Exergy Analysis and Evaluation of the Different Flowsheeting Configurations for CO2 Capture Plant Using 2-Amino-2-Methyl-1-Propanol (AMP)
Abstract
:1. Introduction
2. Modelling Framework
2.1. Model Description of the Capture Plant
2.2. Exergy Analysis
3. Flowsheeting Configurations
3.1. Intercooling Configuration
3.2. Lean Amine Flash Configuration
3.3. Rich Split Configuration
3.4. Vapor Recompression
3.5. Rich Split with Intercooling
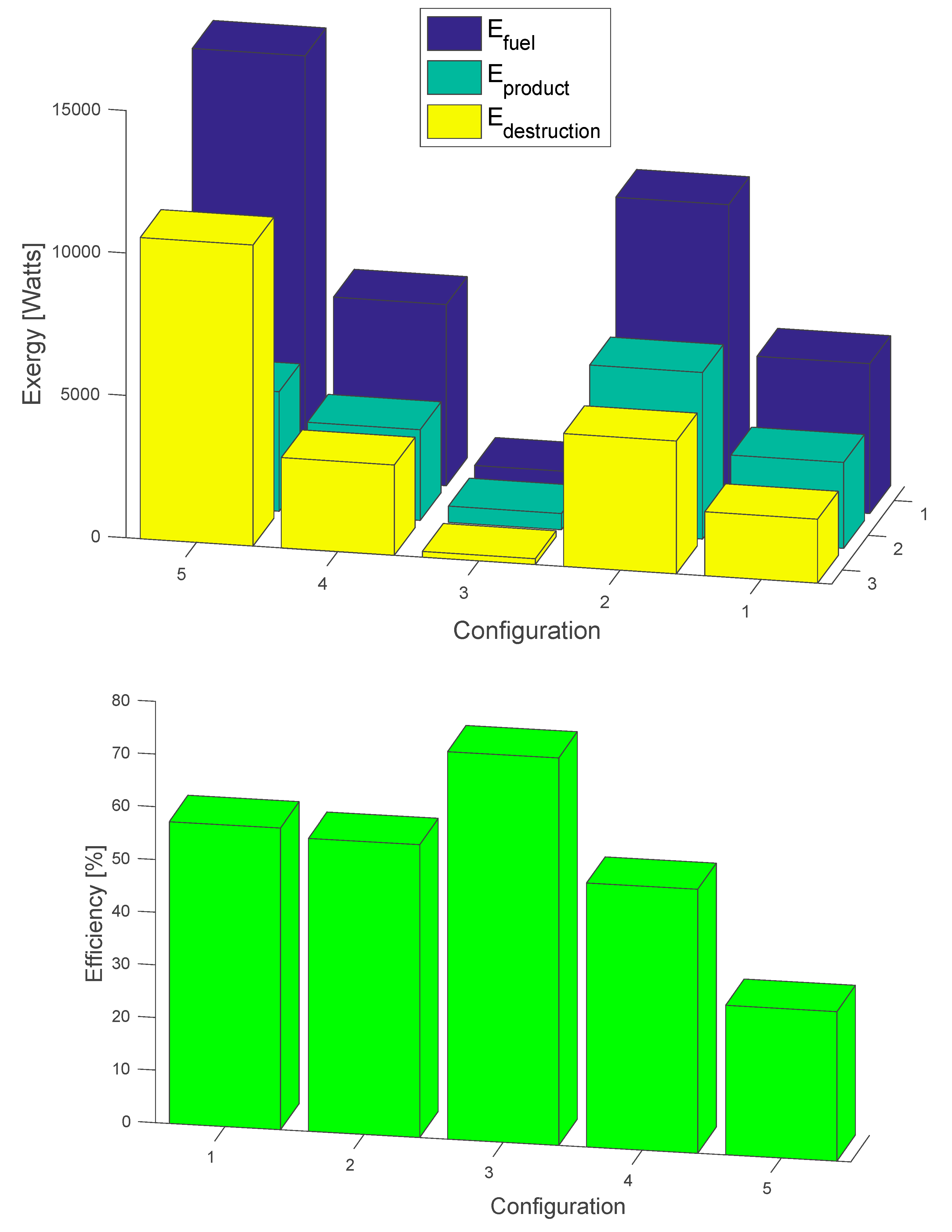
4. Exergy Analysis for the Flowsheeting Configurations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chalmers, H.; Gibbins, J. Initial evaluation of the impact of post-combustion capture of carbon dioxide on supercritical pulverised coal power plant part load performance. Fuel 2007, 86, 2109–2123. [Google Scholar] [CrossRef]
- Rubin, E.S.; Chen, C.; Rao, A.B. Cost and performance of fossil fuel power plants with CO2 capture and storage. Energy Policy 2007, 35, 4444–4454. [Google Scholar] [CrossRef]
- Davison, J. Performance and costs of power plants with capture and storage of CO2. Energy 2007, 32, 1163–1176. [Google Scholar] [CrossRef]
- Jassim, M.S.; Rochelle, G.T. Innovative absorber/stripper configurations for CO2 capture by aqueous monoethanolamine. Ind. Eng. Chem. Res. 2006, 45, 2465–2472. [Google Scholar] [CrossRef]
- Reddy, S.; Scherffius, J.; Freguia, S.; Roberts, C. Fluor’s Econamine FG Plus SM Technology. In Proceedings of the Second Annual Conference on Carbon Sequestration, Alexandria, VA, USA, 6 May 2003. [Google Scholar]
- Leites, I.L.; Sama, D.A.; Lior, N. The theory and practice of energy saving in the chemical industry: Some methods for reducing thermodynamic irreversibility in chemical technology processes. Energy 2003, 28, 55–97. [Google Scholar] [CrossRef]
