Antifungal Activity of Euclea divinorum Root and Study of its Ethnobotany and Phytopharmacology
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethnobotanical Survey
2.2. Plant Material
2.3. Extraction
- Direct extract: 2 g powdered root barks were extracted direct with 100 ml methanol (DM)
- Sequential extracts: a sequential extraction way was demonstrated to produce different subextracts from the dried root bark; 30 g powdered root bark was extracted with different solvents of increasing polarity, 300 mL for each: dichloromethane, ethyl acetate and methanol successively at room temperature for 8 hours.
2.4. Antifungal Test—Agar Diffusion Test
2.5. Determination of Antioxidant Activity
2.6. Determination of Cytotoxicity Activity
2.7. Identification of Naphthoquinones on Thin-Layer Chromatography (TLC)
3. Results
3.1. Ethnobotany of Euclea divinorum
3.2. Antifungal Activity
3.3. Antioxidant Activity
3.4. Cytotoxic Activity
3.5. Identification of Naphthoquinones
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Fatimi, M. Ethnobotanical Survey of Dracaena cinnabari and investigation of the pharmacognostical properties, antifungal and antioxidant activity of its resin. Plants 2018, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Mgbeahuruike, E.E.; Holm, Y.; Vuorela, H.; Amandikwa, C.; Fyhrquist, P. An ethnobotanical survey and antifungal activity of Piper guineense used for the treatment of fungal infections in West-African traditional medicine. J. Ethnopharmacol. 2019, 229, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatimi, M.; Friedrich, U.; Jenett-Siems, K. Cytotoxicity of plants used for traditional medicine in Yemen. Fitoterapia 2005, 76, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Meyer, J.J. Antibacterial activity of water and acetone extracts of the roots of Euclea natalensis. J. Ethnopharmacol. 2000, 72, 313–316. [Google Scholar] [CrossRef]
- Nyambe, M.M.; Hans, R.; Beukes, M.; Morris, J.; Kandawa-Schulz, M. Phytochemical and antibacterial analysis of indigenous chewing sticks, Diospyros lyciodes and Euclea divinorum of Namibia. Biofarmasi J. Nat. Prod. Biochem. 2018, 16, 29–43. [Google Scholar] [CrossRef]
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J. Ethnopharmacol. 2007, 111, 657–666. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Gilbreath, G.G.; Solio, J.; Lutura, M.; Lutuluo, R.; Kunguru, K.; Wood, N.; Mathenge, S.G. Plant use of the Maasai of Sekenani Valley, Maasai Mara, Kenya. J. Ethnobiol. Ethnomed. 2006, 2, 22. [Google Scholar] [CrossRef]
- Ng’ang’a, M.M.; Hussain, H.; Chhabra, S.; Langat-Thoruwa, C.; Al-Harrasi, A.; Krohn, K.; Green, I.R. Eucleanal A and B: Two new napthalene derivatives from Euclea divinorum. Chin. Chem. Lett. 2012, 23, 576–578. [Google Scholar] [CrossRef]
- Maroyi, A. Euclea undulata Thunb.: Review of its botany, ethnomedicinal uses, phytochemistry and biological activities. Asian Pac. J. Trop. Med. 2017, 10, 1030–1036. [Google Scholar] [CrossRef]
- Stander, I.; Van Wyk, C.W. Toothbrushing with the root of Euclea natalensis. J. Biol. Buccale 1991, 19, 167–172. [Google Scholar]
