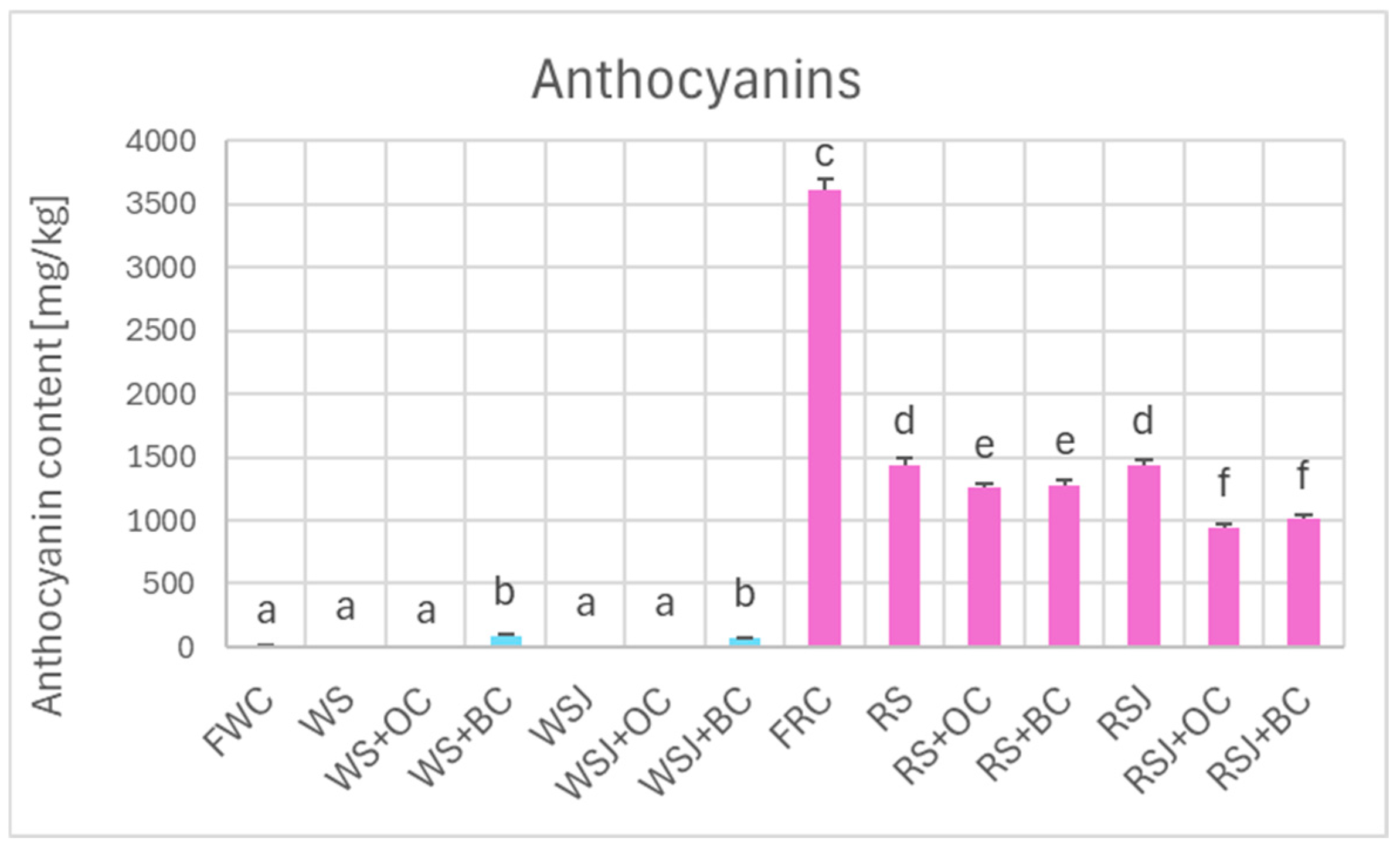
Comparison of the antioxidant properties of fresh and fermented cabbage demonstrated that the fermented cabbage retained most of the polyphenols (Figure 2) and, in the case of red cabbage, anthocyanins (Figure 3). In white cabbage, the total polyphenol content did not differ significantly between fresh cabbage and sauerkraut (821 ± 10 vs. 798 ± 9 mg/kg). In contrast, red sauerkraut had significantly less polyphenols than fresh red cabbage (3990 ± 100 vs. 6915 ± 99 mg/kg). The juice contained comparable amounts of polyphenols and anthocyanins as the solid sauerkraut. The addition of black carrots increased the polyphenol content in the white sauerkraut (up to 1291 ± 57 mg/kg) and white sauerkraut juice (from 689 ± 10 to 1001 ± 19 mg/kg). In contrast, the addition of orange or black carrots decreased the content of anthocyanins and polyphenols in the red sauerkraut (down to 3245 ± 113 and 3387 ± 127 mg/kg, respectively) and red sauerkraut juice (from 3467 ± 204 to 2484 ± 78 and 2777 ± 31 mg/kg, respectively).
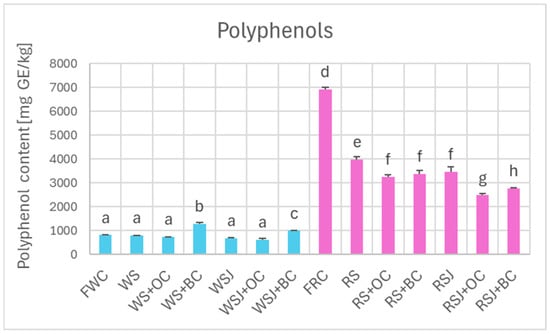
Figure 2.
Polyphenol content of white and red cabbage, fermented cabbage (sauerkraut), and sauerkraut juice. FWC, fresh white cabbage; WS, white sauerkraut; WS+OC, white sauerkraut with orange carrots; WS+BC, white sauerkraut with black carrots; WSJ, white sauerkraut juice; WSJ+OC, juice of white sauerkraut with orange carrots; WSJ+BC, juice of white sauerkraut with black carrots; FRC, fresh red cabbage; RS, red sauerkraut; RS+OC, red sauerkraut with orange carrots; RS+BC, red sauerkraut with black carrots; RSJ, red sauerkraut juice; RSJ+OC, juice of red sauerkraut with orange carrots; RSJ+BC, juice of red sauerkraut with black carrots. Pale blue, white cabbage; purple, red cabbage. Different letters indicate statistically significant differences (p < 0.05). One-way ANOVA with post hoc Tukey test.
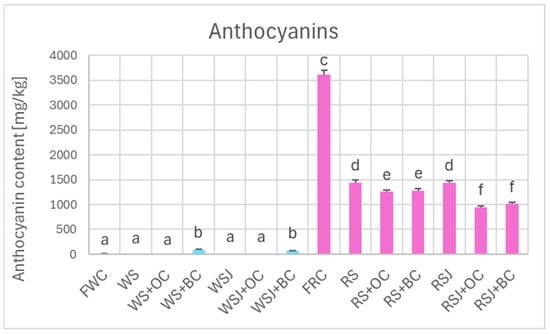
Figure 3.
Anthocyanin content of white and red cabbage, fermented cabbage (sauerkraut) and sauerkraut juice. Pale blue, white cabbage; purple, red cabbage. Different letters indicate statistically significant differences (p < 0.05). One-way ANOVA with post hoc Tukey test.