- Karimi, M.; Hillestad, M.; Svendsen, H.F. Investigation of intercooling effect in CO2 capture energy consumption. Energy Procedia 2011, 4, 1601–1607. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Veawab, A. Integration of CO2 capture unit using single-and blended-amines into supercritical coal-fired power plants: Implications for emission and energy management. Int. J. Greenh. Gas Control. 2007, 1, 143–150. [Google Scholar] [CrossRef]
- Reddy, S.; Scherffius, J.; Gilmartin, J.; Freguia, S. Improved split flow apparatus. Patent No. WO 2004005818 A2, 2004. [Google Scholar]
- Eisenberg, B.; Johnson, R.R. Amine Regeneration Process. U.S. Patent 4,152,217A1, 1 May 1979. [Google Scholar]
- Cousins, A.; Wardhaugh, L.T.; Feron, P.H. Preliminary analysis of process flow sheet modifications for energy efficient CO2 capture from flue gases using chemical absorption. Chem. Eng. Res. Des. 2011, 89, 1237–1251. [Google Scholar] [CrossRef]
- Ahn, H.; Luberti, M.; Liu, Z.; Brandani, S. Process configuration studies of the amine capture process for coal-fired power plants. Int. J. Greenh. Gas Control. 2013, 16, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Quadii, A.; Khalilpouri, R. Modeling and analysis of process configurations for solvent-based post-combustion carbon capture. Asia-Pac. J. Chem. Eng. 2015, 10, 764–780. [Google Scholar] [CrossRef]
- Lars, E.O.; Terje, B.; Christian, B.; Sven, K.B.; Marius, F. Optimization of configurations for amine based CO2 absorption using Aspen HYSYS. 7th Trondheim CCS Conference, TCCS-7. Energy Procedia 2014, 51, 224–233. [Google Scholar]
- Liang, Z.; Gao, H.; Rongwong, W.; Na, Y. Comparative studies of stripper overhead vapor integration-based configurations for post-combustion CO2 capture. Int. J. Greenh. Gas Control. 2015, 34, 75–84. [Google Scholar] [CrossRef]
