1. Introduction
β-lactam antibiotics represent the most important class of antibiotics, as measured by global consumption [
1]. Ampicillin, the first synthetic β-lactam, is a model compound for the development towards more clinically relevant antibiotics such as amoxicillin and cephalexin [
2,
3]. As the pharmaceutical industry (and chemical industry as a whole) embraces biocatalysis as a means of more environmentally friendly API (active pharmaceutical ingredient) production, the synthesis of β-lactams by the enzyme penicillin G acylase (PGA) has become increasingly attractive [
4]. A further optimization of β-lactam production comes from coupling the synthesis and separation of the API by reactive crystallization, which has been shown to increase the selectivity and productivity of PGA [
5].
Growth rate dispersion (GRD) is a deviation from the McCabe Δ
L law, which states that “geometrically similar (i.e., those with the same morphology) crystals of the same material suspended in the same solution grow at the same rate” [
6]. Past research has yielded a number of mechanisms that can lead to or explain GRD [
7]. One explanation is size dependent growth, whereby larger crystals grow faster because of their size. This, however, has been shown to be a manifestation of GRD as crystals with faster growth rates necessarily become larger [
8]. Another model states that crystals undergo random fluctuations in growth rate but all have the same time-average growth rate [
9]. The constant crystal growth model states that nuclei are created with an intrinsic growth rate based on surface features [
10]. Still other models contend that different rates of attrition of different sized crystals [
11] and different crystal faces explains GRD [
12], surface charge distributions can cause GRD [
13], or the presence of additives or adsorbing species can enhance or inhibit growth, leading to growth oscillations and GRD [
14]. In this study we show that GRD observed in ampicillin trihydrate is most easily explained by the constant crystal growth model and that surface morphology and crystal imperfections (such as dislocations) are likely the cause, although the influence of additives, in this case the ampicillin precursors 6-aminopenicillanic acid (6-APA) and D-phenylglycine methyl ester (PGME), may have an important influence.
In this study, the growth rates of individual ampicillin trihydrate crystals were measured by optical microscopy as ampicillin was generated by the PGA-catalyzed condensation of 6-APA and PGME. The PGA-catalyzed generation of supersaturation enabled precise control over the supersaturation of ampicillin [
15]. The catalyst and reactants were homogenous and the reactants were initially present, eliminating mass transfer effects and inhomogeneity that complicate the analysis of reactive crystallizations and anti-solvent crystallizations involving the volumetric addition of different species [
16,
17]. The entire experiment was isothermal, eliminating the heat transfer and subsequent inhomogeneity that complicate analysis of cooling crystallization [
18,
19]. The control over supersaturation also obviated the need for complex flow apparatuses used to maintain a supersaturation set point in other single crystal growth experiments [
10,
20]. One recent study used simulation to look at GRD with these simplified conditions (homogeneous, isothermal, non-nucleating batch) [
21]; here a unique experimental system enables us to probe GRD experimentally with these simplifications. Rather than reconstruct the growth rate distribution from crystal size distributions (CSDs), which requires assumptions about the form of the distribution [
22,
23], the growth rate distribution in the present work is observed directly, enabling simple determination of the mechanism of GRD. Up to ~100 crystals can be analyzed in a single small-volume experiment (<20 microliters) requiring only a microscope and camera.
2. Materials and Methods
Seed crystals were prepared as follows. Ampicillin trihydrate crystals were formed at room temperature by dissolving ampicillin sodium salt (>99%, Fischer Scientific, Geel, Belgium) and neutralized by adding concentrated hydrochloric acid. All seed crystals were grown in the same batch, which was unseeded. Crystals were sieved and the 212- to 300-μm fraction was used as seed crystals. Breakage occurred during sieving and most of the seed crystals were smaller than 212 μm, demonstrating the difficulty in sieving needle shaped crystals (their small cross section allows them to slip through the sieve despite having a long characteristic size). Seeding sometimes connotes addition of a small amount of crystals to breed a large population of crystals via secondary nucleation. As nucleation is suppressed in this study, the seed crystals are from here on referred to as initial crystals. Scanning electron micrographs of the initial crystals are shown in
Figure A1 of
Appendix A.
