Flexible Energy Storage System—An Introductory Review of Textile-Based Flexible Supercapacitors
Abstract
:1. Introduction
1.1. Historical Development of Supercapacitors
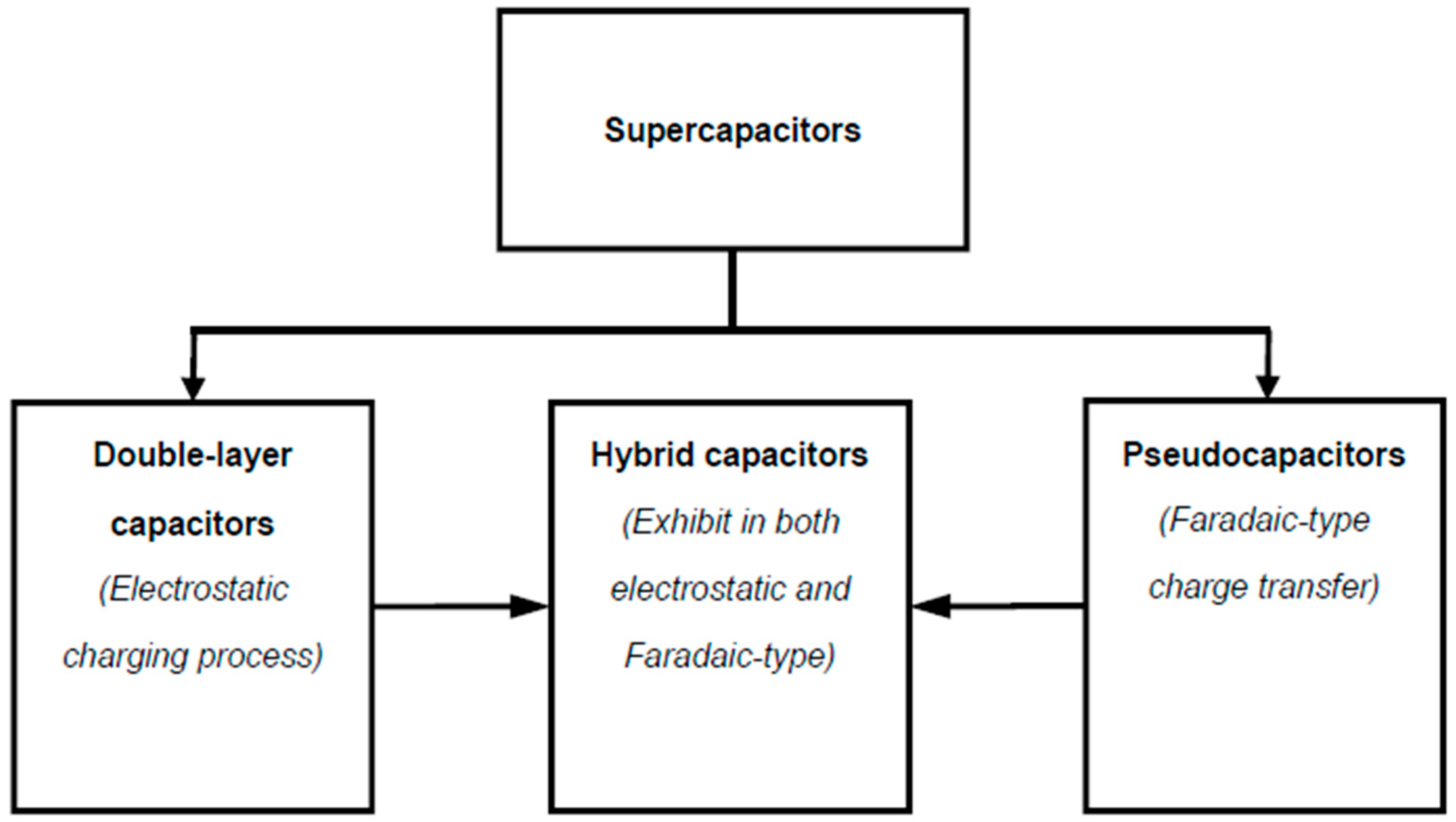
1.2. Nomenclature of Supercapacitors
2. Energy Storage Mechanism in Supercapacitors
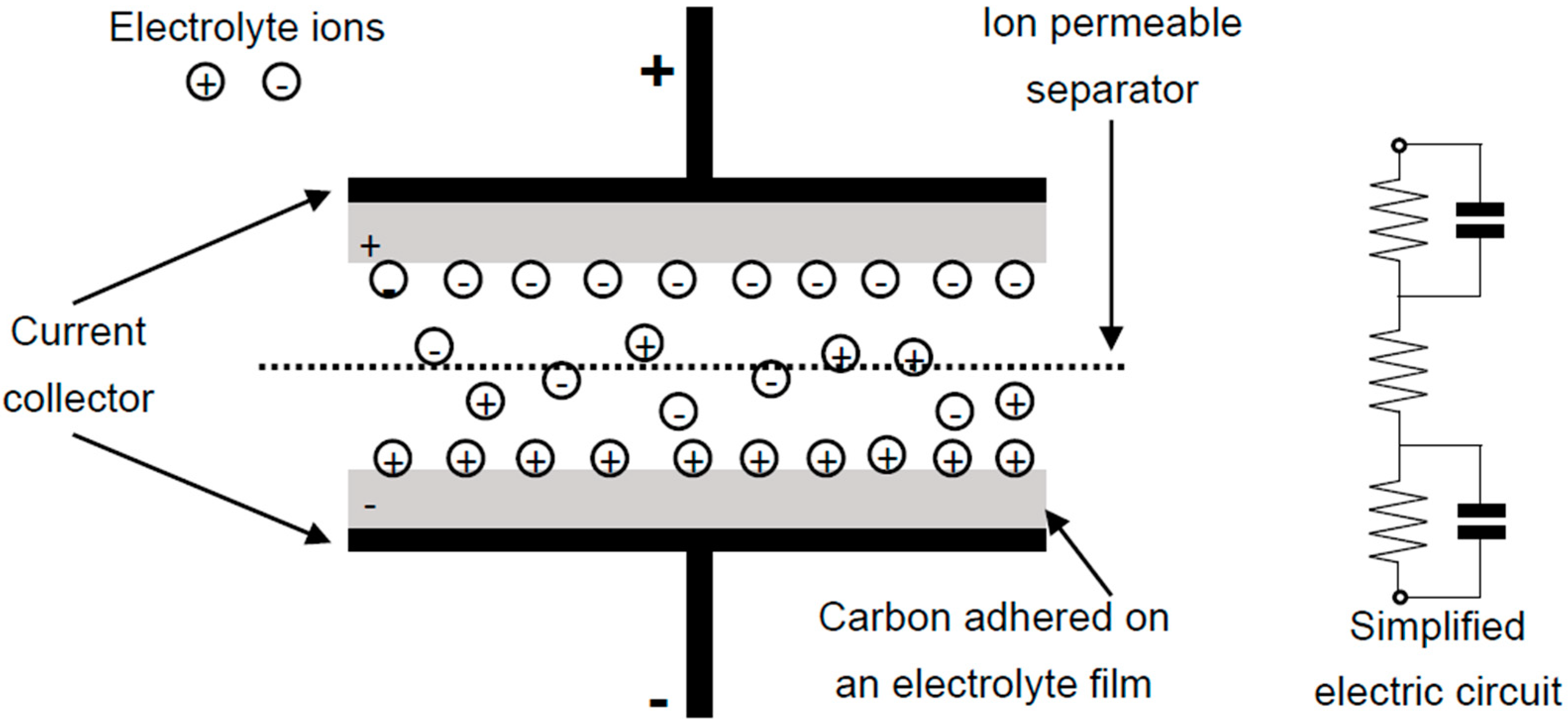
2.1. Electrical Double-Layer Capacitance (EDLC)
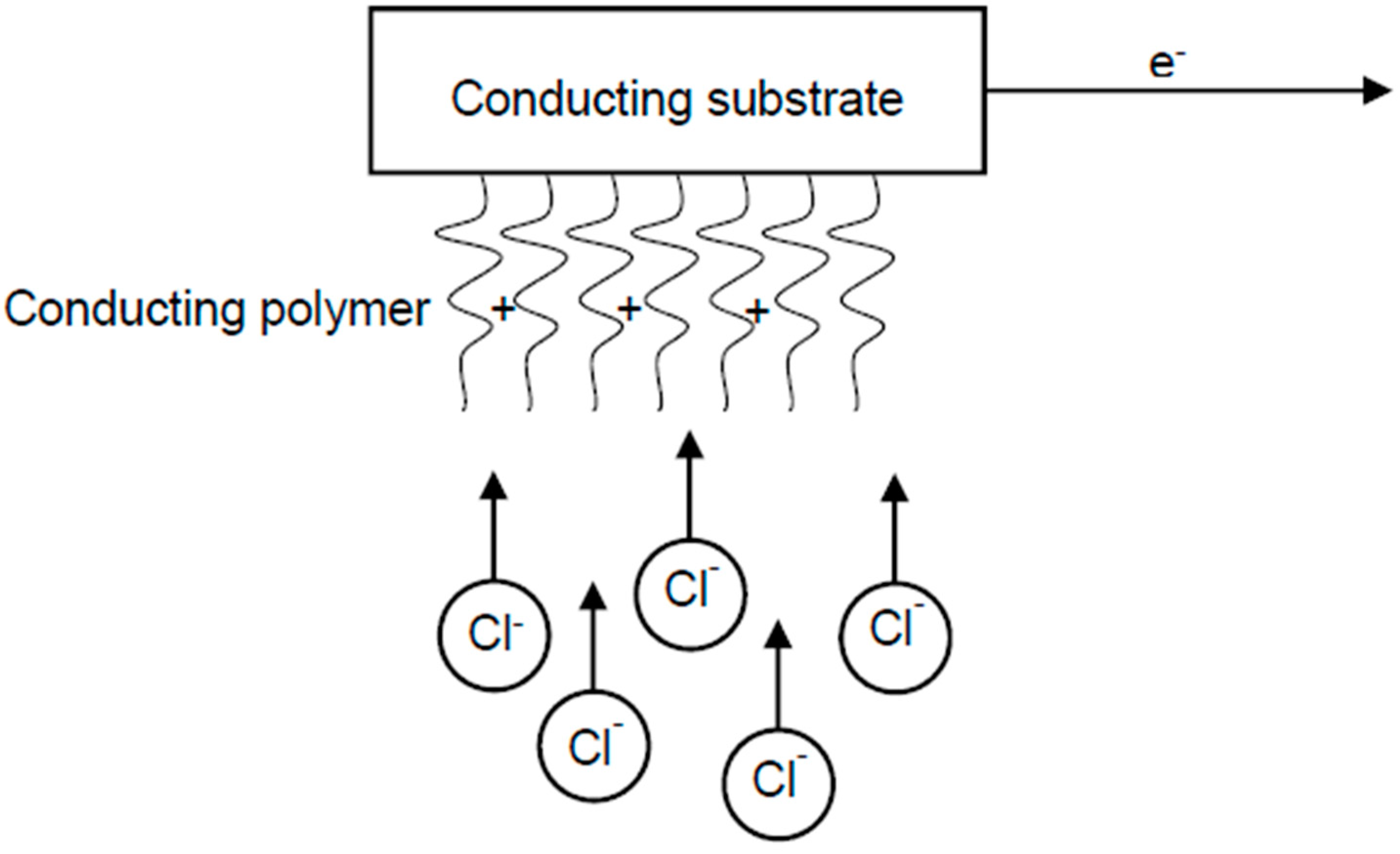
2.2. Pseudocapacitance
3. Electrode Materials
3.1. Carbon Materials
3.2. Metal Oxides
3.3. Conducting Polymers
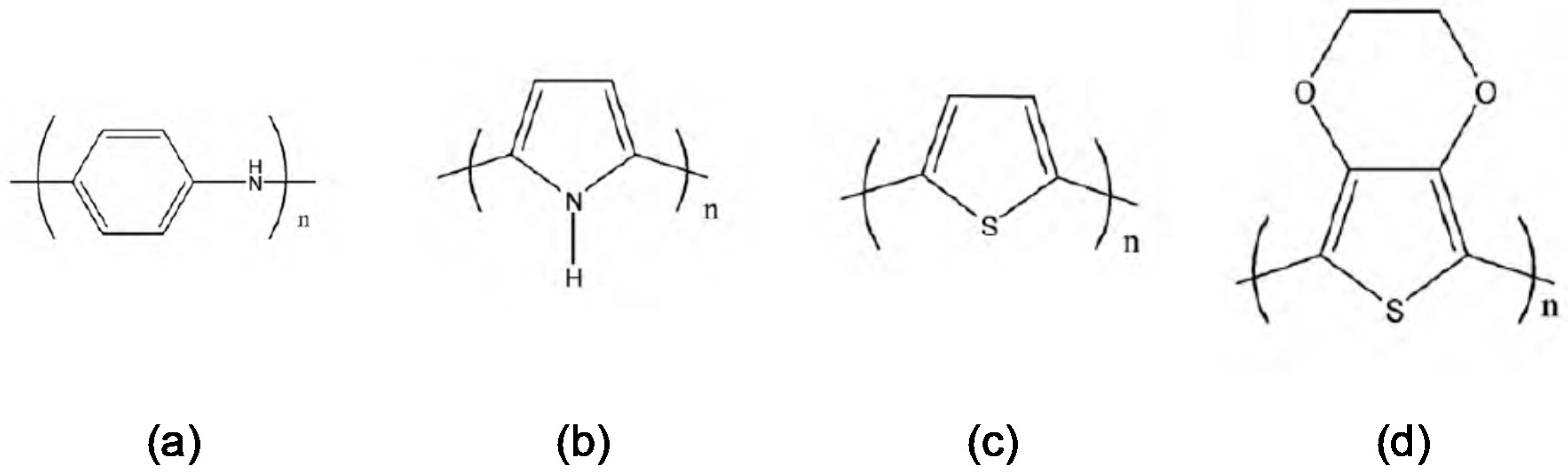
3.3.1. Polyaniline (PAni)
3.3.2. Polypyrrole (Ppy)
3.3.3. Thiophene-Based Conducting Polymer
4. Electrolytic Materials
4.1. Electrolytes in EDLC
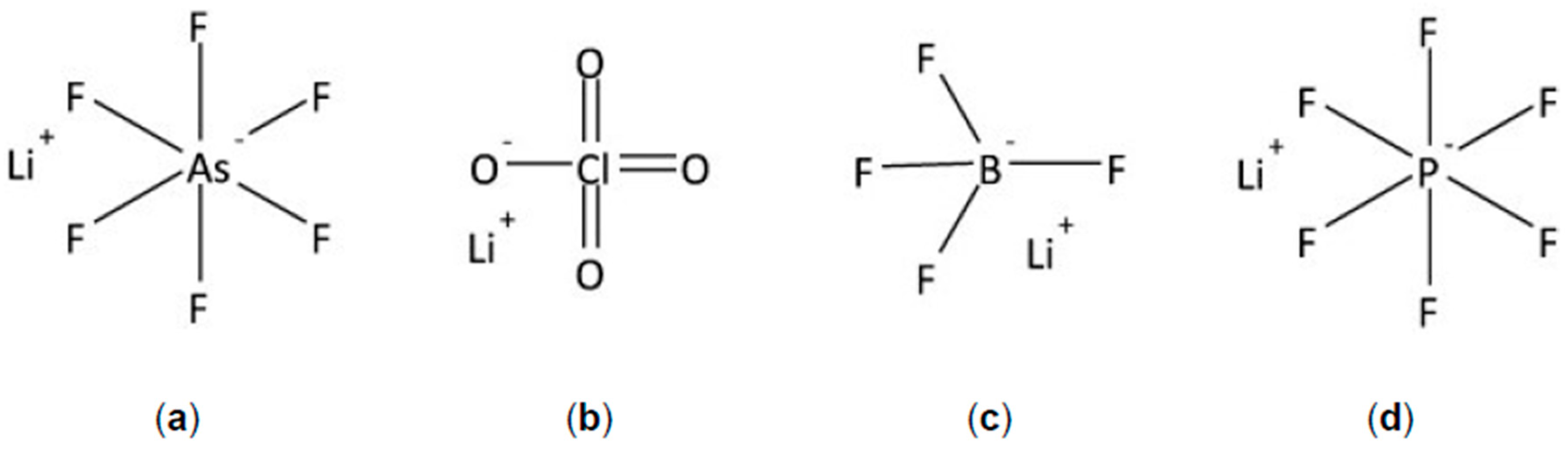
4.2. Electrolytes in Pseudocapacitors
4.3. Polymer Electrolytes
4.3.1. Dry Solid Polymer Electrolytes (Polymer-Salt Complex Electrolytes)
4.3.2. Plasticized Polymer Electrolytes
4.3.3. Gel Polymer Electrolytes
4.3.4. Composite Polymer Electrolytes
5. Current Development in Textile-Based Flexible Supercapacitors
5.1. Coated Textile-Based Supercapacitors
5.2. Fiber and Yarn Electrodes
6. Characterization Techniques for Supercapacitors
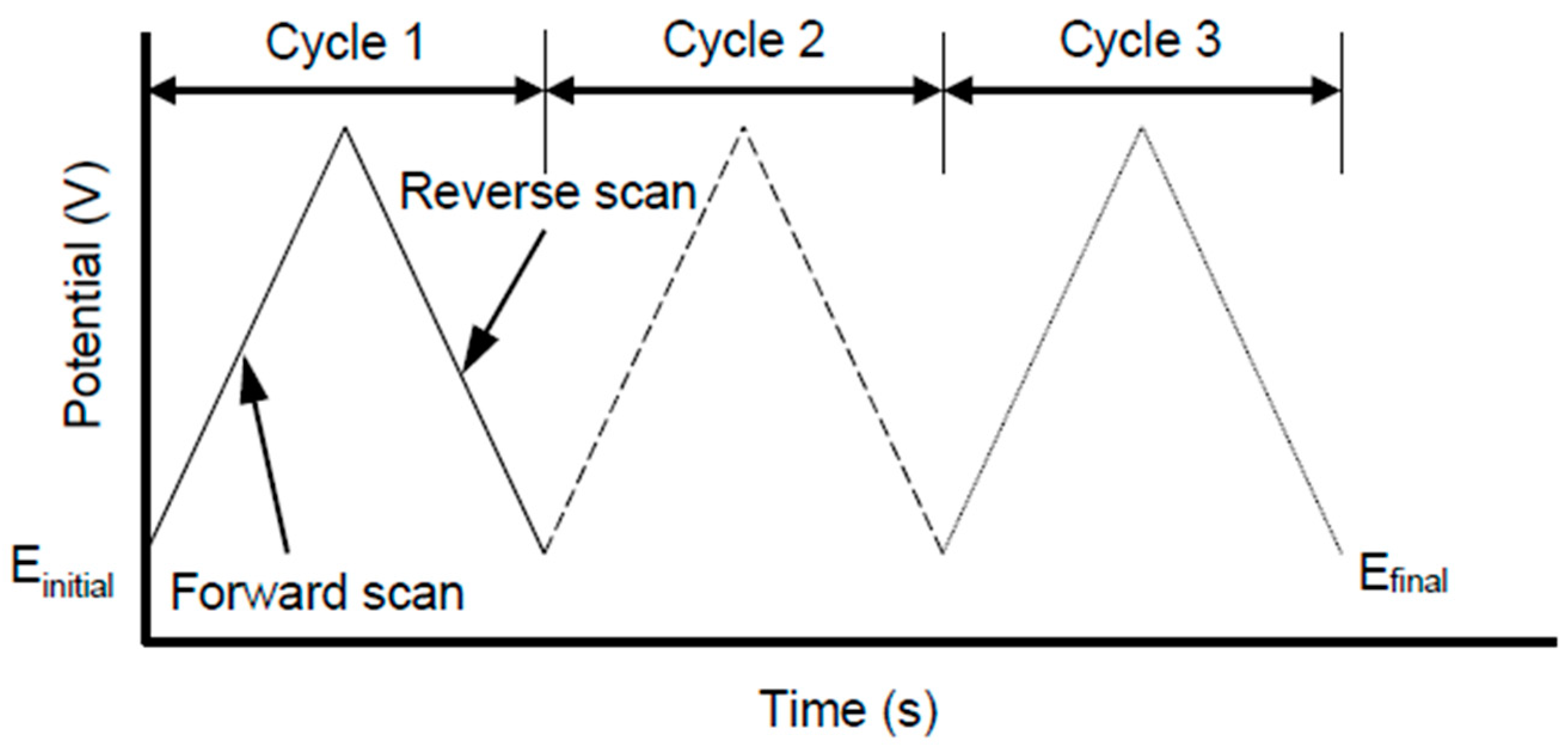
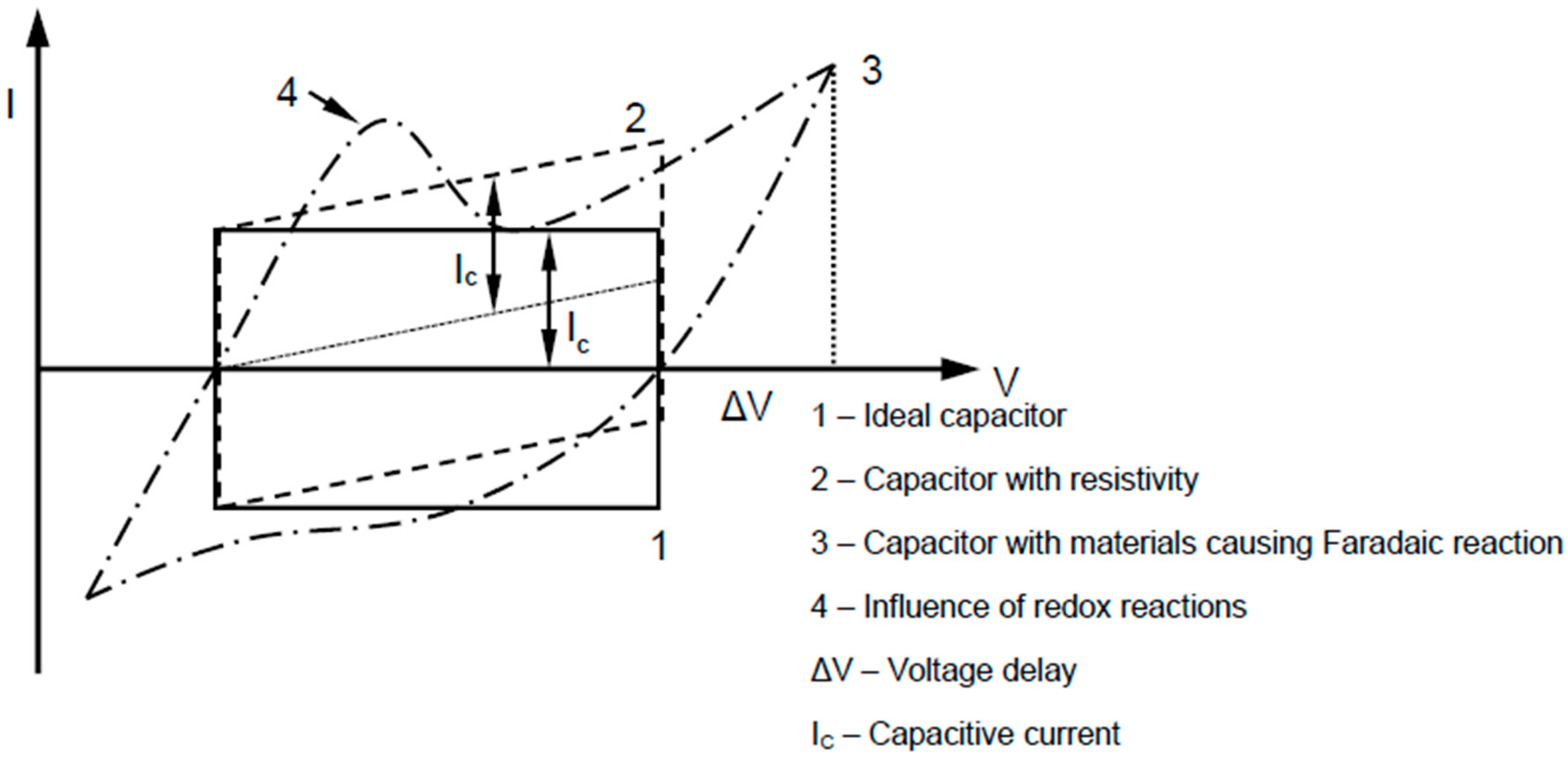
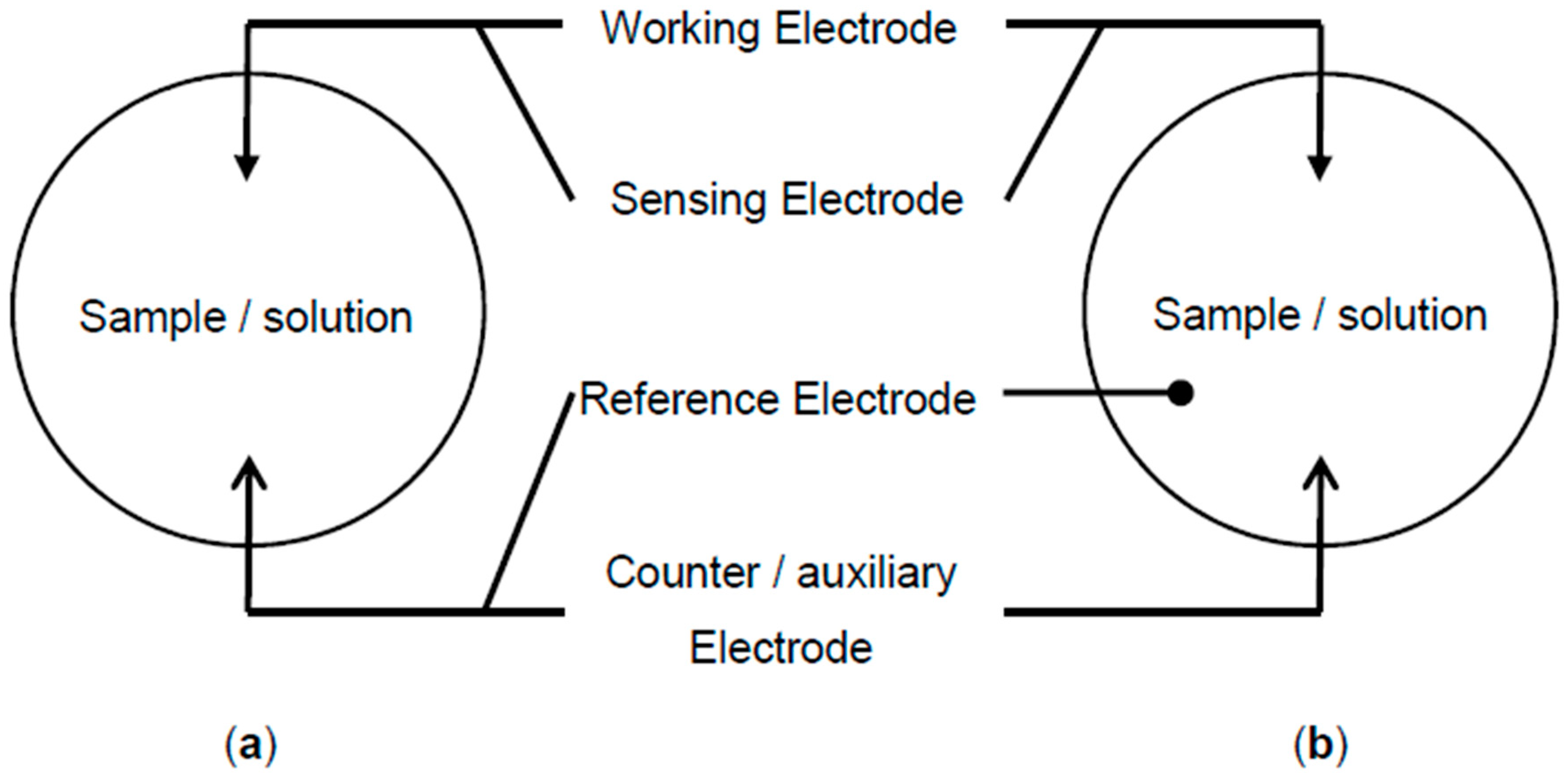
6.1. Cyclic Voltammetry (CV)
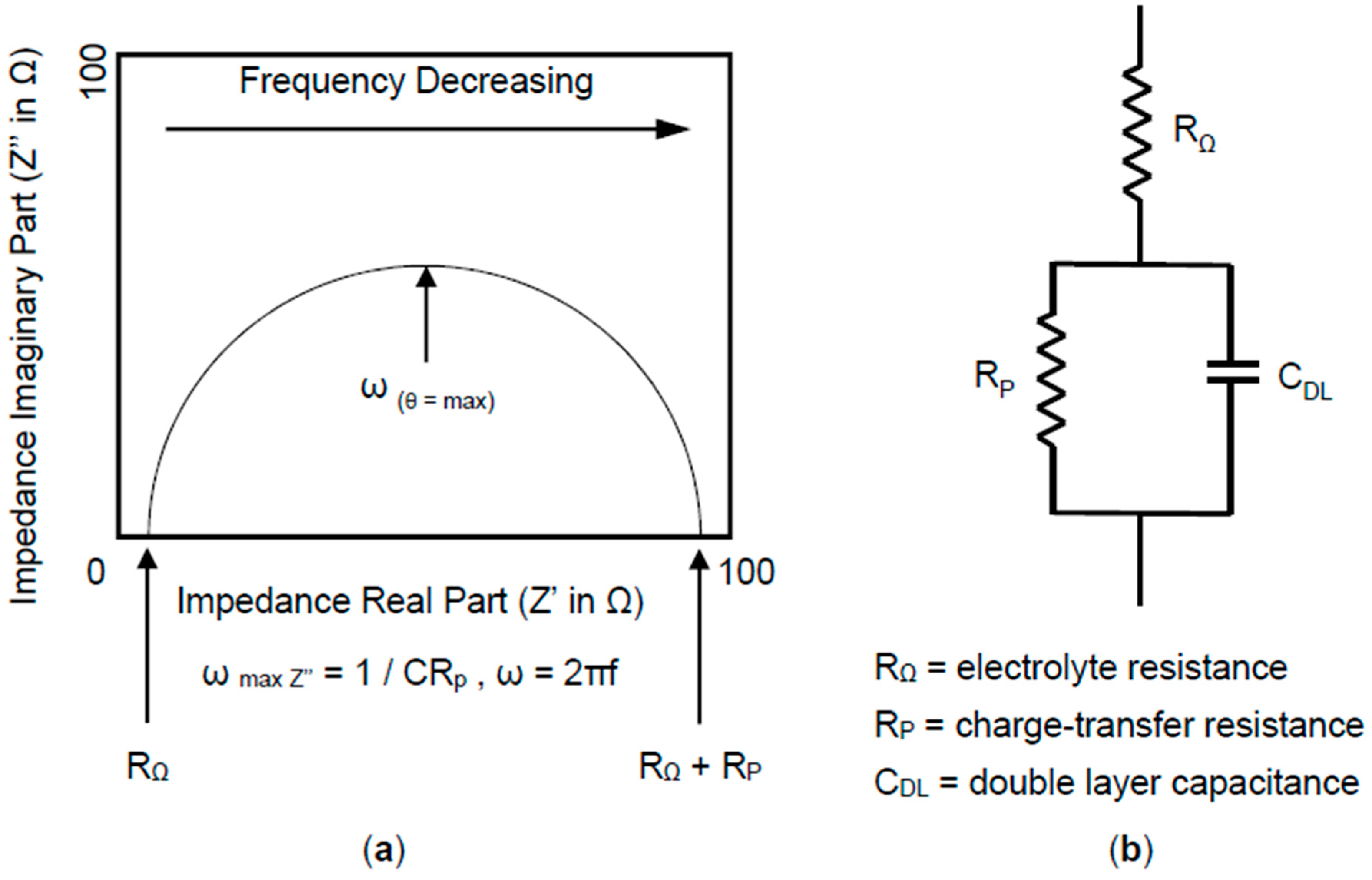
6.2. Electrochemical Impedance Spectroscopy (EIS)
6.2.1. The Nyquist Plot
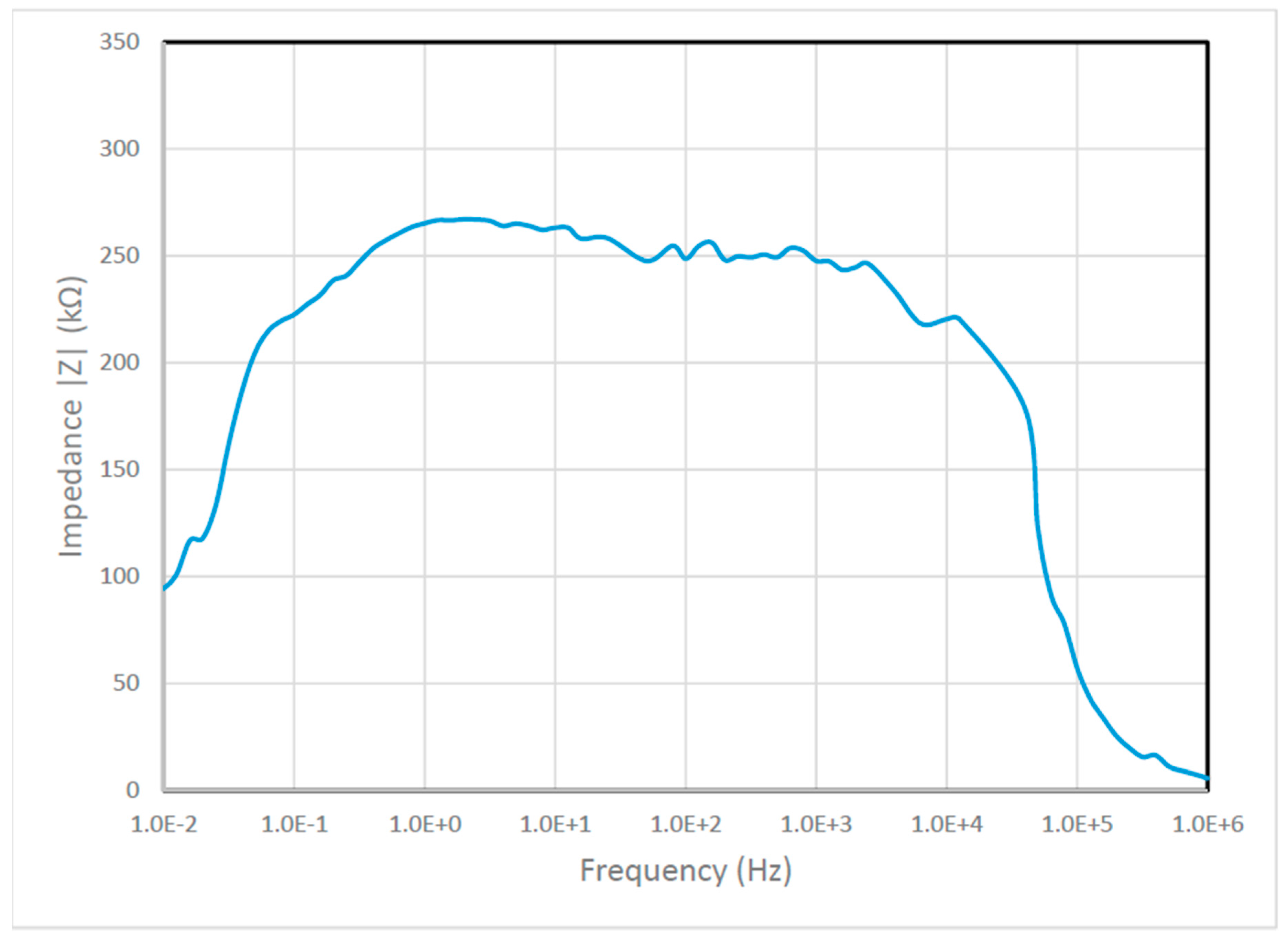
6.2.2. The Bode Plot
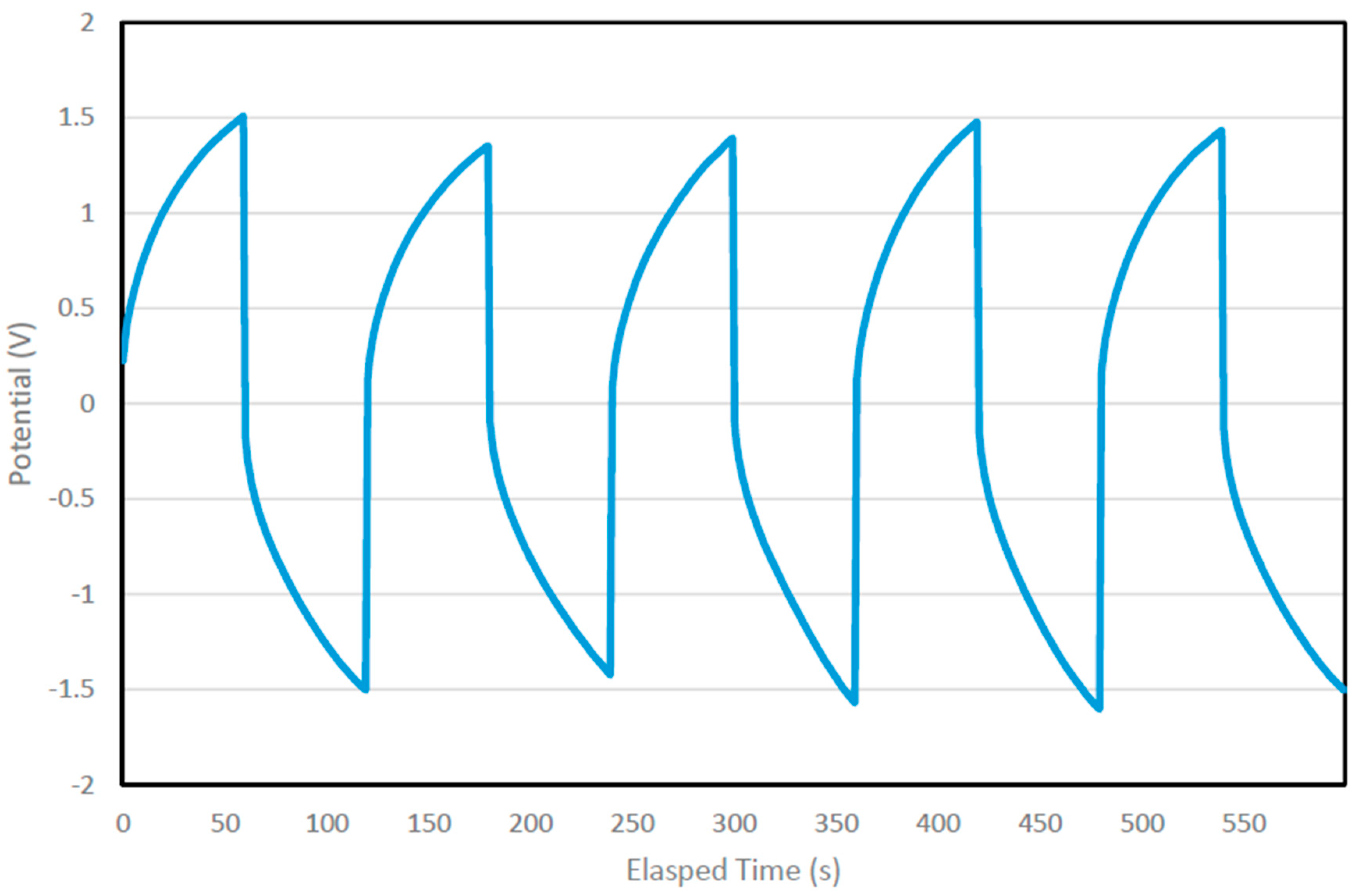
6.3. Cyclic Charge-Discharge Measurement
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.G.; Yu, Z.N.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Balducci, A.; Dugas, R.; Taberna, P.; Simon, P.; Plee, D.; Mastragostino, M. and Passerini, S. High temperature carbon-carbon supercapacitor using ionic liquid as electrolyte. J. Power Sources 2007, 165, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Mastragostino, M.; Arbizzani, C.; Soavi, F. Polymer-based supercapacitors. J. Power Sources 2001, 97–98, 812–815. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Khomenko, V.; Jurewicz, K.; Lota, K.; Beguin, F. Supercapacitors based on conducting polymers/nanotubes composites. J. Power Sources 2006, 153, 413–418. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, B.S.; Chen, S.; Yang, S.H.; Hammond, P.T. Layer-by-layer assembly of all carbon nanotube ultrathin films for electrochemical applications. J. Am. Chem. Soc. 2009, 131, 671–679. [Google Scholar] [CrossRef]