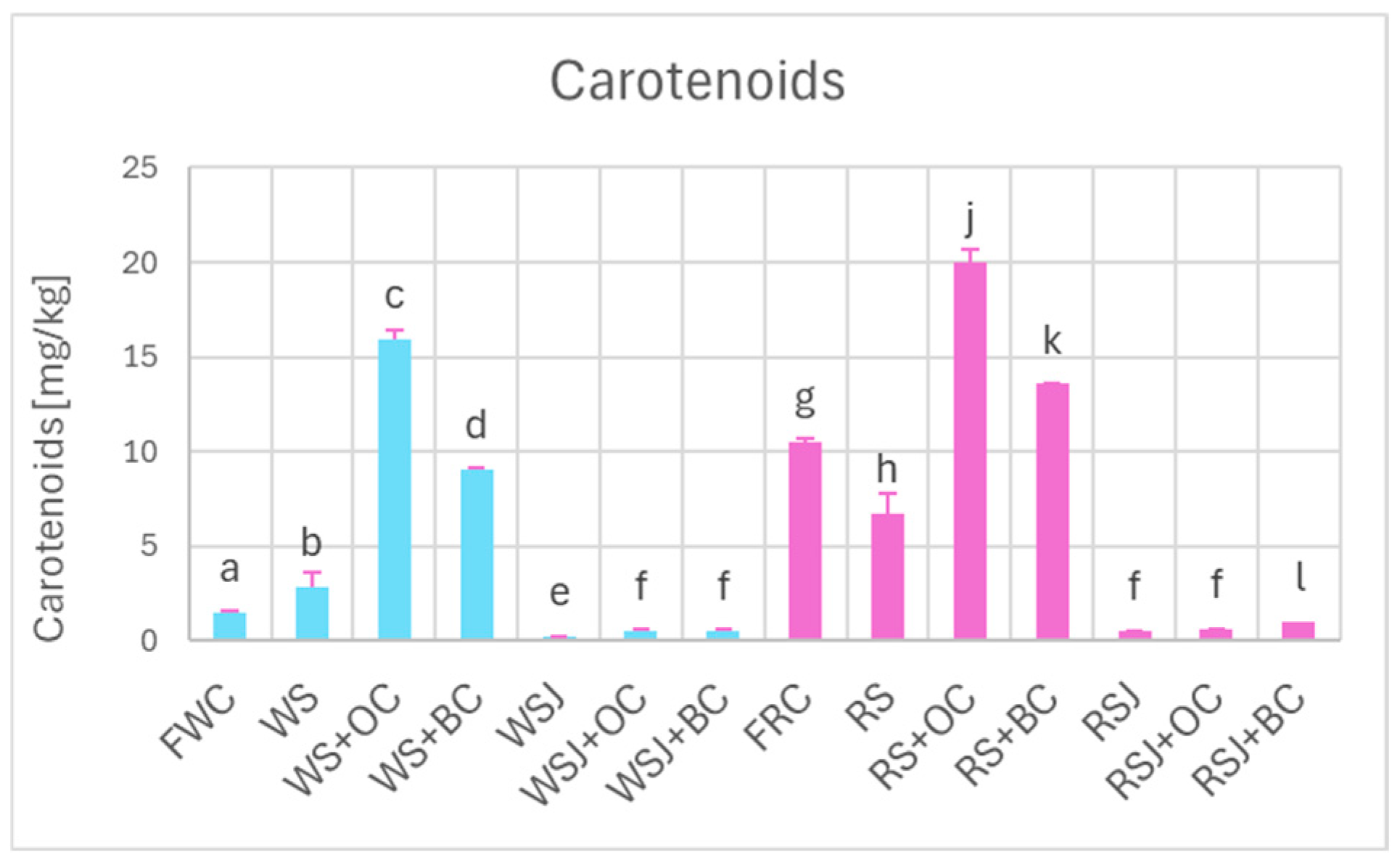
The addition of carrots enriched the sauerkraut in carotenoids—the effect being more significant for orange carrots. The carotenoid concentration did not increase significantly in the sauerkraut juice (Figure 4), apparently due to the hydrophobicity of these compounds and practical insolubility in aqueous solutions.
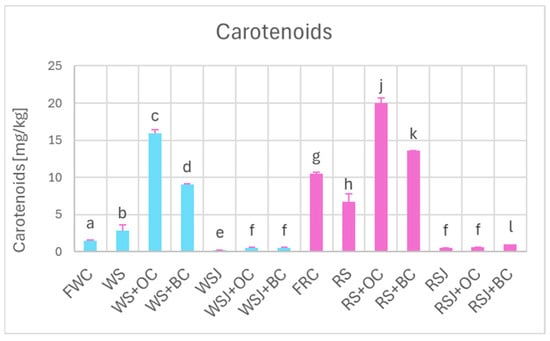
Figure 4.
Carotenoid content of white and red cabbage, fermented cabbage (sauerkraut), and sauerkraut juice. Pale blue, white cabbage; purple, red cabbage. Different letters indicate statistically significant differences (p < 0.05). One-way ANOVA with post hoc Tukey test.
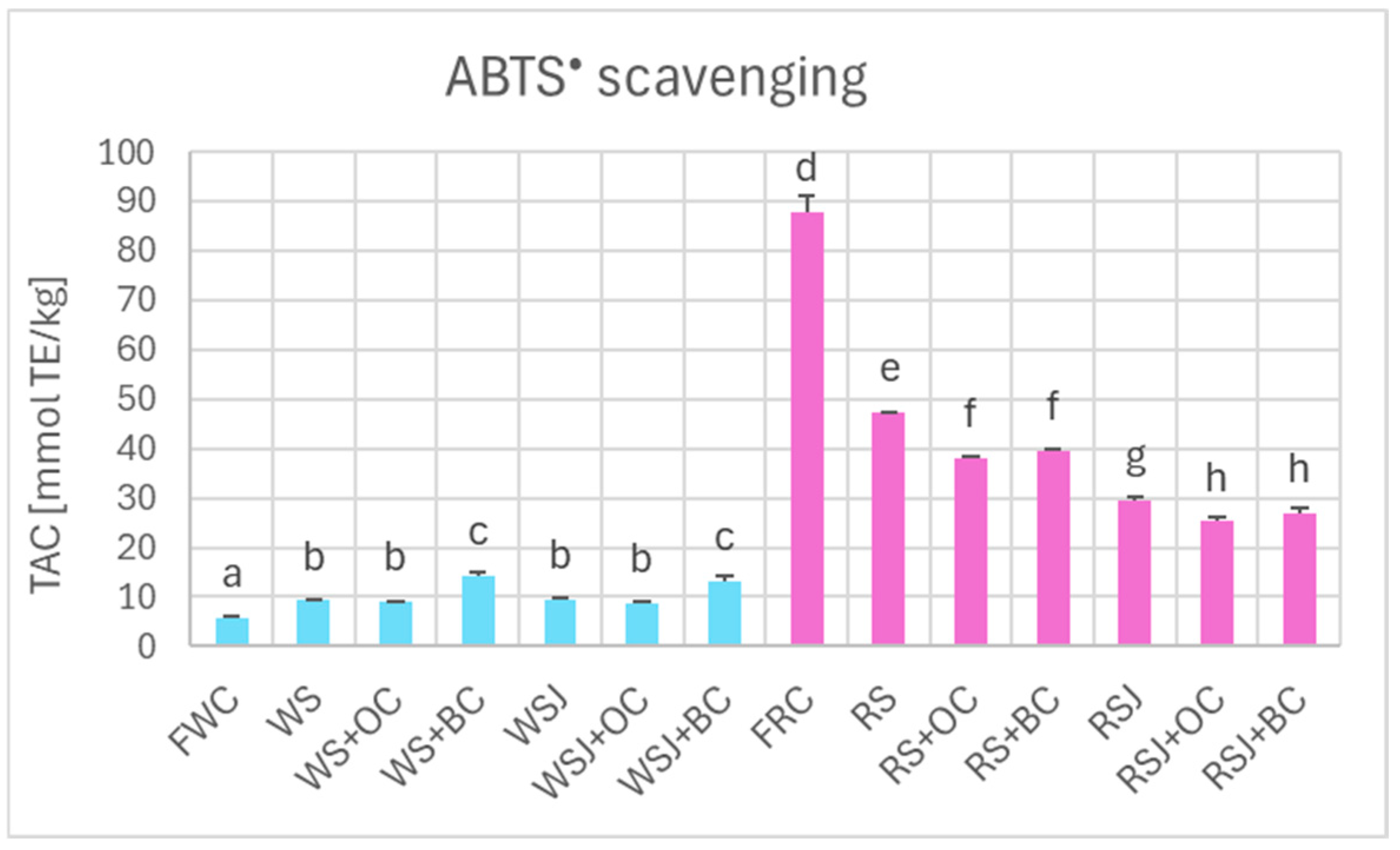
The pattern of Total Antioxidant Capacity of fresh white and red cabbage, white and red sauerkraut, estimated by ABTS• scavenging (Figure 5), was similar to that of polyphenol content. It was much higher for the red cabbage and its products as compared with the white cabbage (87.9 ± 3.3 vs. 5.8 ± 0.2 mmol TE/kg, respectively). TAC of white sauerkraut and white sauerkraut juice (9.2 ± 0.1 and 9.3 ± 0.6 mmol TE/kg, respectively) was higher than that of fresh white cabbage, while TAC of red sauerkraut and red sauerkraut juice (47.3 ± 0.1 and 29.4 ± 0.8 mmol TE/kg, respectively) was considerably lower than that of fresh red cabbage.
