Suppression of Aluminum Dust Explosion by Ca(H2PO4)2/RM Composite Powder with Core–Shell Structure: Effect and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Flame Propagation Suppression Test
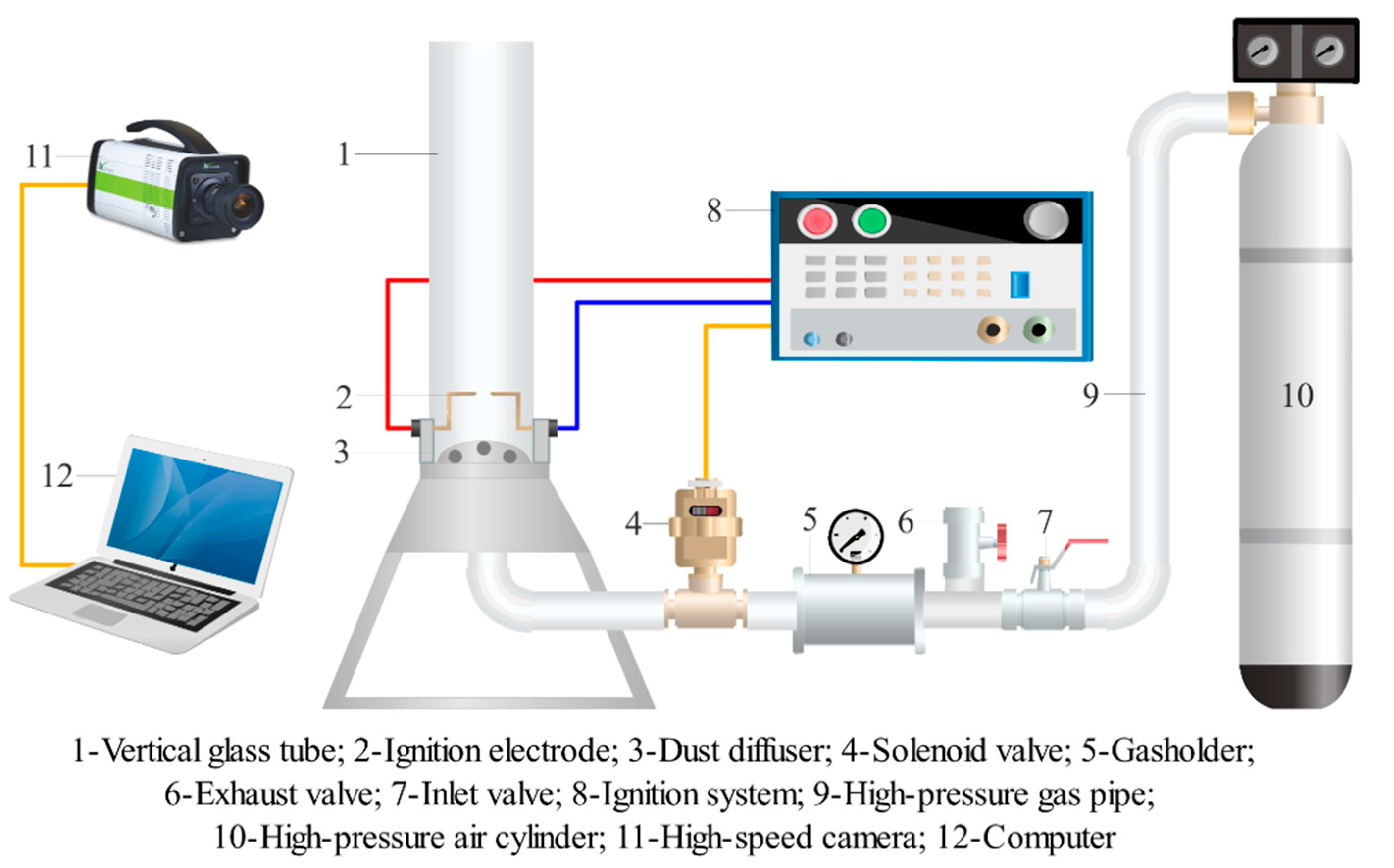
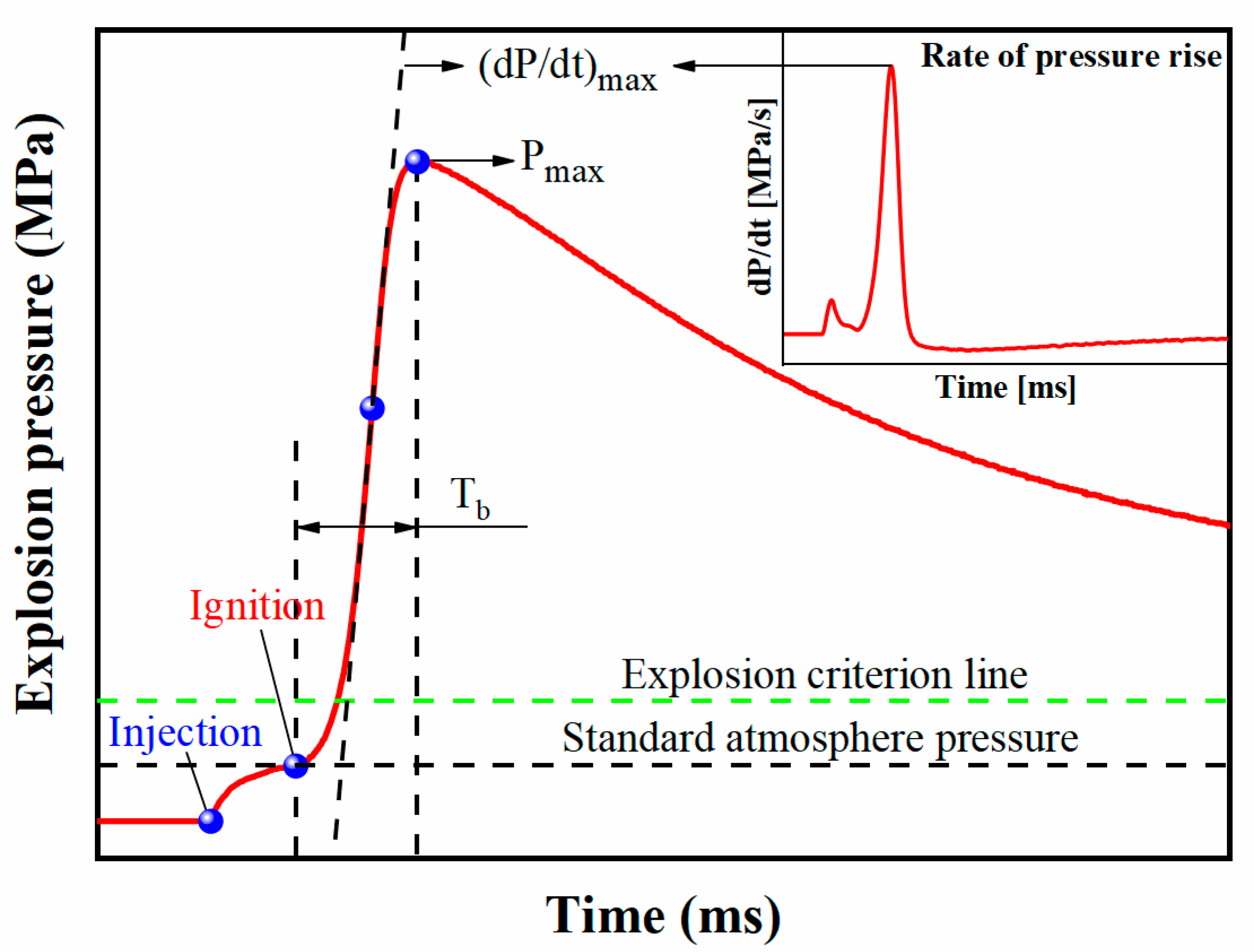
2.3. Explosion Overpressure Suppression Test
3. Results and Discussion
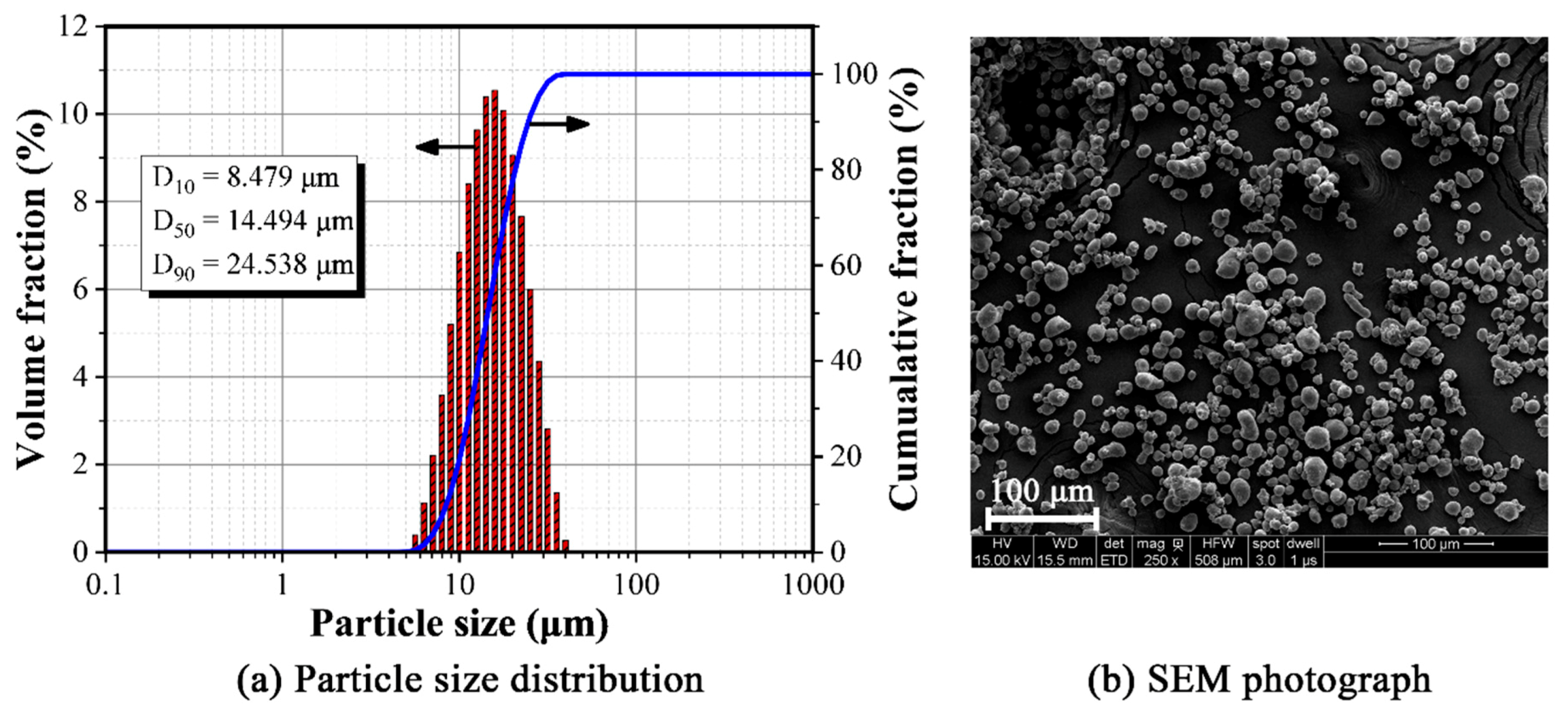
3.1. Structure and Characteristics of Composite Powder
3.2. Suppression Effect of Ca(H2PO4)2/RM Composite Powder for Aluminum Dust Flame Propagation
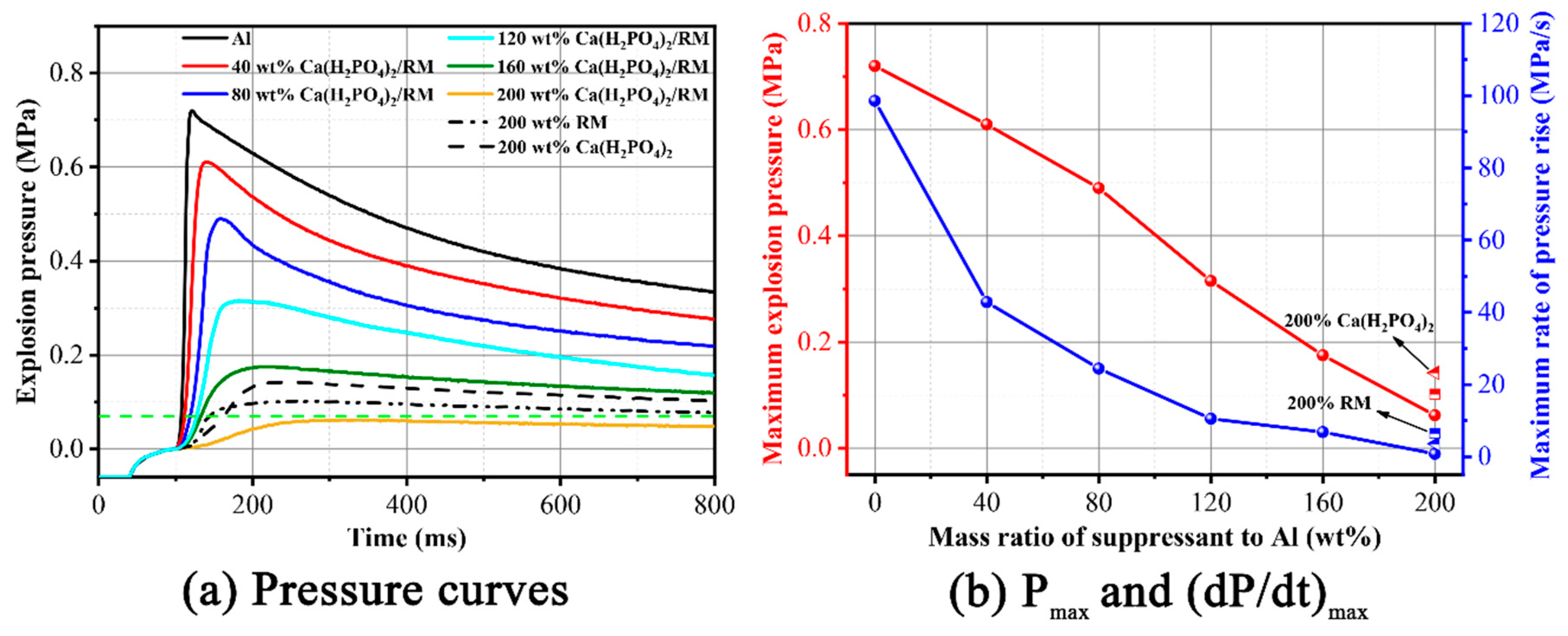
3.3. Suppression Effect of for Aluminum Dust Explosion Overpressure
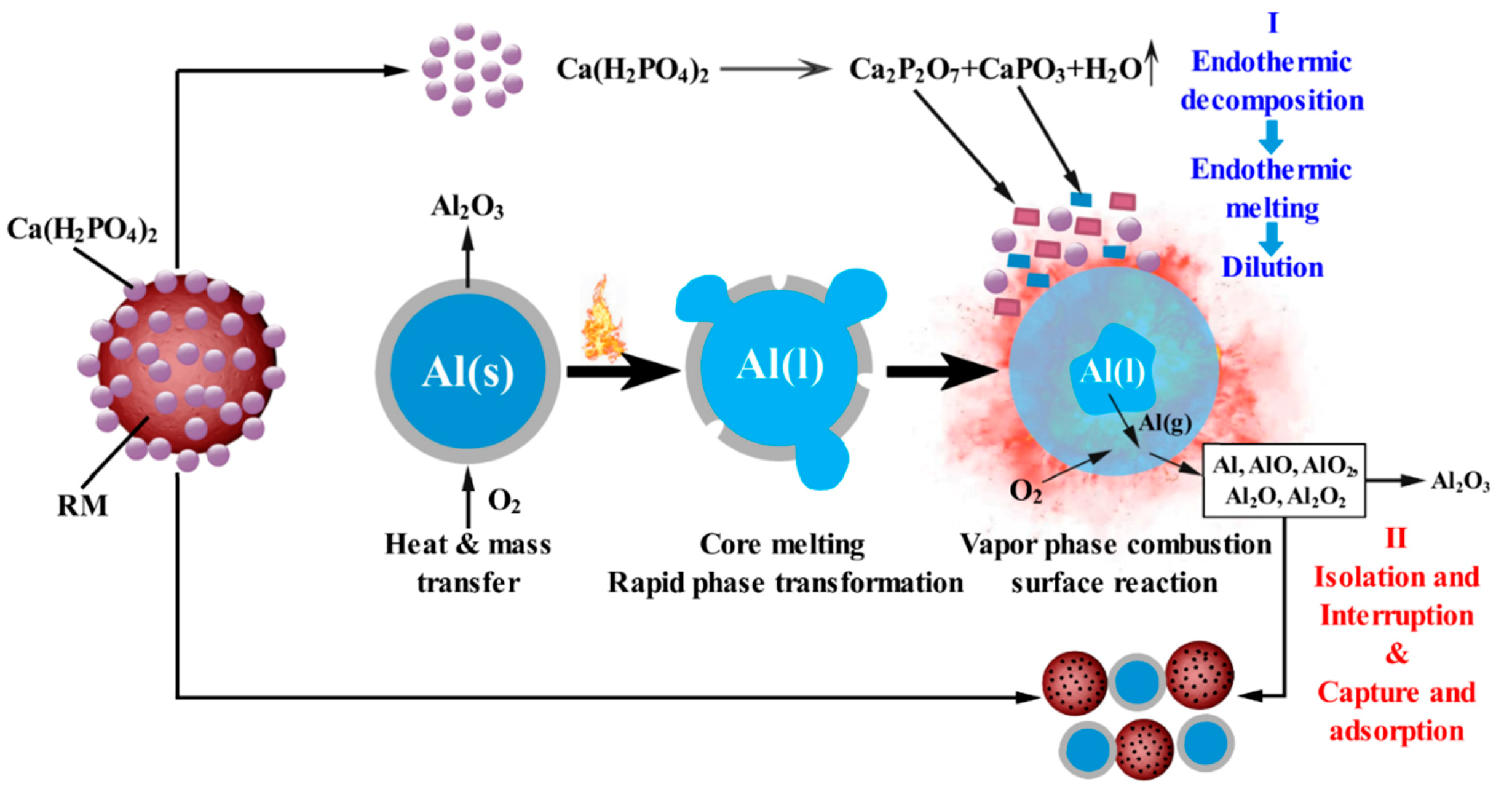
3.4. How Ca(H2PO4)2/RM Composite Powder Suppresses Aluminum Dust Explosion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marmo, L.; Piccinini, N.; Danzi, E. Small magnitude explosion of aluminium powder in an abatement plant: A telling case. Process Saf. Environ. 2015, 98, 221–230. [Google Scholar] [CrossRef]