- Gan, X.P.; Wu, Y.T.; Liu, L.; Shen, B.; Hu, W.B. Electroless copper plating on PET fabrics using hypophosphite as reducing agent. Surf. Coat. Tech. 2007, 201, 7018–7023. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Balasubramanian, K. Simple capacitors to supercapacitors—An Overview. Int. J. Electrochem. Sci. 2008, 3, 1196–1217. [Google Scholar]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Jewell, D.; Chen, G.Z. Carbon nanotube and conducting polymer composites for supercapacitors. Prog. Nat. Sci. 2008, 18, 777–788. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T.S. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Lee, T.; Ooi, C.H.; Othman, R.; Yeoh, F.Y. Activated carbon fiber—The hybrid of carbon fiber and activated carbon. Rev. Adv. Mater. Sci. 2014, 36, 118–136. [Google Scholar]
- Cazorla-Amorós, D.; Alcañiz-Monge, J.; Linares-Solano, A. Characterization of activated carbon fibers by CO2 adsorption. Langmuir 1996, 12, 2820–2824. [Google Scholar] [CrossRef]
- Trasatti, S.; Buzzanca, G. Ruthenium dioxide: A new interesting electrode material. Solid state structure and electrochemical behavior. J. Electroanal. Chem. 1971, 29, A1–A5. [Google Scholar] [CrossRef]
- Sugimoto, W.; Yokoshima, K.; Murakami, Y.; Takasu, Y. Charge storage mechanism of nanostructured anhydrous and hydrous ruthenium-based oxides. Electrochim. Acta 2006, 52, 1742–1748. [Google Scholar] [CrossRef] [Green Version]
- Trasatti, S.; Kurzweil, P. Electrochemical supercapacitors as versatile energy stores. Platin. Met. Rev. 1994, 38, 46–56. [Google Scholar]
- Srinivasan, V.; Weidner, J.W. Capacitance studies of cobalt oxide films formed via electrochemical precipitation. J. Power Sources 2002, 108, 15–20. [Google Scholar] [CrossRef]
- Shinde, V.R.; Mahadik, S.B.; Gujar, T.P.; Lokhande, C.D. Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl. Surf. Sci. 2006, 252, 7487–7492. [Google Scholar] [CrossRef]
- Ganesha, V.; Pitchumanib, S.; Lakshminarayanana, V. New symmetric and asymmetric supercapacitors based on high surface area porous nickel and activated carbon. J. Power Sources 2006, 158, 1523–1532. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Zhang, M.L. Preparation and electrochemical properties of nickel oxide by molten-salt synthesis. Mater. Lett. 2007, 61, 3967–3969. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor Behavior with KCl Electrolyte. J. Solid State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Hu, C.C.; Tsou, T.W. Ideal capacitive behavior of hydrous manganese oxide prepared by anodic deposition. Electrochem. Commun. 2002, 4, 105–109. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. High performance electrochemical supercapacitor from electrochemically synthesized nanostructured polyaniline. Mater. Lett. 2006, 60, 1466–1469. [Google Scholar] [CrossRef]
- Fan, L.Z.; Maier, J. High-performance polypyrrole electrode materials for redox supercapacitors. Electrochem. Commun. 2006, 8, 937–940. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Sun, W.; Wang, S. Capacitance properties of poly(3,4-ethylenedioxythiophene)/polypyrrole composites. J. Power Sources 2006, 159, 370–373. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, Y.J.; Park, N.G.; Kang, M.G.; Chang, S.H. Redox supercapacitor using polyaniline doped with Li salt as electrode. Solid State Ion. 2002, 152–153, 861–866. [Google Scholar] [CrossRef]