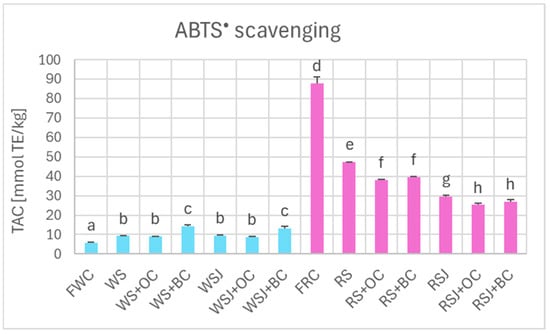
Figure 5.
The TAC of fresh and fermented white and red cabbage estimated by the ABTS• scavenging assay. Pale blue, white cabbage; purple, red cabbage. Different letters indicate statistically significant differences (p < 0.05). One-way ANOVA with post hoc Tukey test.
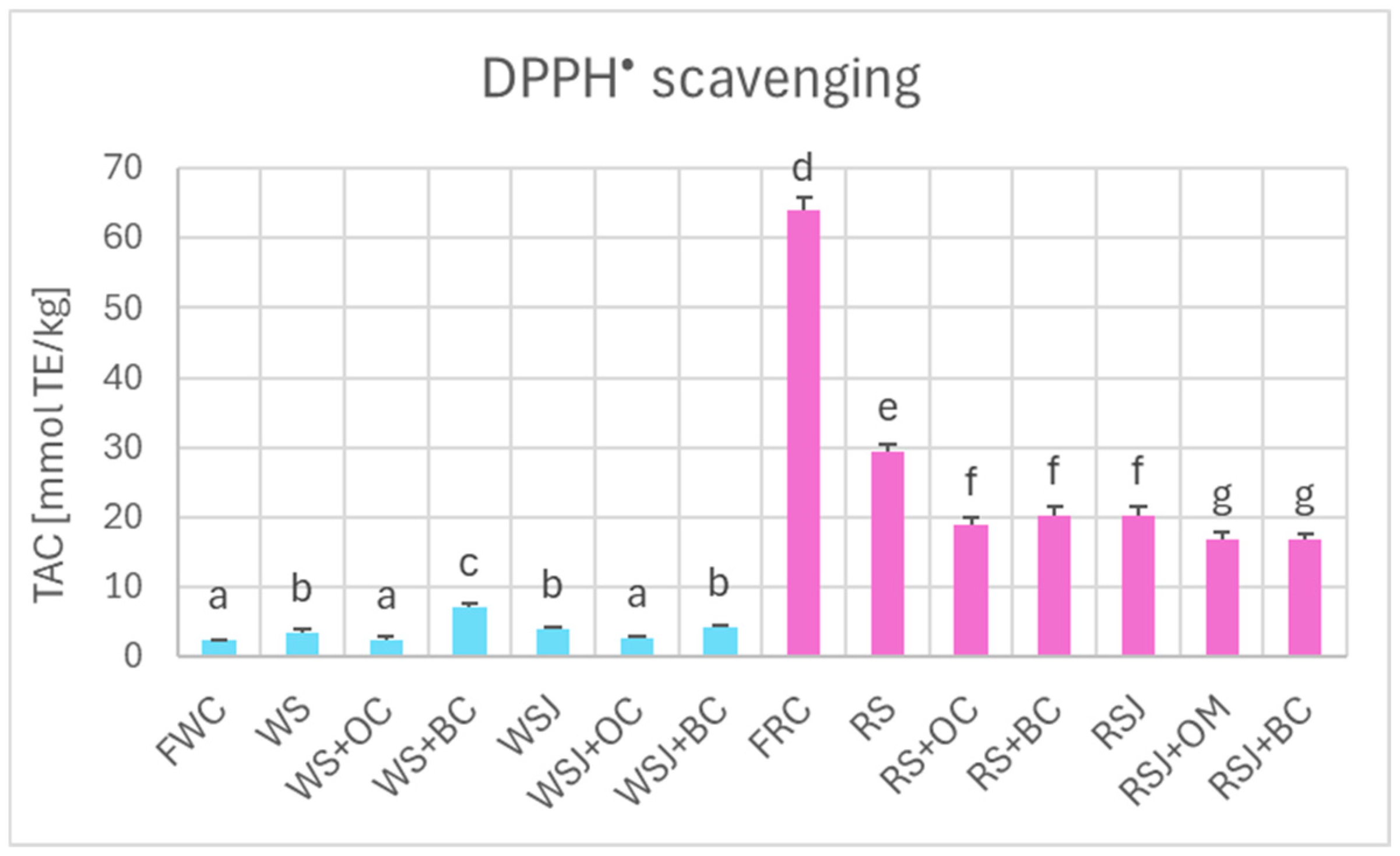
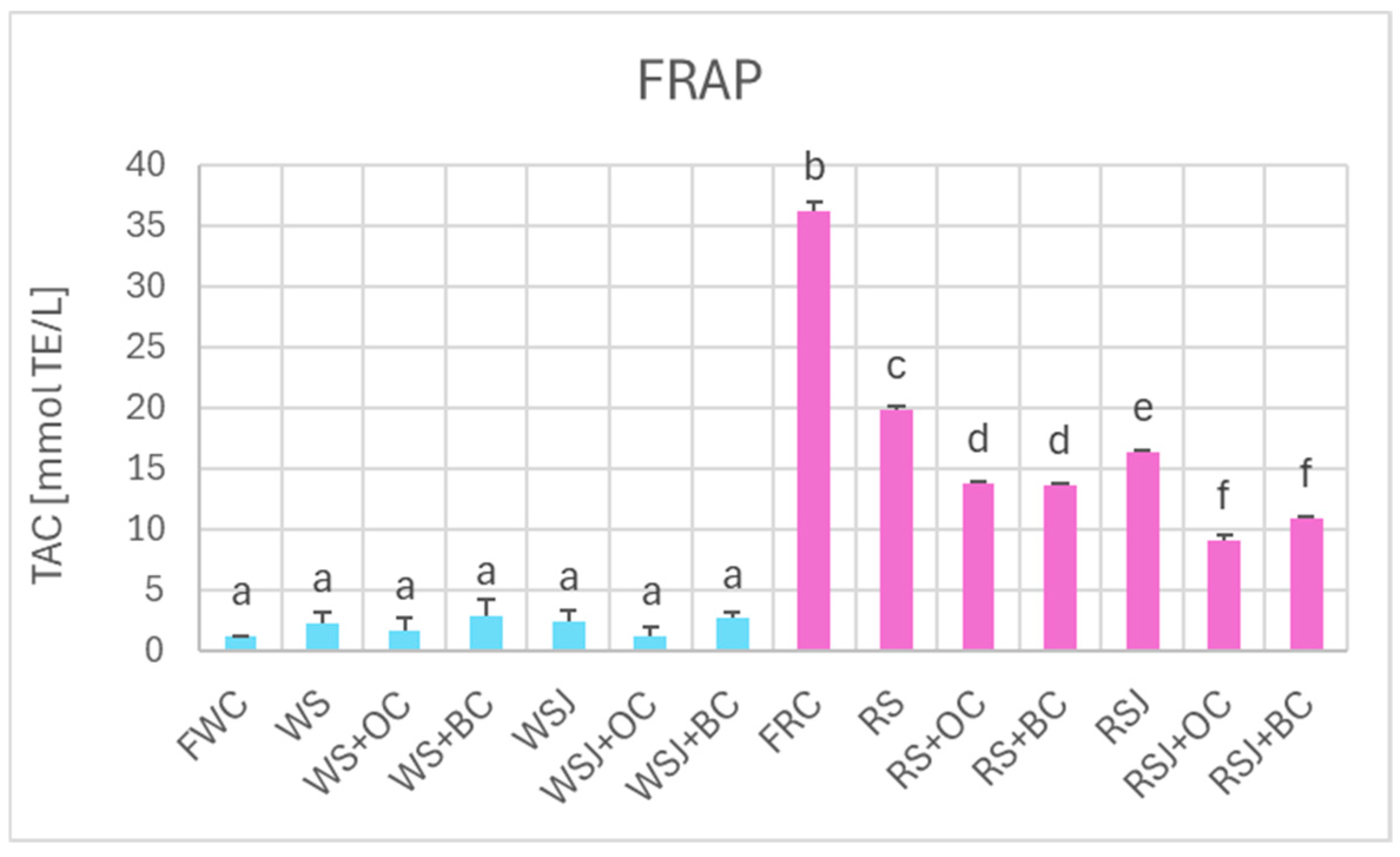
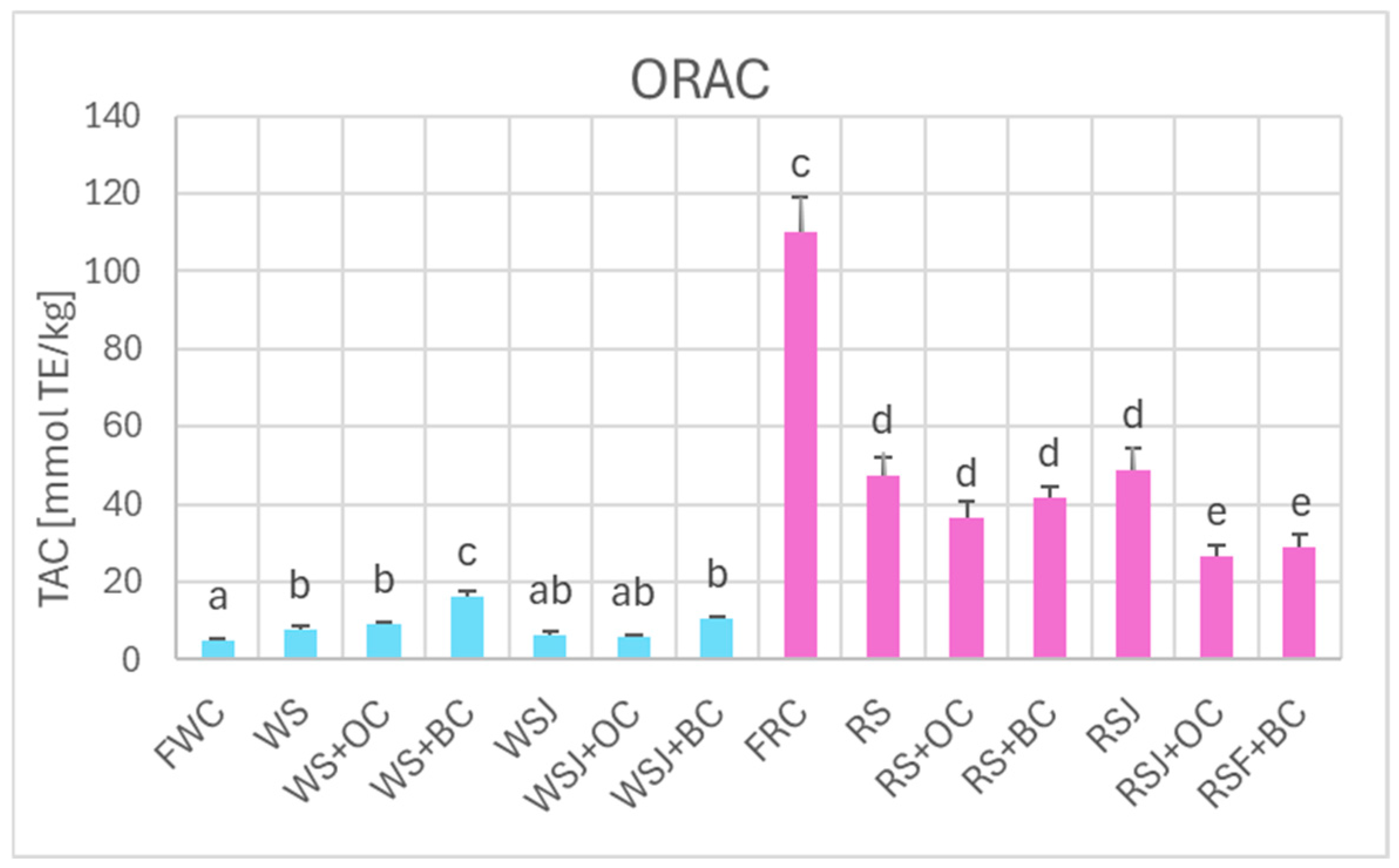
The TAC estimated by DPPH• scavenging (Figure 6), FRAP (Figure 7), and ORAC (Figure 8) showed similar relationships. However, TAC values obtained by DPPH• scavenging (2.3 ± 0.1 and 63.9 ± 2.0 mmol TE/kg for fresh white and red cabbage, respectively) and FRAP (1.2 ± 0.1 and 36.2 ± 0.9 mmol TE/kg for fresh white and red cabbage, respectively) were lower than those found by ABTS• scavenging. The TAC values obtained by ORAC for fresh white cabbage (5.1 ± 0.2 mmol TE/kg) were somewhat lower than the values obtained by ABTS• scavenging, but those found for fresh red cabbage (110.3 ± 