- Amyotte, P.R.; Eckhoff, R.K. Dust explosion causation, prevention and mitigation: An overview. J. Chem. Health Saf. 2010, 17, 15–28. [Google Scholar] [CrossRef]
- Yuan, Z.; Khakzad, N.; Khan, F.; Amyotte, P. Dust explosions: A threat to the process industries. Process Saf. Environ. 2015, 98, 57–71. [Google Scholar] [CrossRef]
- Li, Q.Z.; Wang, K.; Zheng, Y.N.; Mei, X.N.; Lin, B.Q. Explosion severity of micro-sized aluminum dust and its flame propagation properties in 20 L spherical vessel. Powder Technol. 2016, 301, 1299–1308. [Google Scholar] [CrossRef]
- Li, Q.; Lin, B.; Li, W.; Zhai, C.; Zhu, C. Explosion characteristics of nano-aluminum powder–air mixtures in 20L spherical vessels. Powder Technol. 2011, 212, 303–309. [Google Scholar] [CrossRef]
- Jiang, B.; Lin, B.; Shi, S.; Zhu, C.; Li, W. Explosive characteristics of nanometer and micrometer aluminum-powder. Min. Sci. Technol. 2011, 21, 661–666. [Google Scholar] [CrossRef]
- Lin, B.Q.; Li, W.X.; Zhu, C.J.; Lu, H.L.; Lu, Z.G.; Li, Q.Z. Experimental investigation on explosion characteristics of nano-aluminum powder-air mixtures. Combust. Explos. Shock Waves 2010, 46, 678–682. [Google Scholar] [CrossRef]
- Marmo, L.; Cavallero, D.; Debernardi, M.L. Aluminium dust explosion risk analysis in metal workings. J. Loss Prev. Proc. 2004, 17, 449–465. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.X.; Yuan, C.M.; Eckhoff, R.K. A catastrophic aluminium-alloy dust explosion in China. J. Loss Prev. Proc. 2016, 39, 121–130. [Google Scholar] [CrossRef]
- Going, J.E.; Snoeys, J. Explosion protection with metal dust fuels. Process Saf. Prog. 2002, 21, 305–312. [Google Scholar] [CrossRef]
- Taveau, J.; Vingerhoets, J.; Snoeys, J.; Going, J.; Farrell, T. Suppression of metal dust deflagrations. J. Loss Prev. Proc. 2015, 36, 244–251. [Google Scholar] [CrossRef]
- Dastidar, A.; Amyotte, P. Determination of Minimum Inerting Concentrations for Combustible Dusts in a Laboratory-Scale Chamber. Process Saf. Environ. 2002, 80, 287–297. [Google Scholar] [CrossRef]