- Villers, D.; Jobin, D.; Soucy, C.; Cossement, D.; Chahine, R.; Breau, L.; Bélanger, D. The Influence of the Range of Electroactivity and Capacitance of Conducting Polymers on the Performance of Carbon Conducting Polymer Hybrid Supercapacitor. J. Electrochem. Soc. 2003, 150, A747–A752. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Upadhyaya, H.M. Polypyrrole and poly (3-methyl thiophene)-based solid state redox supercapacitors using ion conducting polymer electrolyte. Solid State Ion. 2002, 152–153, 883–889. [Google Scholar] [CrossRef]
- Vol’fkovich, Y.M.; Serdyuk, T.M. Electrochemical Capacitors. Russ. J. Electrochem. 2002, 38, 935–959. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Meneghello, L.; Paraventi, R. Electronically conducting polymers and activated carbon: Electrode materials in supercapacitor technology. Adv. Mater. 1996, 8, 331–334. [Google Scholar]
- Wu, M.Q.; Snook, G.A.; Gupta, V.; Shaffer, M.; Fray, D.J.; Chen, G.Z. Electrochemical fabrication and capacitance of composite films of carbon nanotubes and polyaniline. J. Mater. Chem. 2005, 15, 2297–2303. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, N.G.; Park, Y.J.; Chang, S.H. Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Park, J.H.; Park, O.O. Hybrid electrochemical capacitors based on polyaniline and activated carbon electrodes. J. Power Sources 2002, 111, 185–190. [Google Scholar] [CrossRef]
- Snook, G.A.; Peng, C.; Fray, D.J.; Chen, G.Z. Achieving high electrode specific capacitance with materials of low mass specific capacitance: Potentiostatically grown thick micro-nanoporous PEDOT films. Electrochem. Commun. 2007, 9, 83–88. [Google Scholar] [CrossRef]
- Suematsu, S.; Oura, Y.; Tsujimoto, H.; Kanno, H.; Naoi, K. Conducting polymer films of cross-linked structure and their QCM analysis. Electrochim. Acta 2000, 45, 3813–3821. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Latham, R.J.; Linford, R.G.; Schlindwein, W.S. Conducting polymer-based electrochemical redox supercapacitors using proton and lithium ion conducting polymer electrolytes. Polym. Int. 1998, 47, 28–33. [Google Scholar] [CrossRef]
- Skompska, M.; Mieczkowski, J.; Holze, R.; Heinze, J. In situ conductance studies of p-and n-doping of poly(3,4-dialkoxythiophenes). J. Electroanal. Chem. 2005, 577, 9–17. [Google Scholar] [CrossRef]
- Levi, M.D.; Gofer, Y.; Aurbach, D.; Lapkowski, M.; Vieilc, E.; Serose, J. Simultaneous Voltammetric and In Situ Conductivity Studies of n-Doping of Polythiophene Films with Tetraalkylammonium, Alkali, and Alkaline–Earth Cations. J. Electrochem. Soc. 2000, 147, 1096–1104. [Google Scholar] [CrossRef]