- Jaeheum, J.; Yeong, S.J.; Ung, L.; Youngsub, L.; Chonghun, H. New Configuration of the CO2 Capture Process Using Aqueous Monoethanolamine for Coal-Fired Power Plants. Ind. Eng. Chem. Res. 2015, 54, 3865–3878. [Google Scholar]
- Gabrielsen, F.H. Exergy analysis of alkanolamine-based CO2 removal unit with Aspen Plus. Energy 2004, 29, 1241–1248. [Google Scholar] [CrossRef]
- Lara, Y.; Martinez, A.; Lisbona, P.; Bolea, I.; Gonzalez, A.; Romeo, L.M. Using the second law of thermodynamic to improve CO2 capture systems. Energy Procedia 2011, 4, 1043–1050. [Google Scholar] [CrossRef]
- Olaleye, A.K.; Wang, M.; Kelsall, G. Steady state simulation and exergy analysis of supercritical coal-fired power plant with CO2 capture. Fuel 2015, 151, 57–72. [Google Scholar] [CrossRef]
- Valenti, G.; Bonalumi, D.; Macchi, E. Energy and exergy analyses for the carbon capture with the Chilled Ammonia Process (CAP). Energy Procedia 2009, 1, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Alatiqi, I.; Sabri, M.F.; Bouhamra, W.; Alper, E. Steady-state rate based modelling for CO2 amine absorption—Desorption systems. Gas Sep. Purif. 1994, 8, 3–11. [Google Scholar] [CrossRef]
- Aboudheir, A.; Tontiwachwuthikul, P.; Idem, R. Rigorous model for predicting the behavior of CO2 absorption into AMP in packed-bed absorption columns. Ind. Eng. Chem. Res. 2006, 45, 2553–2557. [Google Scholar] [CrossRef]
- Gabrielsen, J.; Svendsen, H.F.; Michelsen, M.L.; Stenby, E.H.; Kontogeorgis, G.M. Experimental validation of a rate-based model for CO2 capture using an AMP solution. Chem. Eng. Sci. 2007, 62, 2397–2413. [Google Scholar] [CrossRef]
- Gabrielsen, J.; Michelsen, M.L.; Stenby, E.H.; Kontogeorgis, G.M. Modeling of CO2 absorber using and AMP solution. AIChE J. 2006, 52, 3443–3451. [Google Scholar] [CrossRef]