- Van Damme, P.; Van Den Eynden, V.; Vernemmen, P. Plant uses by the Topnaar of the Sesfontein area (Namib Desert). Afr. Focus 1992, 8, 223–252. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Ethnobotanical study of plants used in the management of HIV/AIDS-Related diseases in Livingstone, Southern Province, Zambia. Evid. Based Complement. Altern. Med. 2016, 2016, 4238625. [Google Scholar] [CrossRef] [PubMed]
- Mebe, P.P.; Cordell, G.A.; Pezzuto, J.M. Pentacyclic triterpenes and naphthoquinones from Euclea divinorum. Phytochemistry 1998, 47, 311–313. [Google Scholar] [CrossRef]
- Kaluwa Kaingu, C.; Oduma, J.A.; OduKanuima, T. Preliminary investigation of contractile activity of Ricinus communis and Euclea divinorum extracts on isolated rabbit uterine strips. J. Ethnopharmacol. 2012, 142, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Kigen, G.; Kipkore, W.; Wanjohi, B.; Haruki, B.; Kemboi, J. Medicinal plants used by traditional healers in Sangurur, Elgeyo Marakwet County, Kenya. Pharmacogn. Res. 2017, 9, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Woldemedhin, B.; Nedi, T.; Shibeshi, W.; Sisay, M. Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of the root of Euclea divinorum Hiern (Ebenaceae) in Sprague Dawley rats. J. Ethnopharmacol. 2017, 202, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Geyid, A.; Abebe, D.; Debella, A.; Makonnen, Z.; Aberra, F.; Teka, F.; Kebede, T.; Urga, K.; Yersaw, K.; Biza, T.; et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J. Ethnopharmacol. 2005, 97, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Costa, M.A.; Lopes, M.H.; Paul, M.I.; Ferreira, M.A.; Correia-Alves, A. Naphthaquinones and triterpenoids of Euclea divinorum. Phytochemistry 1976, 15, 829. [Google Scholar] [CrossRef]
- Van der Vijver, L.M.; Gerritsma, K.W. Napthoquinones of Euclea and Diospyros species. Phytochemistry 1974, 13, 2322–2323. [Google Scholar] [CrossRef]
- Hattas, D.; Hjältén, J.; Julkunen-Tiitto, R.; Scogings, P.F.; Rooke, T. Differential phenolic profiles in six African savanna woody species in relation to antiherbivore defense. Phytochemistry 2011, 72, 1796–1803. [Google Scholar] [CrossRef]
- Homer, K.A.; Manji, F.; Beighton, D. Inhibition of peptidase and glycosidase activities of Porphyromonas gingivalis, Bacteroides intermedius and Treponema denticola by plant extracts. J. Clin. Periodontol. 1992, 19, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Homer, K.A.; Manji, F.; Beighton, D. Inhibition of protease activities of periodontopathic bacteria by extracts of plants used in Kenya as chewing sticks (mswaki). Arch. Oral Biol. 1990, 35, 421–424. [Google Scholar] [CrossRef]
- More, G.; Tshikalange, T.E.; Lall, N.; Botha, F.; Meyer, J.J. Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol. 2008, 119, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Samie, A.; Tambani, T.; Harshfield, E.; Green, E.; Ramalivhana, J.N.; Bessong, P.O. Antifungal activities of selected Venda medicinal plants against Candida albicans, Candida krusei and Cryptococcus neoformans isolated from South African AIDS patients. Afr. J. Biotechnol. 2010, 9, 2965–2976. [Google Scholar] [CrossRef]