Growth experiments were prepared as follows: 0.111 grams of 6-aminopenicillanic acid (6-APA, >98% purity, TCI America, Portland, OR, USA, 200 mmol/L) was suspended in 1766 μL of DI water. To dissolve the 6-APA, 634 μL of 1.0 M sodium hydroxide (>98%, Sigma Aldrich, St. Louis, MO, USA, 250 mmol/L) was added to the 6-APA suspension. To the now clear 6-APA solution, 0.103 grams of phenylglycine methyl ester hydrochloride (PGME, >95%, Sigma Aldrich, St. Louis, MO, USA 200 mmol/L) was added. The PGME•HCl dissolved immediately. To this solution, 0.01 to 0.05 grams of ampicillin trihydrate initial crystals were added, and the suspension was then incubated at 25.0 °C for 15 min with agitation to allow the ampicillin to partially dissolve and saturate. The addition of these crystals saturates the solution
and provides initial crystals for observation of growth; at 0.01 g 57% of this initial charge dissolves, at 0.05 g only 11% of the initial charge dissolves. The suspension was divided into three aliquots of 665 μL allowing three repeats of each experiment. To a single aliquot, 35.0 μL of PGA stock solution (10.0 mg/mL, Assemblase®, DSM-SinoChem, Delft, Netherlands) was added. Once the PGA was added, the suspension was mixed with a magnetic stir-bar, 15.0 μL of suspension was rapidly pipetted with a wide-bore pipette tip (to avoid classifying particles by size) onto a glass microscope slide, the droplet was covered with a glass cover slip, the cover slip was sealed with silicon oil, and the microscope began recording images. The entire process from addition of PGA to beginning of data collection took approximately one minute. Images were collected with a temperature-controlled microscope, set at 25.0 °C, at a rate of one frame every two minutes, totaling two hours. The initial conditions for each experiment, based on this procedure, are listed in
Table 1.
A Leica DM LM microscope with a temperature controlled stage was used with incident lighting and 200x total magnification (20x Leica PL Fluotar objective lens and 10x eyepiece magnification). A CoolSNAP-Pro CF camera with 36-bit color imaging was attached to the TV port; the field of view was 1040-by-1392 pixels, corresponding to 1100-by-1480 microns. Images were collected with Image-Pro Plus and exported to MATLAB for analysis.
The images collected were analyzed with GT Fiber [
24,