0.9 mmol TE/kg) were higher than those found by ABTS• scavenging. All methods demonstrated a decreased TAC of red sauerkraut as compared with fresh red cabbage (29.4 ± 0.8, 20.2 ± 1.2, 16.4 ± 0.7, and 48.9 ± 4.7 mmol/kg for ABTS• scavenging, DPPH• scavenging, FRAP and ORAC assays, respectively). In contrast, the TAC of white sauerkraut was higher than that of fresh white cabbage (9.3 ± 0.1, 3.5 ± 0.4 and 7.9 ± 0.9 mmol/kg by ABTS• scavenging, DPPH• scavenging, and ORAC, respectively) or did not differ from the TAC of fresh white cabbage (2.2 ± 0.2 mmol/kg by FRAP). The TAC of white sauerkraut juice did not differ from that of white sauerkraut in all assays; the TAC of red sauerkraut juice did not differ from that of red sauerkraut in the ORAC assay (48.9 ± 5.7 mmol/kg) but was lower than the TAC of the sauerkraut in all other assays (29.4 ± 0.8, 20.2 ± 1.3 and 16.4 ± 0.7 mmol/kg in the ABTS• scavenging, DPPH• scavenging and FRAP assays, respectively).
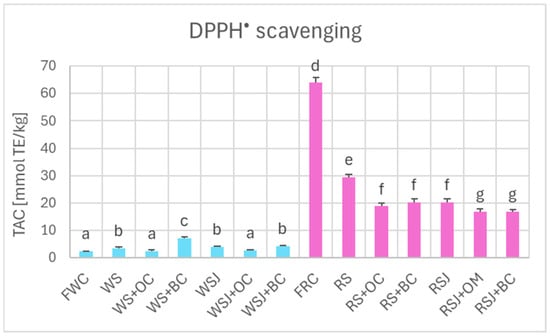
Figure 6.