- Laforgue, A.; Simon, P.; Fauvarque, J.F.; Mastragostino, M.; Soavi, F.; Sarrau, J.F.; Lailler, P.; Conte, M.; Rossi, E.; Saguattie, S. Activated Carbon/Conducting Polymer Hybrid Supercapacitors. J. Electrochem. Soc. 2003, 150, A645–A651. [Google Scholar] [CrossRef]
- Ryu, K.S.; Lee, Y.G.; Hong, Y.S.; Park, Y.J.; Wu, X.L.; Kim, K.M.; Kang, M.G.; Park, N.G.; Chang, S.H. Poly(ethylenedioxythiophene) (PEDOT) as polymer electrode in redox supercapacitor. Electrochim. Acta 2004, 50, 843–847. [Google Scholar] [CrossRef]
- Arbizzani, C.; Catellani, M.; Mastragostino, M.; Mingazzini, C. N- and P-doped Polydithieno [3,4-B:3′4′-D] thiophene: A narrow band gap polymer for redox supercapacitors. Electrochim. Acta 1995, 40, 1871–1876. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Meneghello, L. Characterization by impedance spectroscopy of a polymer-based supercapacitor. Electrochim. Acta 1995, 40, 2223–2228. [Google Scholar] [CrossRef]
- Snook, G.A.; Chen, G.Z. The measurement of specific capacitances of conducting polymers using the quartz crystal microbalance. J. Electroanal. Chem. 2008, 612, 140–146. [Google Scholar] [CrossRef]
- Stenger-Smith, J.D.; Webber, C.K.; Anderson, N.; Chafin, A.P.; Zong, K.K.; Reynolds, J.R. Poly(3,4-alkylenedioxythiophene)-Based Supercapacitors Using Ionic Liquids as Supporting Electrolytes. J. Electrochem. Soc. 2002, 149, A973–A977. [Google Scholar] [CrossRef]
- Hong, J.I.; Yeo, I.H.; Paik, W.K. Conducting Polymer with Metal Oxide for Electrochemical Capacitor: Poly (3,4-ethylenedioxythiophene) RuOx Electrode. J. Electrochem. Soc. 2001, 148, A156–A163. [Google Scholar] [CrossRef]
- Ghosh, S.; Inganäs, O. Networks of Electron-Conducting Polymer in Matrices of Ion-Conducting Polymers Applications to Fast Electrodes. Electrochem. Solid State Lett. 2000, 3, 213–215. [Google Scholar] [CrossRef]
- Arulepp, M.; Permann, L.; Leis, J.; Perkson, A.; Rumma, K.; Jänes, A.; Lust, E. Influence of the solvent properties on the characteristics of a double layer capacitor. J. Power Sources 2004, 133, 320–328. [Google Scholar] [CrossRef]
- Morita, M.; Kaigaishi, T.; Yoshimoto, N.; Egashira, M.; Aida, T. Effects of the Electrolyte Composition on the Electric Double-Layer Capacitance at Carbon Electrodes. Electrochem. Solid State Lett. 2006, 9, A386–A389. [Google Scholar] [CrossRef]
- Lust, E.; Nurk, G.; Jänes, A.; Arulepp, M.; Nigu, P.; Möller, P.; Kallip, S.; Sammelselg, V. Electrochemical properties of nanoporous carbon electrodes in various nonaqueous electrolytes. J. Solid State Electrochem. 2003, 7, 91–105. [Google Scholar] [CrossRef]
- Lazzari, M.; Mastragostino, M.; Soavi, F. Capacitance response of carbons in solvent-free ionic liquid electrolytes. Electrochem. Commun. 2007, 9, 1567–1572. [Google Scholar] [CrossRef]
- Mastragostino, M.; Soavi, F. Strategies for high-performance supercapacitors for HEV. J. Power Sources 2007, 174, 89–93. [Google Scholar] [CrossRef]