- Afkhamipour, M.; Mofarahi, M. Comparison of rate-based and equilibrium-stage models of a packed column for post-combustion CO2 capture using 2-amino-2-methyl-1-propanol (AMP) solution. Int. J. Greenh. Gas Control 2013, 15, 186–199. [Google Scholar] [CrossRef]
- Dash, S.K.; Samanta, A.N.; Bandyopadhyay, S.S. Simulation and parametric study of post-combustion CO2 capture process using (AMP + PZ) blended solvent. Int. J. Greenh. Gas Control 2014, 21, 130–139. [Google Scholar] [CrossRef]
- Kvamsdal, H.M.; Chikukwa, A.; Hillestad, M.; Zakeri, A.; Einbu, A. A comparison of different parameter correlation models and the validation of an MEA-based absorber model. Energy Procedia 2011, 4, 1526–1533. [Google Scholar] [CrossRef] [Green Version]
- Osagie, E.; Biliyok, C.; Di Lorenzo, G.; Hanak, D.; Manovic, V. Techno-economic evaluation of the 2-amino-2-methyl-1-propanol (AMP) process for CO2 capture from natural gas combined cycle power plant. Int. J. Greenh. Gas Control 2018, 70, 45–56. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645. [Google Scholar] [CrossRef]
- Mahamud, R.; Khan, M.M.K.; Rasul, M.G.; Leinster, M.G. Exergy Analysis and Efficiency Improvement of a Coal Fired Thermal Power Plant in Queensland. In Thermal Power Plants—Advanced Applications; IntechOpen Limited: London, UK, 2013. [Google Scholar] [Green Version]
- Aspen Technology. Rate-Based Model of the CO2 Capture Process by AMP Using Aspen Plus; Aspen Technology, Inc.: Cambridge, MA, USA, 2008. [Google Scholar]
- Tsatsaronis, G.; Cziesla, F. Strengths-and-Limitations-of-Exergy-Analysis; Institute of Energy Engineering: Berlin, Germany, 1999; Volume 69, pp. 93–100. [Google Scholar]