The TAC of fresh and fermented white and red cabbage estimated by the DPPH• scavenging assay. Pale blue, white cabbage; purple, red cabbage. Different letters indicate statistically significant differences (p < 0.05). One-way ANOVA with post hoc Tukey test.
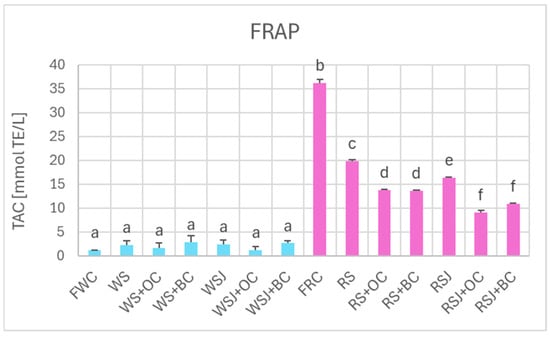
Figure 7.
The TAC of fresh and fermented white and red cabbage estimated by FRAP. Pale blue, white cabbage; purple, red cabbage. Different letters indicate statistically significant differences (p < 0.05). One-way ANOVA with post hoc Tukey test.
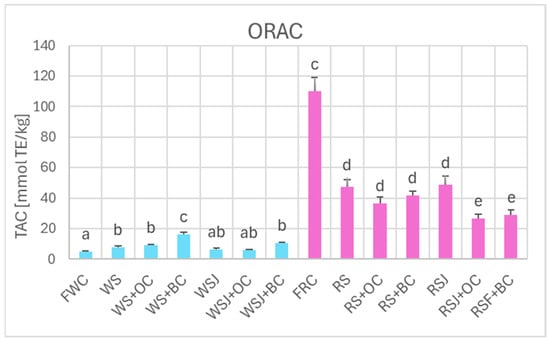
Figure 8.
The TAC of fresh and fermented white and red cabbage estimated by ORAC. Pale blue, white cabbage; purple, red cabbage. Different letters indicate statistically significant differences (p < 0.05). One-way ANOVA with post hoc Tukey test.
Supplementation of cabbage to be fermented with black carrots resulted in an increase in the TAC of the white sauerkraut as estimated by ABTS• scavenging, DPPH• scavenging and ORAC (13.2 ± 0.9, 4.2 ± 0.3 and 10.5 ± 0.8 mmol/kg, respectively), with no significant change observed in the FRAP assay (13.7 ± 0.7 mmol/kg) but a reduction in the TAC of red sauerkraut fermented with carrots, both orange and black (to 36.2 ± 0.1, 18.8 ± 1.0, 13.8 ± 1.3 and 36.6 ± 4.0 mmol/kg for orange carrot and 39.7 ± 0.1, 20.3 ± 1.2, 13.7 ± 0.7 and 41.7 ± 3.0 mmol/kg for black carrot in the ABTS• scavenging, DPPH• scavenging, FRAP and ORAC assays, respectively).
Pearson’s correlation coefficients between the means of the polyphenol content, anthocyanin content, and results of TAC assays were highly significant while those between the carotenoid content and any other parameter were devoid of statistical significance (Table 2).

Table 2.
Correlation coefficients between the parameters describing white and red cabbage and their products.
PCA analysis of variables used to characterize the antioxidant properties of the material studied led to the isolation of two principal components (Table 3).

Table 3.
Eigenvalues, variance contributions, and cumulative variance contributions of two principal components generated by the PCA of parameters used for the characterization of antioxidant properties of cabbage products.
The sequence of the importance of variables for the characterization of cabbage and its products was as follows: TAC-ABTS• (0.973) > TAC-ORAC (0.967) > TAC-DPPH• (0.965) > carotenoid content (0.832) > polyphenol content (0.740) > TAC-FRAP (0.624) > anthocyanin content (0.003).
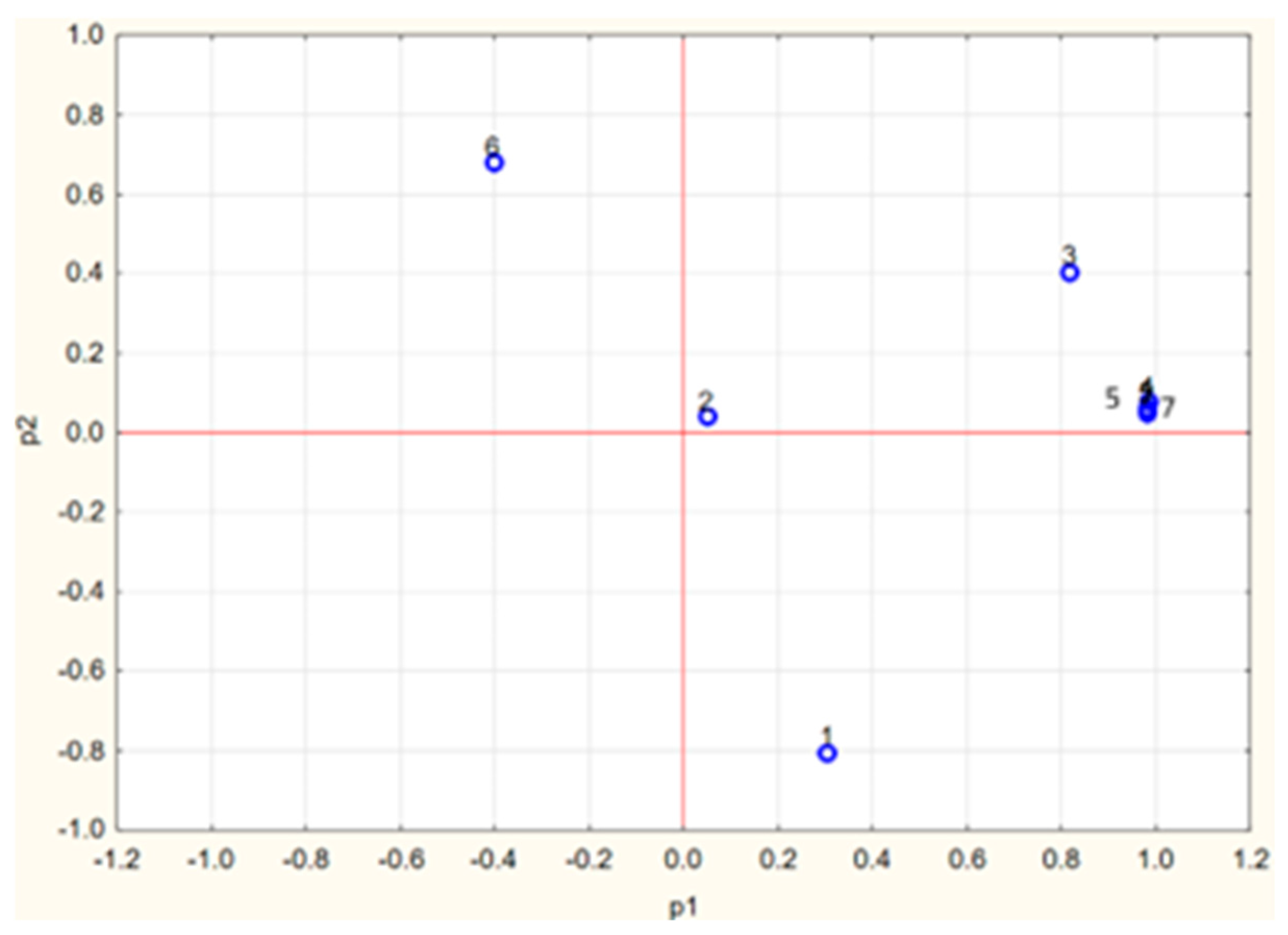
A charge plot of the antioxidant parameters is shown in Figure 9.