- Liu, X.J.; Osaka, T. All-Solid-State Electric Double-Layer Capacitor with Isotropic High-Density Graphite Electrode; Polyethylene Oxide/ LiClO4 Polymer Electrolyte. J. Electrochem. Soc. 1996, 143, 3982–3986. [Google Scholar] [CrossRef]
- Liu, X.J.; Osaka, T. Properties of Electric Double-Layer Capacitors with Various Polymer Gel Electrolytes. J. Electrochem. Soc. 1997, 144, 3066–3071. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Meneghello, L. Polymer-based redox supercapacitors: A comparative study. Electrochim. Acta 1996, 41, 21–26. [Google Scholar] [CrossRef]
- Rudge, A.; Davey, J.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. Conducting polymers as active materials in electrochemical capacitors. J. Power Sources 1994, 47, 89–107. [Google Scholar] [CrossRef]
- Rudge, A.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. A study of the electrochemical properties of conducting polymers for application in electrochemical capacitors. Electrochim. Acta 1994, 39, 273–287. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Latham, R.J.; Linford, R.G.; Schlindwein, W.S. Studies on all solid state electric double layer capacitors using proton and lithium ion conducting polymer electrolytes. J. Chem. Soc. Faraday Trans. 1997, 93, 4177–4182. [Google Scholar] [CrossRef]
- Clemente, A.; Panero, S.; Spila, E.; Scrosati, B. Solid-state, polymer-based, redox capacitors. Solid State Ion. 1996, 85, 273–277. [Google Scholar] [CrossRef]
- Carlberg, J.C.; Inganäs, O. Poly(3,4-ethylenedioxythiophene) as Electrode Material in Electrochemical Capacitors. J. Electrochem. Soc. 1997, 144, L61–L64. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Yue, L.P.; Ma, J.; Zhang, J.J.; Zhao, J.W.; Dong, S.M.; Liu, Z.H.; Cui, G.L.; Chen, L.Q. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer Electrolytes for Lithium-Ion Batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Marcinek, M.; Syzdek, J.; Marczewski, M.; Piszcz, M.; Niedzicki, L.; Kalita, M.; Plewa-Marczewska, A.; Bitner, A.; Wieczorek, P.; Trzeciak, T.; et al. Electrolytes for Li-ion transport—Review. Solid State Ion. 2015, 276, 107–126. [Google Scholar] [CrossRef]
- Mohapatra, S.R.; Thakur, A.K.; Choudhary, R.N.P. Effect of nanoscopic confinement on improvement in ion conduction and stability properties of an intercalated polymer nanocomposite electrolyte for energy storage applications. J. Power Sources 2009, 191, 601–613. [Google Scholar] [CrossRef]
- Dubal, D.P.; Kim, J.G.; Kim, Y.; Holze, R.; Lokhande, C.D.; Kim, W.B. Supercapacitors based on flexible substrates: An overview. Energy Technol. 2014, 2, 325–341. [Google Scholar] [CrossRef]
- Wang, Y.S.; Li, S.M.; Hsiao, S.T.; Liao, W.H.; Chen, P.H.; Yang, S.Y.; Tien, H.W.; Ma, C.C.M.; Hu, C.C. Integration of tailored reduced graphene oxide nanosheets and electrospun polyamide-66 nanofabrics for a flexible supercapacitor with high-volume- and high-area-specific capacitance. Carbon 2014, 73, 87–98. [Google Scholar] [CrossRef]