- Sherman, B.J. Thermodynamic and Mass Transfer Modelling of Aqueous Hindered Amines for Carbon Dioxide Capture. Ph.D. Dissertation, University of Texas, Austin, TX, USA, 2016. [Google Scholar]
- Herrmann, J. Conversion of Experimental Data for Process Modelling of Novel Amine CO2 Capture Solvents. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2014. [Google Scholar]
- Yang, Y. Comprehensive exergy-based evaluation and parametric study of a coal-fired ultra-supercritical power plant. Appl. Energy 2013, 112, 1087–1099. [Google Scholar] [CrossRef]
- Kvamsdal, H.M.; Rochelle, G.T. Effects of the temperature bulge in CO2 absorption from flue gas by aqueous monoethanolamine. Ind. Eng. Chem. Res. 2008, 47, 867–875. [Google Scholar] [CrossRef]
- Anthony, J.L. Carbon dioxide: Generation and capture. In Energy and Environmental Impacts Related to Sustainability Seminar; Kansas State University: Manhattan, KS, USA, 2006. [Google Scholar]
- Woodhouse, S.; Rushfeldt, P. Improved Absorbent Regeneration. Patent No. WO2008/063079 A2, 29 May 2008. [Google Scholar]
- Reddy, S.; Gilmartin, J.; Francuz, V. Integrated Compressor/Stripper Configurations and Methods. U.S. Patent 2009/0205946 A1, 20 August 2009. [Google Scholar]
- Le Moullec, Y.; Kanniche, M. Screening of flowsheet modifications for an efficient monoethanolamine (MEA) based post-combustion CO2 capture. Int. J. Greenh. Gas Control. 2011, 5, 727–740. [Google Scholar] [CrossRef]

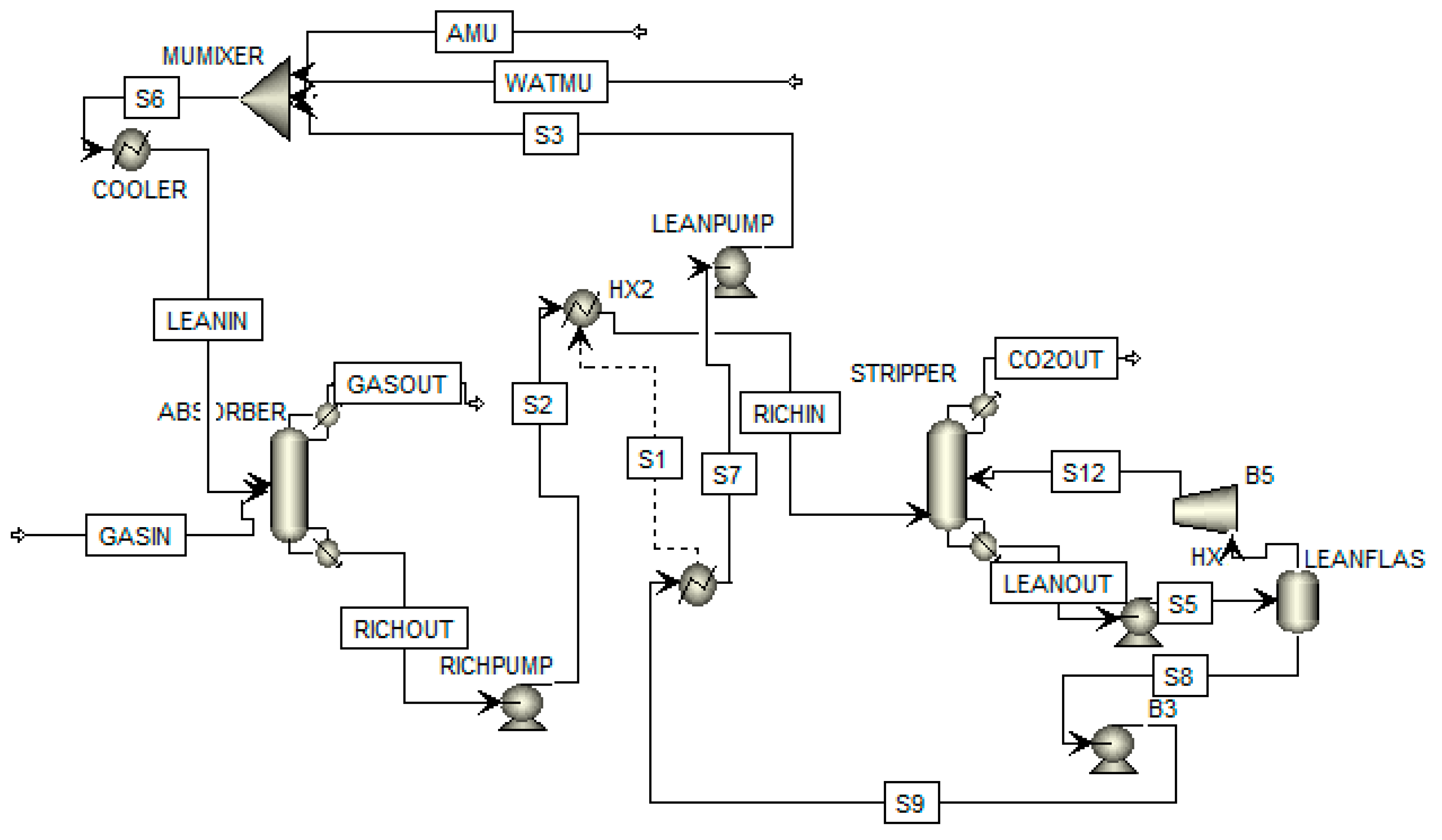

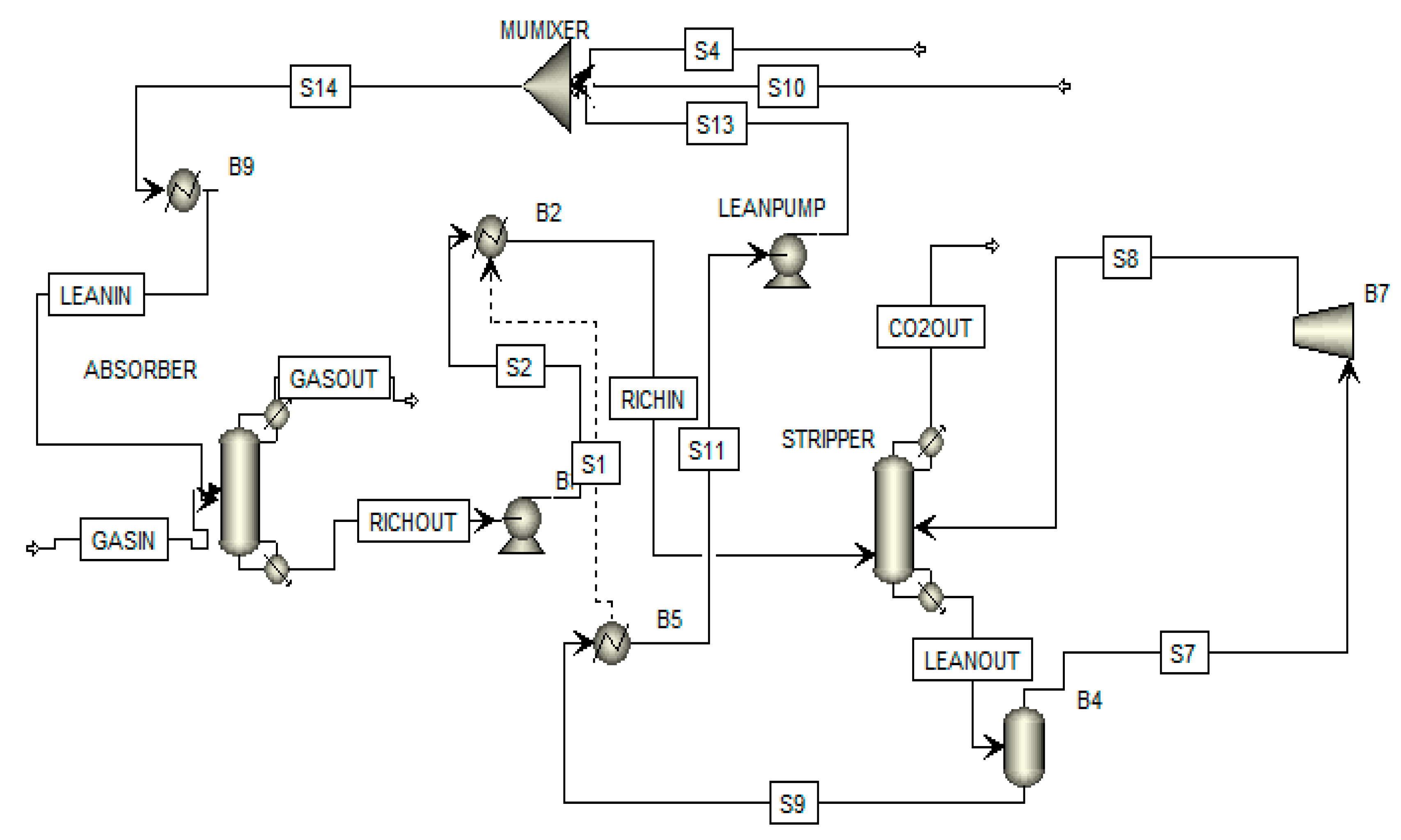
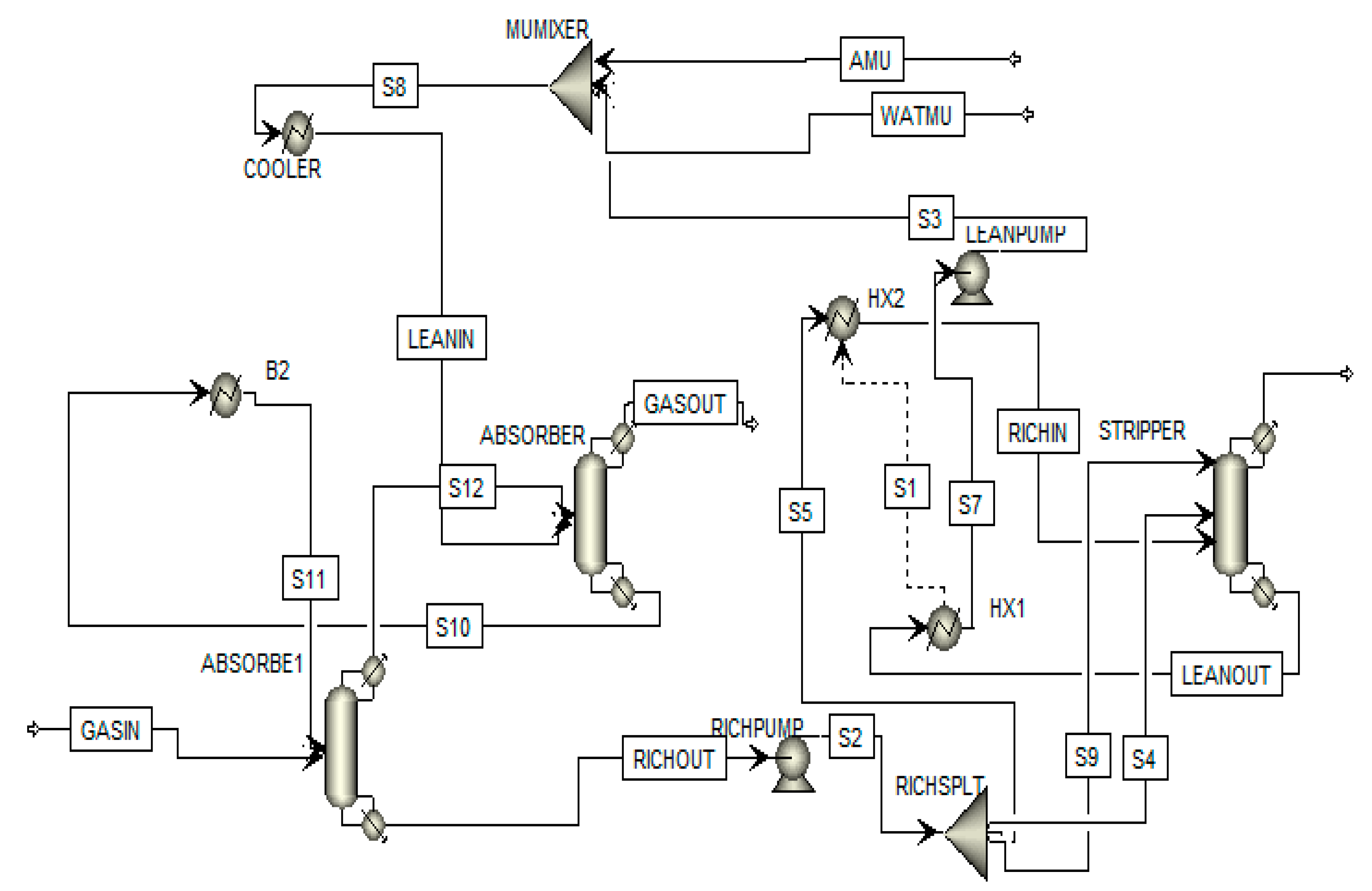
| Author(s) | Detail of Study | Solvent | Exergy Analysis |
|---|---|---|---|
| Leites et al. [6] | Evaluated intercooler at 40–80 °C, operation at 40 °C gave a maximum effect on reboiler duty using monoethanolamine (MEA) | MEA | No |