25]. GT Fiber is a program designed to measure the lengths of fibers in images and has the advantage that it can distinguish when two fibers (or crystals) overlap or cross, an issue which has rendered image analysis of other needle-like crystals difficult [
26,
27]. As there was no convection during the experiment, individual crystals did not move substantially between frames and could be tracked over the course of the entire experiment. With the initial frame, individual crystals to be tracked were selected, by hand, and their length and width calculated with GT Fiber.
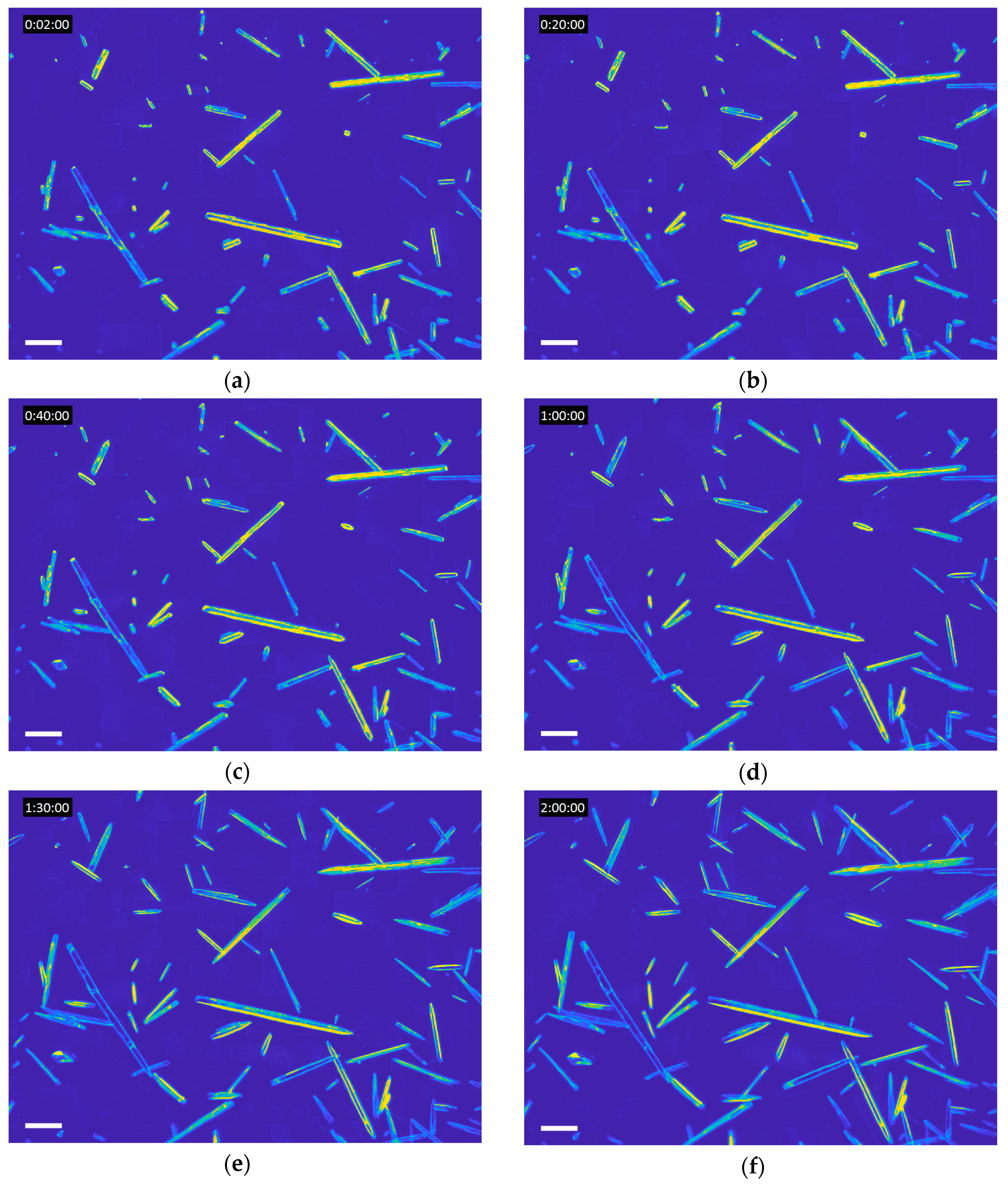
Figure 1a shows the initial frame from a single experiment; every additional frame was fit with GT Fiber, using the previous frame to identify the crystals of interest.
Figure 1b–f shows the same experiment over 2 h of growth. The lengths and widths of each individual crystal over time were extracted, from which growth rates could be determined. While the length calculation is robust, the width measurement is more uncertain; the crystals are transparent (only their edges are visible, see
Figure 1) so there is no obvious edge along which to measure the width. In total, 1003 ampicillin trihydrate crystals were observed by this method. Occasionally, two crystals would grow end to end (confusing the image analysis) or out of the image frame (resulting in unobservable growth); these crystals were removed from the dataset, giving a working set of 887 crystals. Videos of growing crystals, including the video
Figure 1 was taken from, are
Figures S1–S3 in the Supplemental Material.
To make inferences about the properties of the entire population of crystals, as well as the properties of ampicillin trihydrate itself, from this sample of 887 crystals, required the use of several different statistical tools. A discussion of those tools is included here to enable the reader to quickly understand the coming results. The Lilliefors statistical test tests the null hypothesis that the data are sampled from a normally distributed population without specifying the mean or variance of the population. The test statistic is the maximum distance between the sample cumulative distribution and the cumulative normal distribution with mean equal to the sample mean and variance equal to the sample variance. Tables of critical values have been computed by Monte Carlo methods and are widely available [
28]. The coefficient of determination,
R2, is a measure of the amount of variance explained by the model.