- Jost, K.; Perez, C.R.; McDonough, J.K.; Presser, V.; Heon, M.; Dion, G.; Gogotsi, Y. Carbon coated textiles for flexible energy storage. Energy Environ. Sci. 2011, 4, 5060–5067. [Google Scholar] [CrossRef]
- Dong, L.; Xu, C.; Li, Y.; Huang, Z.H.; Kang, F.; Yang, Q.H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar] [CrossRef]
- Yong, S.; Owen, J.R.; Tudor, M.J.; Beeby, S.P. Fabric based supercapacitor. J. Phys. Conf. Ser. 2013, 476, 012114. [Google Scholar] [CrossRef]
- Ding, Y.; Invernale, M.A.; Sotzing, G.A. Conductivity trends of PEDOT-PSS impregnated fabric and the effect of conductivity on electrochromic textile. ACS Appl. Mater. Interfaces 2010, 2, 1588–1593. [Google Scholar] [CrossRef]
- Gan, X.P.; Wu, Y.T.; Liu, L.; Shen, B.; Hu, W.B. Electroless plating of Cu-Ni-P alloy on PET fabrics and effect of plating parameters on the properties of conductive fabrics. J. Alloys Compd. 2008, 455, 308–313. [Google Scholar] [CrossRef]
- Han, E.G.; Kim, E.A.; Oh, K.W. Electromagnetic interference shielding effectiveness of electroless Cu-plated PET fabrics. Synth. Met. 2001, 123, 469–476. [Google Scholar] [CrossRef]
- Gasana, E.; Westbroek, P.; Hakuzimana, J.; De Clerck, K.; Priniotakis, G.; Kiekens, P.; Tseles, D. Electroconductive texile structures through electroless deposition of polypyrrole and copper at polyaramide surfaces. Surf. Coat. Technol. 2006, 201, 3547–3551. [Google Scholar] [CrossRef]
- Park, N.W.; Kim, I.W. and Kim, J.Y. Copper Metallization of Poly (ethylene terephthalate) Fabrics via Intermediate Polyaniline Layers. Fibers Polym. 2009, 10, 310–314. [Google Scholar] [CrossRef]
- Shoji, E.; Takagi, S.; Araie, H. Novel conducting fabric polymer composites as stretchable electrodes: One-step fabrication of chemical actuators. Polym. Adv. Technol. 2009, 20, 423–426. [Google Scholar] [CrossRef]
- Sumboja, A.; Liu, J.; Zheng, W.G.; Zong, Y.; Zhang, H.; Liu, Z. Electrochemical energy storage devices for wearable technology: A rationale for materials selection and cell design. Chem, Soc. Rev. 2018, 47, 5919–5945. [Google Scholar] [CrossRef] [PubMed]
- Kofod, G.; Stoyanov, H.; Gerhard, R. Multilayer coaxial fibre dielectric elastomers for actuation and sensing. Appl. Phys. A 2011, 102, 577–581. [Google Scholar] [CrossRef]
- Jost, K.; Stenger, D.; Perez, C.R.; McDonough, J.K.; Lian, K.; Gogotsi, Y.; Dion, G. Knitted and screen printed carbon-fiber supercapacitors for applications in wearable electronics. Energy Environ. Sci. 2013, 6, 2698–2705. [Google Scholar] [CrossRef]
- Dong, L.; Xu, C.; Yang, Q.; Fang, J.; Li, Y.; Kang, F. High-performance compressible supercapacitors based on functionally synergic multiscale carbon composite textiles. J. Mater. Chem. A 2015, 3, 4729–4737. [Google Scholar] [CrossRef]