- Jiang, H.; Bi, M.; Li, B.; Zhang, D.; Gao, W. Inhibition evaluation of ABC powder in aluminum dust explosion. J. Hazard. Mater. 2019, 361, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.P.; Bi, M.S.; Gao, W.; Gan, B.; Zhang, D.W.; Zhang, Q. Inhibition of aluminum dust explosion by NaHCO3 with different particle size distributions. J. Hazard. Mater. 2018, 344, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Bi, M.; Ma, D.; Li, B.; Cong, H.; Gao, W. Flame suppression mechanism of aluminum dust cloud by melamine cyanurate and melamine polyphosphate. J. Hazard. Mater. 2019, 368, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, X.; Ma, X.; Xiao, Q.; Liu, B.; Zhang, G. Experimental study on whether and how particle size affects the flame propagation and explosibility of oil shale dust. Process Saf. Prog. 2019, 38, e12075. [Google Scholar] [CrossRef]
- Cao, W.G.; Qin, Q.F.; Cao, W.; Lan, Y.H.; Chen, T.; Xu, S.; Cao, X. Experimental and numerical studies on the explosion severities of coal dust/air mixtures in a 20-L spherical vessel. Powder Technol. 2017, 310, 17–23. [Google Scholar] [CrossRef]
- Li, Q.Z.; Yuan, C.C.; Tao, Q.L.; Zheng, Y.N.; Zhao, Y. Experimental analysis on post-explosion residues for evaluating coal dust explosion severity and flame propagation behaviors. Fuel 2018, 215, 417–428. [Google Scholar] [CrossRef]
- Yu, L.F.; Li, G.; Liu, W.C.; Yu, J.N.; Yuan, C.M. Experimental investigations on ignition sensitivity of hybrid mixtures of oil shale dust and syngas. Fuel 2017, 210, 1–7. [Google Scholar] [CrossRef]
- Li, G.; Yuan, C.M.; Fu, Y.; Zhong, Y.P.; Chen, B.Z. Inerting of magnesium dust cloud with Ar, N2 and CO2. J. Hazard. Mater. 2009, 170, 180–183. [Google Scholar] [CrossRef]
- Sen, H.S.; Liu, Z.T.; Zhao, E.L.; Lin, S.; Qiu, L.M.; Qian, J.F.; Liu, H.X.; Xia, S.K. Comparison of behavior and microscopic characteristics of first and secondary explosions of coal dust. J. Loss Prev. Proc. 2017, 49, 382–394. [Google Scholar] [CrossRef]