| Karimi et al. [7] | Evaluated intercooling effect. The optimal location for intercooling was 1/4th to 1/5th of the height of the column from the bottom, nearly 3% savings in reboiler energy. | MEA | No |
| Aroonwilas and Veawab [8] | Evaluated intercooling design. Removal of liquid done at 1/5th of column height from the bottom. Cooling at 45 °C resulted in 10% energy savings in the reboiler. | MEA | No |
| Reddy et al. [9] | Evaluated lean amine flash configuration. Hot lean amine temperature reduced from 120 to 103 °C, the low temperature, however, increases the energy consumption in the stripper. | MEA | No |
| Eisenberg and Johnson [10] | Evaluated rich split configuration resulting in 7.1% reboiler duty savings over reference case. | MEA | No |
| Cousins et al. [11] | Reviewed fifteen flowsheeting configurations, these include multi-component columns, inter-stage temperature control, heat-integrated stripping column, split flow process, vapor recompression, matrix stripping, heat integration. Results showed significant energy savings. | MEA | No |
| Ahn et al. [12] | Evaluated ten different configurations capture plants, this included the multiple alterations (absorber intercooling combined with condensate evaporation and lean amine flash). The multiple strategies achieved a greater reduction in the energy requirement reducing steam consumption by up to 37% when compared to the simple absorber/stripper configurations. | MEA | No |
| Sharma et al. [13] | Reviewed and assessed the advantages of fourteen different flow sheeting configurations. Results showed pump-around was more beneficial than intercooling. | MEA | No |
| Lars et al. [14] | Evaluated and compared different configurations; vapor recompression with split stream gave the best reduction of 11% compared to the conventional. | MEA | No |