- Feyissa, T.; Asres, K.; Engidawork, E. Renoprotective effects of the crude extract and solvent fractions of the leaves of Euclea divinorum Hierns against gentamicin-induced nephrotoxicity in rats. J. Ethnopharmacol. 2013, 145, 758–766. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M.D. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lindl, T.; Bauer, J. Zell- und Gewebekultur, Einführung in die Grundlagen Sowie Ausgewählte Methoden und Anwendungen; Gustav-Fischer-Verlag Jena: Stuttgart, Germany, 1989; p. 181. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis. A Thin Layer Chromatography Atlas; Springer: Heidelberg, Germany, 2009; pp. 275–279. [Google Scholar]
- Miller, A.G.; Morris, M. Ethnoflora of the Soqotra Archipelago; Charlesworth Group: Huddersfield, UK, 2004; pp. 540–541. [Google Scholar]
- Evans, W.C. Trease and Evans Pharmacognosy, 15th ed.; Saunders Ltd.: London, UK, 2002; pp. 432–434. [Google Scholar]
- Bapela, M.J.; Lall, N.; Meyer, J.J.M. Seasonal variation of naphthoquinones in Euclea natalensis subspecies natalensis. S. Afr. J. Bot. 2008, 74, 218–224. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Havel, L.; Kizek, R. Noteworthy secondary metabolites naphthoquinones-their occurrence, pharmacological properties and analysis. Curr. Pharm. Anal. 2009, 5, 47–68. [Google Scholar] [CrossRef]
- Krenn, L.; Blaeser, U.; Hausknost-Chenicek, N. Determination of Naphthoquinones in Droserae herba by Reversed-Phase High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 1998, 20, 3149–3160. [Google Scholar] [CrossRef]
- Joubert, A.; van der Kooy, F.; Meyer, J.J.M.; Lall, N. HPLC in the comparative study of the content of naphthoquinones (quinonoid constituents) in Euclea species of South Africa. Chroma 2006, 64, 399–403. [Google Scholar] [CrossRef]
- Uddin, G.; Rauf, A.; Arfan, M.; Rehman, T.U.; Khan, A.Z.; Ali, G.; Rehman, B.; Zia-ul-Haq, M. Molecular docking of Diospyrin as a LOX inhibitory compound. J. Saudi Chem. Soc. 2016, 20, S448–S450. [Google Scholar] [CrossRef]
- Weigenand, O.; Hussein, A.A.; Lall, N.; Meyer, J.J. Antibacterial activity of naphthoquinones and triterpenoids from Euclea natalensis root bark. J. Nat. Prod. 2004, 67, 1936–1938. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Q.; Graf, T.N.; Lee, D.; Chai, H.B.; Mi, Q.; Kardono, L.B.; Setyowati, F.M.; Ismail, R.; Riswan, S.; Farnsworth, N.R.; et al. Cytotoxic and antimicrobial constituents of the bark of Diospyros maritima collected in two geographical locations in Indonesia. J. Nat. Prod. 2004, 67, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Ziaratnia, S.M.; Kunert, K.J.; Lall, N. Elicitation of 7-methyljuglone in Drosera capensis. S. Afr. J. Bot. 2009, 75, 97–103. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Habib, E.; León, F.; Radwan, M.M.; Tabanca, N.; Gao, J.; Wedge, D.E.; Cutler, S.J. Antifungal metabolites from the roots of Diospyros virginiana by overpressure layer chromatography. Chem. Biodivers. 2011, 8, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Weiganand, O.; Hussein, A.A.; Meyer, J.J.M. Antifungal activity of naphthoquinones and triterpenes isolated from the root bark of Euclea natalensis. S. Afr. J. Bot. 2006, 72, 579–583. [Google Scholar] [CrossRef]