R2 will be close to unity when there is little noise (compared to signal) and the model has high predictive power; when there is significant noise (compared to signal)
R2 will be close to zero regardless of the model. The root mean squared error, RMSE, measures the differences between estimated values and observed values, making it more robust against noise compared to
R2. However, RMSE is scale dependent, and therefore is not used to compare an estimator’s predictions on different sets of observed values; rather it should be used to compare different estimator’s performance on a single set of observed values. A
t-test can be used to assess if the means of two sets of data are significantly different, given that the means come from a normally distributed population. Spearman’s rho,
ρS, is a rank correlation coefficient that assesses whether or not a monotonic (i.e., one-to-one) functional relationship exists between two variables. When
ρS is zero, there is no monotonic relationship between the variables and when
ρS is unity, there is a perfect monotonic relationship; a
t-test can be used to determine if
ρS is significantly different from zero, thus assessing the significance of the correlation between two variables. Finally, the results are reported as 95% confidence intervals, meaning that if the study was repeated multiple times on different sample crystals, 95% of the calculated confidence intervals would cover the true value. Higher confidence levels will give larger confidence interval widths, 95% confidence is common and was therefore chosen to be the level used in this study.
4. Discussion
Over the course of 2 h, the mean crystal width increased from 18.9 μm to 21.5 μm. This change in width corresponds to an increase of approximately two pixels. All measurements are discretized by the camera making it impossible to measure accurate width trends for individual crystals, but for the population the two pixel change in width is significant at the 99% confidence level. As the width change is so small, it is assumed most of the growth occurs at the ends of the needles. Equation (2) characterizes the rate of accumulation of dissolved ampicillin
c as solute is generated by the reaction
R and consumed by the ends of the crystals.
where
ρ is the density of the crystal,
A is cross-sectional area of the crystal, and
G is growth rate of the crystal.
Shortly after the critical time,
tcr, the linear increase in length over time suggests that the concentration reaches a pseudo steady state such that the left-hand side of Equation (2) goes to zero. As the reaction rate is constant across experiments, the product of area and growth rate must be constant. At higher loadings there is more area, therefore, one expects to observe a decrease in growth rate. The relationship between area and initial loading is not obvious. At a loading of 0.01 g, 57% of the initial crystal mass dissolved during the initial dissolution step to form a saturated solution (see Methods). Many small crystals may have dissolved entirely, leaving mostly large crystals, evidenced by the broader distribution of initial sizes in
Figure 4. At a loading of 0.05 g, only 11% of the initial crystal mass dissolved. Less dissolution of each crystal was required to saturate the solution; many of the small crystals that would have entirely dissolved in the 0.01 g loading were observable in the growth phase of the 0.05 g loading experiments.
Figure 5a shows that the growth rate is negatively correlated with the initial loading, but that initial loading is a poor predictor for growth rate given the large scatter in the data and the low
R2 = 0.10. The 95% prediction interval given by the dashed curves are correspondingly wide to capture the variance in the growth rate. It is also possible that the simple hyperbolic relationship,
, may not be appropriate for the aforementioned reasons.
There also exists a weak but statistically significant (at the 95% confidence level) correlation between critical time and growth rate. Longer critical times are correlated with slower growth rates, independent of initial loading, as can be seen in
Figure 5b where the 95% confidence interval on the slope is −8.1 × 10
−3 to −5.9 × 10
−3. There is evidence that the presence of other species, in this case the reactants 6-APA and PGME, can inhibit the growth of ampicillin crystals [
29,
30], which may lead to lack of growth below a critical supersaturation. The lack of growth is postulated to arise from the other species adsorbing to the edge of growth steps and preventing the step from advancing; halting the layer-by-layer growth mechanism [
31,
32,
33]. The advance of edges along the length of the crystal is visible on some crystals in the videos provided with the
Supplemental Material, although to be visible at this magnification they must be groups or bunches of growth steps.