- Liu, Z.T.; Lin, S.; Zhang, S.S.; Wang, E.Y.; Liu, G.H. Observations of microscopic characteristics of post-explosion coal dust samples. J. Loss Prev. Proc. 2016, 43, 378–384. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Meng, X.; Yan, K.; Chen, J. Suppression of Aluminum Dust Explosion by Ca(H2PO4)2/RM Composite Powder with Core–Shell Structure: Effect and Mechanism. Processes 2019, 7, 761. https://doi.org/10.3390/pr7100761
Wang J, Meng X, Yan K, Chen J. Suppression of Aluminum Dust Explosion by Ca(H2PO4)2/RM Composite Powder with Core–Shell Structure: Effect and Mechanism. Processes. 2019; 7(10):761. https://doi.org/10.3390/pr7100761
Chicago/Turabian StyleWang, Junfeng, Xiangbao Meng, Ke Yan, and Jinshe Chen. 2019. "Suppression of Aluminum Dust Explosion by Ca(H2PO4)2/RM Composite Powder with Core–Shell Structure: Effect and Mechanism" Processes 7, no. 10: 761. https://doi.org/10.3390/pr7100761
APA StyleWang, J., Meng, X., Yan, K., & Chen, J. (2019). Suppression of Aluminum Dust Explosion by Ca(H2PO4)2/RM Composite Powder with Core–Shell Structure: Effect and Mechanism. Processes, 7(10), 761. https://doi.org/10.3390/pr7100761