- Gafner, S.; Wolfender, J.L.; Nianga, M.; Stoeckli-Evans, H.; Hostettmann, K. Antifungal and antibacterial naphthoquinones from Newbouldia laevis roots. Phytochemistry 1996, 42, 1315–1320. [Google Scholar] [CrossRef]
- Sasaki, K.; Abe, H.; Yoshizaki, F. In vitro antifungal activity of naphthoquinone derivatives. Biol. Pharm. Bull. 2002, 25, 669–670. [Google Scholar] [CrossRef]
- Futuro, D.O.; Ferreira, P.G.; Nicoletti, C.D.; Borba-Santos, L.P.; Silva, F.C.D.; Rozental, S.; Ferreira, V.F. The antifungal activity of naphthoquinones: An integrative review. An. Acad. Bras. Cienc. 2018, 90, 1187–1214. [Google Scholar] [CrossRef]
- Errante, G.; La Motta, G.; Lagana, C.; Wittebolle, V.; Sarciron, M.E.; Barret, R. Synthesis and evaluation of antifungal activity of naphthoquinone derivatives. Eur. J. Med. Chem. 2006, 41, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooy, F.; Meyer, J.J.M.; Lall, N. Antimycobacterial activity and possible mode of action of newly isolated neodiospyrin and other naphthoquinones from Euclea natalensis. S. Afr. J. Bot. 2006, 72, 349–352. [Google Scholar] [CrossRef]

| Used Part/ Extract Solvent | Pharmacological Activities/Details [References] | Model of Test |
|---|---|---|
| 1. Antibacterial activity against oral pathogens | ||
| Root/Methanol | Activity against Streptococcus mutans and S. sanguinis by MIC < 2.5 mg/mL [5] | in vitro |
| Stick (Stem)/ Dichloromethane | Weak activity against Staphyloccocus aureus, Escherichia coli, Bacillus subtilis [21] | in vitro |
| Stem/Aqueous | Inhibition of enzymes activity of Porphyromonas gingivalis, Treponema denticola; Bacteroides gingivalis, B. intermedius up to 200 μg/mL [22] | in vivo |
| Bark, Leaves/Ethanol | Activity against Actinomyces naeslundii, Streptococcus mutans and Actinobacillus actinomycetemcomitans (MIC: 6.2–25.0 mg/mL) [23] | in vitro |
| Fruit/Methanol | Moderate activity against Neisseria gonorrhoea (250–2000 µg/mL) [17] | in vitro |
| 2. Antifungal activity | ||
| Leaves/Hexane | No activity against Candida albicans, and C. krusei [24] | in vitro |
| Leaves/Methanol | Weak activity against C. albicans; Cryptococcus neoformans (4000 µg/mL). No activity against Aspergillus flavus; A. niger; Tricophyton mentagrophytes and T. violacium [17] | in vitro |
| Bark, Leaves/Ethanol | No effect on C. albicans [23] | in vitro |
| 3. Cytotoxicity activity | ||
| Bark, Leaves/Ethanol | Moderate cytotoxicity (inhibitory concentration (IC50): 142.3 μg/mL) on vero cell line [23] | in vitro |
| Root/Petrol ether/ Ethyl acetate 1:1; Methanol | The lipophilic extract displayed marked toxicity (IC50 value 11.6 µg/mL) [3]; The polar extract showed less toxicity (IC50 36.0 µg/mL) against ECV-304 cells [3] | in vitro |
| 4. Oxytocic activity | ||
| Root, Bark/Aqueous, Ethanol | Direct stimulation of the uterus contraction [14] | in vivo |
| 5. Diuretic activity | ||
| Root/80% Methanol | Diuretic effect at 200–400 mg/kg [16] | in vivo |
| 6. Antioxidant activity | ||