Again, the scatter in the data is large,
R2 = 0.15, making critical time only weakly predictive of growth rate, however both are affected by similar surface phenomena. An underlying property of the crystal surface could be affecting both growth rate and critical time but by different mechanisms, resulting in the observed correlation and providing evidence that the distribution of growth rates results from a distribution of crystal properties (the spacing of the adsorbed species on the edge of advancing growth steps determines the critical supersaturation required to overcome the impinging adsorbed molecule [
31]). The distance between adsorbed molecules on the edge should follow a geometric distribution (assuming each adsorption site is identical), as the length and number of edges increases the probability of gaps between adsorbed molecules large enough to support growth also increases, even with the same fractional coverage of sites. Therefore, in this experimental procedure, crystals with longer growth step edges are expected to have shorter critical times and higher growth rates. Different numbers and arrangements of dislocations in the crystal can lead to different lengths of growth step edges as well as different growth rates [
10,
34].
Finally, given that the system is not mixed, we considered whether the distribution of growth rates could be attributed to the slight differences in local supersaturation due to the random scattering of crystals. For this analysis, we continued to assume that growth occurs predominately at the ends of the crystals, such that only the ends of the crystals act as a sink for ampicillin generated in the bulk (see
Appendix B for analysis of this assumption). It was also assumed that there is no bulk flow and that the height dimension is insignificant (sampled volumes measure 1290 × 967 × 43 microns L × W × H). With these assumptions, the images can be divided into areas such that each area contains a single growing crystal end that is the only sink for dissolved ampicillin in that area.
Figure 6 shows how one such image was divided.
As the reaction rate is the same throughout the bulk and if GRD is the result of the random placement of crystals only, the growth rate of each crystal should be correlated with the area over which that crystal serves as the dissolved ampicillin sink (controlled for the growing area of the sink). Instead, no correlation was found between the area of influence and the growth rate.
Figure 7 shows the expected growth rate (see
Appendix C for details of calculation) versus the measured growth rate for the experiment shown in
Figure 6. The calculation under-predicts the growth rate, likely because the width is overestimated during the image analysis. It is visually obvious that the data are not correlated, with a 95% confidence interval on the slope ranging from −0.50 to 0.99, indicating no linear relationship (Spearman’s rho indicates no one-to-one relationship, linear or otherwise). No correlation was found when the same analysis was applied to other experiments not shown in
Figure 6. To describe the growth rate of an individual crystal requires knowledge of the collective system properties (i.e., supersaturation, temperature, etc.)
and intrinsic crystal properties (dislocation frequency, surface features). While many correlations exist and can partly be explained by system properties, the large scatter in the data are in line with the constant crystal growth model where GRD is explained in terms of intrinsic crystal properties.
Previous studies have incorporated a distribution of growth rates into a population balance model to examine the effect of GRD on the CSD from a continuous crystallizer [
22]. The population density function,
n, from a mixed-suspension, mixed-product removal (MSMPR) crystallizer is given by
where
Bo is the nucleation rate,
f(
G) is the probability density function describing the growth rates of nuclei, and τ is the mean residence time of the MSMPR. To compare CSDs, it is assumed that the rate of generation of crystals, given by the suspension density
MT divided by the residence time, is the same in systems following the Δ
L law and systems exhibiting GRD.
MT is defined by
where
kv is a volumetric shape factor. With Equation (4) the nucleation rate could be adjusted to ensure the same rate of crystal generation in any crystallizer. It was shown in
Figure 2b that the growth rates of the crystals follow a normal distribution, with a mean
μ = 0.84 μm/min and
σ = 0.43 μm/min, and it is assumed that this distribution is appropriate for the nuclei in the MSMPR exhibiting GRD,
f(
G). Using these results, an MSMPR producing ampicillin by the PGA-catalyzed reactive crystallization was modeled. A residence time of 42 min has previously been shown to optimize yield while meeting purity constraints, therefore τ = 42 was used in the model [
35].
Figure 8 shows the population density function of the crystals produced by an MSMPR following the Δ
L law (no GRD,
σ = 0) and an MSMPR with GRD. With GRD, the population density function becomes skewed towards larger crystals compared to the Δ
L law growth rate; there are an order of magnitude more crystals at 400 μm length with GRD than without GRD. Such a substantial increase in large crystals could require a further processing step, such as grinding, if the process were designed to meet product size specifications with the assumption that the growth rates of all crystals are the same.