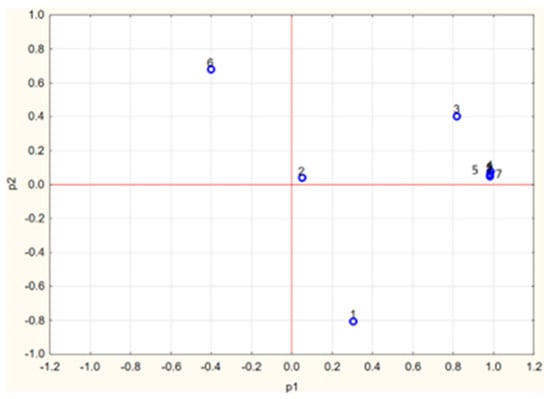
Figure 9.
Charge plot of the parameters used for the description of cabbage antioxidant properties. 1, polyphenol content; 2, anthocyanin content; 3, carotenoid content; 4, TAC-ABTS•; 5, TAC-DPPH•; 6, TAC-FRAP; 7, TAC-ORAC.
The t-values for individual cabbage preparations with respect to new variables 1 and 2 are shown in Table 4.

Table 4.
The t-values for individual cabbage preparations with respect to new variables 1 and 2.
4. Discussion
The present results point to the much higher antioxidant capacity of red cabbage, red sauerkraut, and red sauerkraut juice as compared with white cabbage. Similar conclusions were reached in other studies, usually employing a smaller range of assays [41,42,43,44], although ethanolic extracts of white cabbage were reported to have a higher TAC and higher H2O2-scavenging activity than extracts of red cabbage [45].
As expected, various assays provided different values of TACs of cabbage and its products. This may be partly due to different reaction mechanisms on which these assays are based. FRAP is based on single electron transfer (SET) reactions, utilizing antioxidants, as well as DPPH• and ABTS• scavenging assays, which include both SET and hydrogen atom transfer (HAT) reactions. In contrast, ORAC focuses on HAT reactions [46,47,48]. Lower TAC values of cabbage were observed for the FRAP and DPPH scavenging assays, while the ORAC and ABTS• scavenging assays yielded higher values. It suggests a significant contribution of antioxidants reacting through the HAT mechanism to the TAC of cabbage. In the FRAP assay, addition of black carrots did not elevate the TAC of sauerkraut, while such elevation was observed with the three other methods. It suggests that black carrots and white cabbage have a similar content of Fe3+-reducing antioxidants. It is known that glutathione and other thiols show poor reactivity in this assay [49,50]. While ABTS• and DPPH• are radicals of synthetic molecules, not found in nature, and reactions of antioxidants with these radicals depend on their stereoselectivity, the peroxyl radicals acting as oxidants in the ORAC assay, generated at a relatively low rate, resemble to a higher extent the antioxidant activity in vivo and in food products. The much higher TAC of red cabbage, red sauerkraut, and red sauerkraut juice in comparison with white cabbage and products of its fermentation evidences the higher ability of antioxidants present in red cabbage to react with the physiologically relevant peroxyl radicals.
The increase in TAC of white cabbage after fermentation, also observed by others [25], is intriguing and contrasts with the decrease in the TAC after fermentation of red cabbage. This may be due to (i) the better extractability of antioxidants present in white cabbage after fermentation, (ii) the generation of antioxidants during fermentation, combined with the resistance of antioxidants to lactic fermentation. The TAC of red cabbage is apparently conditioned to a considerable extent by anthocyanins. From 9 to 36 different anthocyanins, predominantly cyanidin derivatives have been detected in various red cabbages. Among them, a large number occurs in acylated forms [51,52]. Anthocyanins are well extractable to aqueous solutions and relatively unstable. A decrease in the anthocyanin content found in this study confirms previous observations [28,52]. The composition of anthocyanins was also demonstrated to be altered by the fermentation of red cabbage [30]. Fermentation was reported to cause a small (about 10%) decrease in the anthocyanin bioavailability as well [52].
The phenolic content, anthocyanin content and results of TAC assays showed high correlation (r ≥ 0.98), while correlating poorly with the carotenoid content. PCA analysis showed a possibility of compression of antioxidant parameters to two principal components. The t-values for principal component 1 were negative for white cabbage and its products, and positive for red cabbage and its products.
The red cabbage contains more polyphenols and anthocyanins than black carrots, since the replacement of 20% cabbage with black carrots decreased the anthocyanin content in the sauerkraut. Indeed, data from aqueous extracts of red carrots pointed to the polyphenol content of black carrots of 1189 [53] and 78.9–2915 mg/kg [54], significantly lower than that found here for red cabbage (6914 ± 99 mg/kg). Our previous study estimated the anthocyanin content of black carrot to be 256 ± 11 mg/kg [55] as compared to 3610 ± 93 found for the red cabbage in this study. In contrast, it is advisable to add vegetables rich in antioxidants, like carrots, to the white cabbage subject to fermentation, to elevate its antioxidant capacity. The considerable antioxidant capacity of red sauerkraut juice, comparable to that of solid red sauerkraut, found in this study, is worth emphasizing since, in contrast to white sauerkraut juice, its consumption is far from popular.
The high anthocyanin content of red cabbage is not its only benefit as, compared with white cabbage, it was reported to contain also more vitamin C and DL-α-tocopherol [56]. It has been emphasized that the assets of red cabbage include high contents of antioxidants, phytochemicals, vitamins (especially C, E, A, K) and mineral components such as calcium, manganese, magnesium, iron, and potassium. We have not studied the ascorbate content since its decrease during cabbage fermentation and concomitant formation of ascorbigen is well documented [27,57,58,59]. It is also low in saturated fats and cholesterol [60]. However, it should be kept in mind that the cabbage contents of antioxidants and other components depend on the growth conditions and fertilization of cabbage [61,62,63,64].
Cabbage (Brassica oleracea var. capitata) belongs to the most widely grown and consumed vegetables in the world, especially in areas where climatic conditions restrict the list of cultivated species [65,66]. Numerous health effects of red cabbage were reported, including anti-inflammatory [67], antimicrobial [68,69] and anticancer action (inhibition of proliferation of cancer cells) [70,71,72]. Red cabbage administered to rats with diabetes induced by high-fat/high-fructose diet ameliorated oxidative stress and improved metabolic profile and hepatic function [73]. Red cabbage reduced diabetic nephropathy in rats with diabetes induced by streptozotocin [74]. In rats fed high-cholesterol diet red cabbage diminished hepatic injury and dyslipidemia [75]. Aqueous extract of red cabbage reduced oxidative stress induced by Triton WR-1339 in rats [76]. Red cabbage extract rich in anthocyanins decreased cognitive impairment in aging mice and had beneficial effects on gut microbiota [77]. Red cabbage juice, but not white cabbage juice, increased the stress resistance and lifespan of Caenorhabditis elegans [78]. These findings speak in favor of the broader consumption of red cabbage and products of its fermentation.
5. Conclusions
This study confirmed the much higher TAC of red cabbage and red sauerkraut as compared with white cabbage and white sauerkraut. The TAC of white sauerkraut was increased by the addition of black carrots to the cabbage while the TAC of red sauerkraut and red sauerkraut juice was decreased by the addition of orange and red carrots. Enrichment of fermented cabbage with carrots increased the carotenoid content of both white and red sauerkraut. The high TAC of red sauerkraut juice is noteworthy and suggests its broader use.