- Lyu, X.; Su, F.; Miao, M. Two-ply yarn supercapacitor based on carbon nanotube/stainless steel core-sheath yarn electrodes and ionic liquid electrolyte. J. Power Sources 2016, 307, 489–495. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Y.; Fang, Y.; Lu, R.; Sha, J. Performance characteristics of supercapacitor electrodes made of silicon carbide nanowires grown on carbon fabric. J. Power Sources 2013, 243, 648–653. [Google Scholar] [CrossRef]
- Yun, T.G.; Oh, M.; Hu, L.; Hyun, S.; Han, S.M. Enhancement of electrochemical performance of textile based supercapacitor using mechanical pre-straining. J. Power Sources 2013, 244, 783–791. [Google Scholar] [CrossRef]
- Jost, K.; Dion, G.; Gogotsi, Y. Textile energy storage in perspective. J. Mater. Chem. A 2014, 2, 10776–10787. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, H.; Huang, Y.; Zhu, M.; Meng, W.; Liu, C.; Pei, Z.; Hao, C.; Wang, Z.; Zhi, C. From Industrially Weavable and Knittable Highly Conductive Yarns to Large Wearable Energy Storage Textiles. ACS Nano 2015, 9, 4766–4775. [Google Scholar] [CrossRef]
- Le, V.T.; Kim, H.; Ghosh, A.; Kim, J.; Chang, J.; Vu, Q.A.; Pham, D.T.; Lee, J.H.; Kim, S.W.; Lee, Y.H. Coaxial fiber supercapacitor using all-carbon material electrodes. ACS Nano 2013, 77, 5940–5947. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-graphene core-sheath microfibers for all-solid-state, stretchable fibriform supercapacitors and wearable electronic textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, P.T.; Heineman, W.R. Cyclic Voltammetry. J. Chem. Educ. 1983, 60, 702–706. [Google Scholar] [CrossRef]
- Heinze, J. Cyclic Voltammetry—Electrochemical Spectroscopy. Angew. Chem. 1984, 23, 831–847. [Google Scholar] [CrossRef]
- Homolka, D.; Hung, L.Q.; Hofmanova, A.; Khalil, M.W.; Koryta, J.; Marecek, V.; Samec, Z.; Sen, S.K.; Vanysek, P.; Weber, J.; et al. Faradaic ion transfer across the interface of two immiscible electrolyte solutions: Chronopotentiometry and cyclic voltammetry. Anal. Chem. 1980, 52, 1606–1610. [Google Scholar] [CrossRef]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Huang, V.M.W.; Wu, S.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Local electrochemical impedance spectroscopy: A review and some recent developments. Electrochim. Acta 2011, 56, 8048–8057. [Google Scholar] [CrossRef] [Green Version]
- Ates, M. Review study of electrochemical impedance spectroscopy and equivalent electrical circuits of conducting polymers on carbon surfaces. Prog. Org. Coat. 2011, 71, 1–10. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Harrington, D.A.; van den Driessche, P. Mechanism and equivalent circuits in electrochemical impedance spectroscopy. Electrochim. Acta 2011, 56, 8005–8013. [Google Scholar] [CrossRef] [Green Version]