| Liang et al. [15] | Five different flow sheeting configurations studied, the new innovation was the combination of split flow with overhead exchanger and improved split flow with vapor recompression These innovations decreased equivalent work by 17.21% and 17.52% respectively | MEA | No |
| Jung et al. [16] | Evaluated rich vapor recompression and cold solvent split. Results showed that reboiler heat was reduced from 3.44 MJ/kg CO2 to 2.75 MJ/kg CO2. | MEA | No |
| Geuzebroek [17], Lara et al. [18], Olaleye et al. [19] | Exergy analysis of CO2 capture plant | MEA | Yes |
| Valenti et al. [20] | Exergy analysis of CO2 capture plant | Ammonia | Yes |
(Watts) | (Watts) | (Watts) | (%) | |
|---|---|---|---|---|
| Absorber | 45.19 | 27.02 | 18.17 | 59.79 |
| Stripper | 5085.56 | 4224.59 | 860.97 | 83.07 |
| Pump | 393.27 | 389.29 | 3.98 | 98.99 |
| Cooler | 1037.07 | 307.0 | 730.06 | 29.60 |
| Heat exchanger | 4174.62 | 1037.07 | 3137.55 | 24.84 |
| Total | 10,735.7 | 5984.98 | 4750.72 | 55.75 |
| Rich Loading (mol/mol) | Absorber (ABS) Capacity (mol/mol) | Reboiler Duty (kW) | ABS Temp (°C) | |
|---|---|---|---|---|
| Reference | 0.388 | 0.123 | 4.77 | 63.07 |
| AMP-cooled | 0.503 | 0.173 | 3.60 | 47.11 |
| Loading of Lean Out (mol/mol) | Reboiler Duty (kW) | Cross-Heat Exchanger Duty (kW) | |
|---|---|---|---|
| Reference | 0.265 | 4.77 | 17.46 |
| AMP-lean amine flash | 0.252 | 2.79 | 10.92 |
| Split-Fraction (%) | Reboiler Duty (kW) | Cross Heat Exchanger Flowrate (kg/s) | Cross Heat Exchanger Duty (kW) | |
|---|---|---|---|---|
| Reference | 0 | 4.77 | 0.10089 | 17.46 |
| AMP-rich Split | 30 | 4.7 | 0.09989 | 17.30 |
| Reboiler Duty (kW) | Cross-Heat Exchanger Duty (kW) | |
|---|---|---|
| Reference plant | 4.77 | 17.46 |
| AMP Vap-Recompression | 1.93 | 5.69 |