Author Contributions
Conceptualization, I.S.-B.; methodology, I.S.-B., M.R. and G.B.; investigation, M.R. and I.S.-B.; resources, I.S.-B. and M.R.; writing—original draft preparation, I.S.-B., M.R. and G.B.; writing—review and editing. I.S.-B. and G.B.; supervision, I.S.-B.; project administration, I.S.-B.; funding acquisition, I.S.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed within the project “Modification of anthocyanins/anthocyanidins as new markers of food oxidation” (application number 2023/51/B/NZ9/02490) financed by the National Science Centre (NCN), Poland, within the “OPUS 26” program.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are indebted to Edyta Bieszczad-Bedrejczuk for the technical help.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH• | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | gallic acid equivalent(s) |
| ORAC | Oxygen Radical Absorbing Capacity |
| TAC | Total Antioxidant Capacity |
| TE | Trolox equivalent(s) |
| FWC | fresh white cabbage |
| WS | white sauerkraut |
| WS+OC | white sauerkraut with orange carrots |
| WS+BC | white sauerkraut with black carrots |
| WSJ | white sauerkraut juice |
| WSJ+OC | juice of white sauerkraut with orange carrots |
| WSJ+BC | juice of white sauerkraut with black carrots |
| FRC | fresh red cabbage |
| RS | red sauerkraut |
| RS+OC | red sauerkraut with orange carrots |
| RS+BC | red sauerkraut with black carrots |
| RSJ | red sauerkraut juice |
| RSJ+OC | juice of red sauerkraut with orange carrots |
| RSJ+BC | juice of red sauerkraut with black carrots |
References
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant activity of anthocyanins and anthocyanidins: A critical review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins and heart health. Ann. Ist. Super. Sanità 2007, 43, 369–374. [Google Scholar] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of anthocyanins on vascular health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Llauradó, E.; Valls, R.M.; Salamanca, P.; Rubió, L.; Yuste, S.; Solà, R. The health benefits of anthocyanins: An umbrella review of systematic reviews and meta-analyses of observational studies and controlled clinical trials. Nutr. Rev. 2022, 80, 1515–1530. [Google Scholar] [CrossRef]
- Santacroce, L.; Bottalico, L.; Charitos, I.A.; Haxhirexha, K.; Topi, S.; Jirillo, E. Healthy diets and lifestyles in the world: Mediterranean and blue zone people live longer. Special focus on gut microbiota and some food components. Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 1774–1784. [Google Scholar] [CrossRef]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2024, 4, 7563–7588. [Google Scholar] [CrossRef]
- Willcox, D.C.; Willcox, B.J.; Todoriki, H.; Suzuki, M. The Okinawan diet: Health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J. Am. Coll. Nutr. 2009, 28, 500S–516S. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Dwivedi, S.L.; Dutt, S.; Singh, B.; Garg, M.; Ortiz, R. Anthocyanin-rich vegetables for human consumption—Focus on potato, sweetpotato and tomato. Int. J. Mol. Sci. 2022, 23, 2634. [Google Scholar] [CrossRef]
- Gandikota, M.; De Kochko, A.; Chen, L.; Ithal, N.; Fauquet, C.; Reddy, A.R. Development of transgenic rice plants expressing maize anthocyanin genes and increased blast resistance. Mol. Breed. 2001, 7, 73–83. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, H. Anthocyanins in plant food: Current status, genetic modification, and future perspectives. Molecules 2023, 28, 866. [Google Scholar] [CrossRef] [PubMed]
- Bazzani, C.; Canavari, M. Is local a matter of food miles or food traditions? Ital. J. Food Sci. 2017, 29, 505–517. [Google Scholar] [CrossRef]
- Panlaqui, C.C.; Abad, C. Zero kilometer food project: An analysis of food demand and supply structure of high-value crops. Int. J. Innov. Sci. Res. Rev. 2024, 6, 7372–7379. [Google Scholar]
- Owens, S.R. Feeding prejudice: Reluctance within the European Union to accept genetically modified crops may hinder the benefits of this technology reaching the developing world. EMBO Rep. 2003, 4, 229–232. [Google Scholar] [CrossRef]
- Herring, R.J. Opposition to transgenic technologies: Ideology, interests and collective action frames. Nat. Rev. Genet. 2008, 9, 458–463. [Google Scholar] [CrossRef]
- Folta, K.M. Acceptance of crop biotechnology requires a change in communication strategy. Plant Physiol. 2025, 198, kiaf167. [Google Scholar] [CrossRef] [PubMed]
- Heinzelmann, U. Food Culture in Germany; Bloomsbury Publishing: Westport, CT, USA, 2008. [Google Scholar]
- Halawa, M.; Parasecoli, F. Designing the future of Polish food: How cosmopolitan tastemakers prototype a national gastronomy. Gastronomica 2022, 22, 8–18. [Google Scholar] [CrossRef]
- Draghici, G.A.; Lupu, M.A.; Borozan, A.B.; Nica, D.; Alda, S.; Alda, L.; Gogoasa, I.; Gergen, I.; Bordean, D.M. Red cabbage, millennium’s functional food. J. Hortic. For. Biotechnol. 2013, 17, 52–55. [Google Scholar]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Żarski, W. Culinary identity as the determinant of cultural distinctiveness in Silesia and the Vilnius region. In Estonia and Poland: Creativity and Tradition in Cultural Communication; Laineste, L., Brzozowska, D., Chłopicki, W., Eds.; ELM Scholarly Press: Tartu, Estonia, 2013; Volume 2, pp. 147–160. [Google Scholar]
- Świtała-Trybek, D. New trends in culinary tourism–regional (Silesian) fusion cuisine. Geog. Tour. 2020, 1, 47–54. Available online: https://czasopisma.ukw.edu.pl/index.php/gat/article/view/132 (accessed on 31 July 2025).
- Kusznierewicz, B.; Śmiechowska, A.; Bartoszek, A.; Namieśnik, J. The effect of heating and fermenting on antioxidant properties of white cabbage. Food Chem. 2008, 108, 853–861. [Google Scholar] [CrossRef]
- Sun, Y.P.; Chou, C.C.; Yu, R.C. Antioxidant activity of lactic-fermented Chinese cabbage. Food Chem. 2009, 115, 912–917. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Sidro, B.; Ullate, M.; Frías, J.; Vidal-Valverde, C. White cabbage fermentation improves ascorbigen content, antioxidant and nitric oxide production inhibitory activity in LPS-induced macrophages. LWT 2012, 46, 77–83. [Google Scholar] [CrossRef]
- Hunaefi, D.; Akumo, D.N.; Smetanska, I. Effect of fermentation on antioxidant properties of red cabbages. Food Biotechnol. 2013, 27, 66–85. [Google Scholar] [CrossRef]
- Parada, R.B.; Emilio, M.; Campos, C.A.; Marisol, V. Improving the nutritional properties of Brassica L. vegetables by spontaneous fermentation. Foods Raw Mater. 2022, 10, 97–105. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Changes in the content and composition of anthocyanins in red cabbage and its antioxidant capacity during fermentation, storage and stewing. Food Chem. 2015, 167, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Świder, O.; Roszko, M.Ł.; Wójcicki, M. The inhibitory effects of plant additives on biogenic amine formation in fermented foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 12935–12960. [Google Scholar] [CrossRef]
- Mam, S.; Rudra, S.G.; Singh, S. Comparison of starter spices for retention of sensory attributes, appearance, and antioxidants in red cabbage sauerkraut. J. Sci. Ind. Res. 2024, 83, 1214–1222. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q.; Li, H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A modification of the ABTS• decolorization method and an insight into its mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Furdak, P.; Bartosz, G.; Sadowska-Bartosz, I. Effect of thermal treatment on the antiproliferative and antioxidant activities of garlic. Food Sci. Nutr. 2025, 13, e70375. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Chun, O.K.; Smith, N.; Sakagawa, A.; Lee, C.Y. Antioxidant properties of raw and processed cabbages. Int. J. Food Sci. Nutr. 2004, 55, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Kamińska, I.; Kołton, A. Phenolic compounds as the major antioxidants in red cabbage. Folia Hort. 2010, 22, 19–24. [Google Scholar] [CrossRef]