- Pajkossy, T.; Jurczakowski, R. Electrochemical impedance spectroscopy in interfacial studies. Curr. Opin. Electrochem. 2017, 1, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Gueshi, T.; Tokuda, K.; Matsuda, H. Voltammetry at partially covered electrodes: Part I. Chronopotentiometry and chronoamperometry at model electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1978, 89, 247–260. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Churikov, A.V.; Ushakov, A.V. Lithium transport processes in electrodes on the basis of Li3V2(PO4)3 by constant current chronopotentiometry, cyclic voltammetry and pulse chronoamperometry. Electrochim. Acta 2014, 122, 187–196. [Google Scholar] [CrossRef]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. Studies on the cycle life of commercial lithium ion batteries during rapid charge–discharge cycling. J. Power Sources 2001, 102, 294–301. [Google Scholar] [CrossRef]
- Prosini, P.P.; Zane, D.; Pasquali, M. Improved electrochemical performance of a LiFePO4-based composite cathode. Electrochim. Acta 2001, 46, 3517–3523. [Google Scholar] [CrossRef]
- Lingane, P.J. Chronopotentiometry and Chronoamperometry with Unshielded Planar Electrodes. Anal. Chem. 1964, 36, 1723–1726. [Google Scholar] [CrossRef]
- Pyun, S.I.; Shin, H.C.; Lee, J.W.; Go, J.Y. Chapter 2: Electrochemical Methods. In Electrochemistry of Insertion Materials for Hydrogen and Lithium; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 11–32. [Google Scholar]


| Characteristics | Electrolytic Capacitor | Carbon Supercapacitor | Battery |
|---|---|---|---|
| Specific energy (Wh kg−1) | <0.1 | 1–10 | 10–100 |
| Specific Power (W kg−1) | >>10,000 | 500–10,000 | <1000 |
| Discharging time | 10–6 to 10–3 s | s to min | 0.3–3 h |
| Charging time | 10–6 to 10–3 s | s to min | 1–5 h |
| Charge/discharge efficiency (%) | ~100 | 85–98 | 70–85 |
| Cycle-life (cycles) | Infinite | >500,000 | ~1000 |
| Max voltage (Vmax) determinants | Dielectric thickness and strength | Electrode and electrolyte stability window | Thermodynamics of phase reactions |
| Charge stored determinants | Electrode area and dielectric | Electrode microstructure and electrolyte | Active mass and thermodynamics |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, C.-y.; Kan, C.-w.; Mak, C.-l.; Chau, K.-h. Flexible Energy Storage System—An Introductory Review of Textile-Based Flexible Supercapacitors. Processes 2019, 7, 922. https://doi.org/10.3390/pr7120922
Hui C-y, Kan C-w, Mak C-l, Chau K-h. Flexible Energy Storage System—An Introductory Review of Textile-Based Flexible Supercapacitors. Processes. 2019; 7(12):922. https://doi.org/10.3390/pr7120922
Chicago/Turabian StyleHui, Chi-yuen, Chi-wai Kan, Chee-leung Mak, and Kam-hong Chau. 2019. "Flexible Energy Storage System—An Introductory Review of Textile-Based Flexible Supercapacitors" Processes 7, no. 12: 922. https://doi.org/10.3390/pr7120922
APA StyleHui, C.-y., Kan, C.-w., Mak, C.-l., & Chau, K.-h. (2019). Flexible Energy Storage System—An Introductory Review of Textile-Based Flexible Supercapacitors. Processes, 7(12), 922. https://doi.org/10.3390/pr7120922