| Reboiler Duty (kW) | Cross-Heat Exchanger Flowrate (kg/s) | Cross-Heat Exchanger Duty (W) | |
|---|---|---|---|
| Reference | 4.77 | 0.10089 | 17.46 |
| AMP Rich split | 0.55 | 2.55 × 10−4 | 5.30 |
| Configurations | Efuel (Watts) | Eproduct (Watts) | Edestruction (Watts) | Eeff (%) |
|---|---|---|---|---|
| Intercooling | 5261.33 | 3015.67 | 2245.66 | 57.32 |
| Rich split | 10,515.44 | 5851.37 | 4664.07 | 55.65 |
| Rich slit + intercooling | 780.11 | 573.87 | 206.23 | 73.56 |
| Lean amine flash | 6360.78 | 3189.95 | 3170.83 | 50.15 |
| Vapor recompression | 14,767.7 | 4197.07 | 10,570.65 | 28.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osagie, E.; Aliyu, A.M.; Nnabuife, S.G.; Omoregbe, O.; Etim, V. Exergy Analysis and Evaluation of the Different Flowsheeting Configurations for CO2 Capture Plant Using 2-Amino-2-Methyl-1-Propanol (AMP). Processes 2019, 7, 391. https://doi.org/10.3390/pr7060391
Osagie E, Aliyu AM, Nnabuife SG, Omoregbe O, Etim V. Exergy Analysis and Evaluation of the Different Flowsheeting Configurations for CO2 Capture Plant Using 2-Amino-2-Methyl-1-Propanol (AMP). Processes. 2019; 7(6):391. https://doi.org/10.3390/pr7060391
Chicago/Turabian StyleOsagie, Ebuwa, Aliyu M. Aliyu, Somtochukwu Godfrey Nnabuife, Osaze Omoregbe, and Victor Etim. 2019. "Exergy Analysis and Evaluation of the Different Flowsheeting Configurations for CO2 Capture Plant Using 2-Amino-2-Methyl-1-Propanol (AMP)" Processes 7, no. 6: 391. https://doi.org/10.3390/pr7060391
APA StyleOsagie, E., Aliyu, A. M., Nnabuife, S. G., Omoregbe, O., & Etim, V. (2019). Exergy Analysis and Evaluation of the Different Flowsheeting Configurations for CO2 Capture Plant Using 2-Amino-2-Methyl-1-Propanol (AMP). Processes, 7(6), 391. https://doi.org/10.3390/pr7060391