- Ashfaq, F.; Butt, M.S.; Bilal, A.; Tehseen, S.; Suleria, H.A. Comparative assessment of free radical scavenging ability of green and red cabbage based on their antioxidant vitamins and phytochemical constituents. Curr. Bioact. Compd. 2020, 16, 1231–1241. [Google Scholar] [CrossRef]
- Ha, J.; Park, S.E.; Hwang, I.G.; Bang, K.W.; Kim, S.-H.; Lee, J.G.; Choi, C.-S.; Kang, H.J. Evaluation of the antioxidant activities in cabbage (Brassica oleracea var. capitata) accessions. J. Korean Soc. Food Sci. Nutr. 2023, 52, 679–690. [Google Scholar] [CrossRef]
- Al Jabr, F.A.; Saif, M.A.; Al Zaid, A.S.; Al Homood, M.I.; Al Thani, H.A.; Al Qadheeb, A.M. Red and white cabbage extracts: Antioxidant effects on bovines albumins. Int. J. Pharm. Res. Allied Sci. 2020, 9, 97–104. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 2. Hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid peroxidation assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Resat Apak, R., Esra Capanoglu, E., Fereidoon, S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 77–106. [Google Scholar] [CrossRef]
- Furdak, P.; Kut, K.; Bartosz, G.; Sadowska-Bartosz, I. Comparison of various assays of antioxidant activity/capacity: Limited significance of redox potentials of oxidants/indicators. Int. J. Mol. Sci. 2025, 26, 7069. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Romaszko, J. The impact of red cabbage fermentation on bioavailability of anthocyanins and antioxidant capacity of human plasma. Food Chem. 2016, 190, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Shams, R.; Dash, K.K.; Shaikh, A.M.; Kovács, B. Anthocyanin extraction from black carrot: Health promoting properties and potential applications. J. Agric. Food Res. 2025, 19, 101533. [Google Scholar] [CrossRef]
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Bartosz, G.; Baran, S.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. Antioxidant capacity and hydrogen peroxide formation by black and orange carrots: Black and orange carrots. Agric. Food Sci. 2022, 31, 71–77. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, A.K.; Bahadur, A.; Singh, B.; Singh, K.P.; Rai, M. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata). Sci. Hortic. 2006, 108, 233–237. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Frías, J.; Ciska, E.; Honke, J.; Piskula, M.K.; Kozlowska, C.; Vidal-Valverde, C. Influence of fermentation conditions on glucosinolates, ascorbigen, and ascorbic acid content in white cabbage (Brassica oleracea var. capitata cv. Taler) cultivated in different seasons. J. Food Sci. 2009, 74, C62–C67. [Google Scholar] [CrossRef]
- Pandey, S.; Garg, F.C. Preparation of spiced sauerkraut by using lactic acid bacteria and by natural fermentation. Int. J. Sci. Res. 2013, 4, 2753–2761. [Google Scholar]
- Du, R.; Song, G.; Zhao, D.; Sun, J.; Ping, W.; Ge, J. Lactobacillus casei starter culture improves vitamin content, increases acidity and decreases nitrite concentration during sauerkraut fermentation. Int. J. Food Sci. Technol. 2018, 53, 1925–1931. [Google Scholar] [CrossRef]
- Sarkar, D.; Rakshit, A. Red cabbage as potential functional food in the present perspective. Int. J. Bioresource Sci. 2017, 4, 7–8. [Google Scholar] [CrossRef][Green Version]
- Pavla, B.; Pokluda, R. Influence of alternative organic fertilizers on the antioxidant capacity in head cabbage and cucumber. Not. Bot. Horti Agrobot. Cluj-Na. 2008, 36, 63–67. [Google Scholar] [CrossRef]
- Bimova, P.; Pokluda, R. Impact of organic fertilizers on total antioxidant capacity in head cabbage. Hortic. Sci. 2009, 36, 21–25. [Google Scholar] [CrossRef]
- Biesiada, A.; Nawirska-Olszanska, A.; Kucharska, A.; Sokol-Letowska, A.; Kedra, K. The effect of nitrogen fertilization on nutritive value and antioxidative activity of red cabbage. Acta Sci. Pol. Hortorum Cultus 2010, 9, 13–21. [Google Scholar]
- Phahlane, C.J.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Development, yield, and antioxidant content in red cabbage as affected by plant density and nitrogen rate. Int. J. Veg. Sci. 2018, 24, 160–168. [Google Scholar] [CrossRef]
- Ștefan, I.M.A.; Ona, A.D. Cabbage (Brassica oleracea L.). Overview of the health benefits and therapeutical uses. Hop Med. Plants 2020, 28, 150–169. Available online: https://www.researchgate.net/publication/353411136 (accessed on 8 September 2025).
- Mabry, M.E.; Turner-Hissong, S.D.; Gallagher, E.Y.; McAlvay, A.C.; An, H.; Edger, P.P.; Moore, J.D.; Pink, D.A.C.; Teakle, G.R.; Stevens, C.J.; et al. The evolutionary history of wild, domesticated, and feral Brassica oleracea (Brassicaceae). Mol. Biol. Evol. 2021, 38, 4419–4434. [Google Scholar] [CrossRef]
- Ha, H.J.; Lee, C.B. Antioxidant and anti-inflammation activity of red cabbage extract. Culi. Sci. Hos. Res. 2014, 20, 16–26. [Google Scholar]
- Demirdöven, A.; Karabıyıklı, Ş.; Tokatlı, K.; Öncül, N. Inhibitory effects of red cabbage and sour cherry pomace anthocyanin extracts on food borne pathogens and their antioxidant properties. LWT 2015, 63, 8–13. [Google Scholar] [CrossRef]
- Guan, Y.; Ji, Y.; Yang, X.; Pang, L.; Cheng, J.; Lu, X.; Zheng, J.; Yin, L.; Hu, W. Antioxidant activity and microbial safety of fresh-cut red cabbage stored in different packaging films. LWT 2023, 175, 114478. [Google Scholar] [CrossRef]
- Gaafar, A.A.; Aly, H.F.; Salama, Z.A.; Mahmoud, K.M. Characterizing the antioxidant and anticancer properties of secondary metabolites from red and white cabbages Brassica oleracea L. var. capitata. World J. Pharm. Res. 2014, 3, 171–186. [Google Scholar]
- Tajalli, F.; Saeedi, M.; Malekabadi, A.V. Anticancer and antioxidant effects of red cabbage on three cancerous cell lines and comparison with a normal cell line (HFF-3). J. Genes Cells 2020, 6, 12–20. [Google Scholar] [CrossRef]
- Drozdowska, M.; Leszczyńska, T.; Koronowicz, A.; Piasna-Słupecka, E.; Dziadek, K. Comparative study of young shoots and the mature red headed cabbage as antioxidant food resources with antiproliferative effect on prostate cancer cells. RSC Adv. 2020, 10, 43021–43034. [Google Scholar] [CrossRef] [PubMed]
- Dal, S.; Van der Werf, R.; Walter, C.; Bietiger, W.; Seyfritz, E.; Mura, C.; Peronet, C.; Legrandois, J.; Werner, D.; Ennahar, S.; et al. Treatment of NASH with antioxidant therapy: Beneficial effect of red cabbage on type 2 diabetic rats. Oxid. Med. Cell. Longev. 2018, 2018, 7019573. [Google Scholar] [CrossRef]
- Kataya, H.A.; Hamza, A.A. Red cabbage (Brassica oleracea) ameliorates diabetic nephropathy in rats. Evid. Based Complement. Altern. Med. 2008, 5, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, M.S. Red cabbage (Brassica oleracea L.) mediates redox-sensitive amelioration of dyslipidemia and hepatic injury induced by exogenous cholesterol administration. Am. J. Chin. Med. 2014, 42, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Veber, B.; Camargo, A.; Dalmagro, A.P.; Bonde, H.L.P.; Magro, D.D.D.; Lima, D.D.D.; Zeni, A.L.B. Red cabbage (Brassica oleracea L.) extract reverses lipid oxidative stress in rats. An. Acad. Bras. Ciênc. 2020, 92, e20180596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jing, P. Red cabbage anthocyanins attenuate cognitive impairment by attenuating neuroinflammation and regulating gut microbiota in aging mice. J. Agric. Food Chem. 2023, 71, 15064–15072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiao, S.; Jing, P. Red cabbage rather than green cabbage increases stress resistance and extends the lifespan of Caenorhabditis elegans. Antioxidants 2021, 10, 930. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).