| Root/Aqueous; Methanol | Antioxidant activity at 2000 µg/mL with 74.5–82.5% DPPH inhibition [25] | in vitro |
| 7. Toxicity effect | ||
| Root/80% Methanol | Safe extract at 2000 mg/kg [16] | in vivo |
| 8. Antidote effect | ||
| Leaves/Methanol | Antidote for nephrotoxicity caused by gentamicin at (100 mg/kg) [25] | in vivo |
| Used Part/ Preparation Form/Administration Route | Ethnobotanical Uses | Ct % | Ref |
|---|---|---|---|
| 1. Medicinal uses | |||
| 1.1. Dental diseases | 100 | ||
| Root/dried, raw, cut pieces are used as toothbrush (misawk)/topically | dental diseases: mouth and dental fungal infection | [3] | |
| Root/dried powder of root barks/ rubbing on the teeth | tooth cleaning | [3,30], | |
| Root/root bark is powdered with little water and salt and inserted into the tooth cavity | toothache, tooth cavity | [or], [30] | |
| 1.2. Dermal diseases | 70 | ||
| Roots/dried powder of the root bark in little water as paste/topically | fresh wound, abscesses and kin diseases | [3,30] | |
| Root/a piece is chewed and salvia used or Root barks powdered and mixed in little water ass paste/ topically | skin complaints, sores, fungal skin complaints, (ringworm) | [or], [30] | |
| 1.3. Blood diseases | 20 | ||
| Root/a piece of root is chewed and the produced salvia is swallowed/internal | to purify blood | [30] | |
| 2. Cosmetic | 90 | ||
| Root pieces/fresh or dried root pieces used as chewing/ topically | red colouring of lip and mouth | [or], [30] | |
| 3. Economic uses | 50 | ||
| Leaves, fruit/ dried or fresh parts | fodder for livestock and for firewood | [or], [30] | |
| Dried stems | firewood | [or] | |
| Dried stems | house ceiling | [or] | |
| Fungi Strains | IZ Diameter (mm) | ||||
|---|---|---|---|---|---|
| SD | SE | SM | DM | Nys | |
| Absidia corymbifera | 17 | 10 | 17 | 18 | 25 |
| Aspergillus fumigatus | 16 | 15 | 16 | 15 | 24 |
| Candida krusei | 16 | 15 | 16 | 18 | 24 |
| Microsporum gypseum | 25 | 16 | 15 | 20 | 22 |
| Mucor sp. | 10 | 10 | 10 | 15 | 21 |
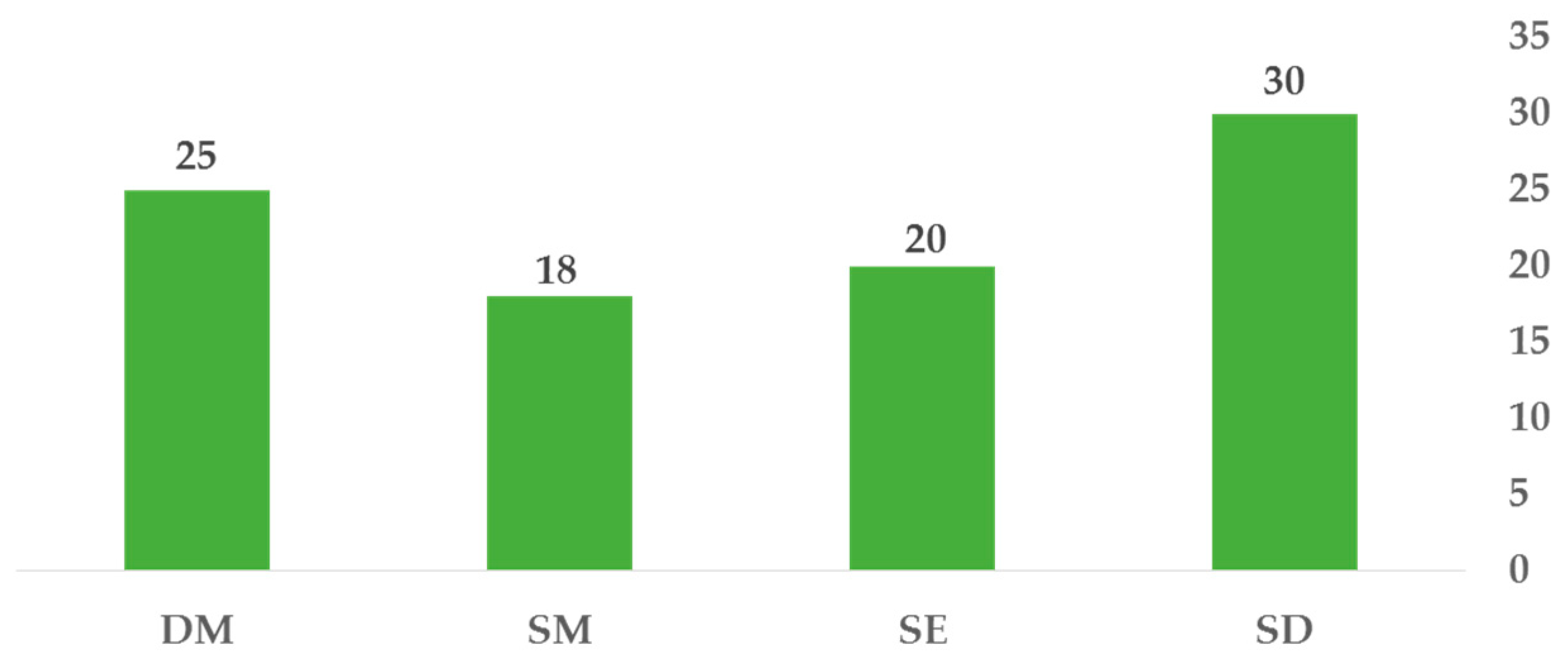
| Trichophyton mentagrophytes | 30 | 20 | 18 | 25 | 25 |
| Extracts Solvents | IC50 (μg/mL) | |
|---|---|---|
| Antioxidant activity | Cytotoxicity | |
| Dichloromethane | 690.5 | 240.0 |
| Sequential ethyl acetate | 680.8 | 387.7 |
| Sequential methanol | 225.0 | 800.6 |
| Direct methanol | 550.0 | 900.5 |
| Ascorbic acid a | 15.5 | - |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Fatimi, M. Antifungal Activity of Euclea divinorum Root and Study of its Ethnobotany and Phytopharmacology. Processes 2019, 7, 680. https://doi.org/10.3390/pr7100680
Al-Fatimi M. Antifungal Activity of Euclea divinorum Root and Study of its Ethnobotany and Phytopharmacology. Processes. 2019; 7(10):680. https://doi.org/10.3390/pr7100680
Chicago/Turabian StyleAl-Fatimi, Mohamed. 2019. "Antifungal Activity of Euclea divinorum Root and Study of its Ethnobotany and Phytopharmacology" Processes 7, no. 10: 680. https://doi.org/10.3390/pr7100680
APA StyleAl-Fatimi, M. (2019). Antifungal Activity of Euclea divinorum Root and Study of its Ethnobotany and Phytopharmacology. Processes, 7(10), 680. https://doi.org/10.3390/pr7100680





