Biodegradation Potential and Diversity of Diclofenac-degrading Microbiota in an Immobilized Cell Biofilter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diclofenac-Based Wastewater Composition
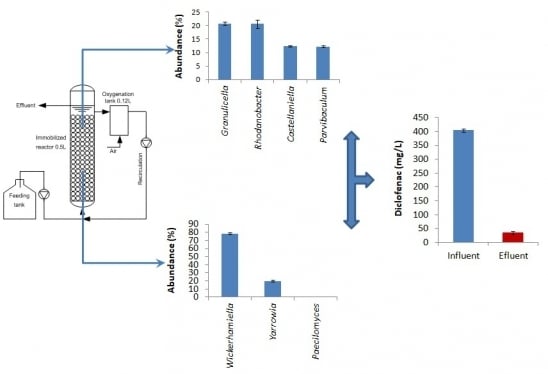
2.2. Bioreactor Configuration
2.3. Physicochemical Analyses
2.4. HPLC Determination of Diclofenac
2.5. Amplicon Sequencing Analysis of the Diclofenac-degranding Microbiota in the Immobilized Cell Biofilter
2.6. Statistical Analysis
3. Results and Discussion
3.1. Operating Behavior and Effectiveness of the Immobilized Cell Biofilter to Degrade Diclofenac-Based Wastewater
3.2. Bacterial Community Structure in the Immobilized Cell Biofilter Fed with Diclofenac-Based Wastewater
Author Contributions
Funding
Conflicts of Interest
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Ginebreda, A.; Muñoz, I.; Alda, M.L.; Brix, R.; Doval, J.L.; Barceló, D. Environmental risk assessment of pharmaceuticals in rivers: Relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain). Environ. Int. 2010, 36, 153–162. [Google Scholar] [CrossRef]
- Nagler, J.J.; Bouma, J.; Thorgaard, G.H.; Dauble, D.D. High incidence of a male-specific genetic marker in phenotypic female chinook salmon from the Columbia River. Environ. Health Perspect. 2001, 109, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Zorita, S.; Martensson, L.; Mathiasson, L. Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the South of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Rao, Y. Diclofenac and its transformation products: Environmental occurrence and toxicity. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Al-Rajab, A.J.; Sabourin, L.; Lapen, D.R.; Topp, E. The non-steroidal anti-inflammatory drug diclofenac is readily biodegradable in agricultural soils. Sci. Total Environ. 2010, 409, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; González-Doncel, M.; Pro, J.; Carbonell, G.; Tarazona, J.V. Occurrence of pharmaceutically active compounds in surface waters of the Henares–Jarama–Tajo river system (Madrid, Spain) and a potential risk characterization. Sci. Total Environ. 2010, 408, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T.; Schmidt-Baumler, K.; Stan, H.J. Occurrence and distribution of organic contaminants in the aquatic system in Berlin. Part I: Drug residues and other polar contaminants in Berlin surface and groundwater. Acta Hydrochim. Hydrobiol. 1998, 26, 272–278. [Google Scholar] [CrossRef]
- Rabiet, M.; Togola, A.; Brissaud, F.; Seidel, J.L.; Budzinski, H.; Elbaz-Poulichet, F. Consequences of treated water recycling as regards pharmaceuticals and drugs in surface and ground waters of a medium-sized Mediterranean catchment. Environ. Sci. Technol. 2006, 40, 5282–5288. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Commission Implementing Decision (EU) 2015/495. Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, L 78/40. [Google Scholar]
- Directive 2008/105/EC. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Off. J. Eur. Union 2008, L 348/84. [Google Scholar]
- Liu, J.; Lu, G.; Xie, Z.; Zhang, Z.; Li, S.; Yan, Z. Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci. Total Environ. 2015, 511, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Damasceno de Oliveira, L.L.; Nunes, B.; Antunes, S.C.; Campitelli-Ramos, R.; Rocha, O. Acute and chronic effects of three pharmaceutical drugs on the tropical freshwater cladoceran Ceriodaphnia silvestrii. Water Air Soil Pollut. 2018, 229, 116. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Chaudhry, M.J.I.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Hapeshi, E.; Achilleos, A.; Meric, S.; Gros, M.; Petrovic, M.; Barcelo, D. Existence of pharmaceutical compounds in tertiary treated urban wastewater that is utilized for reuse applications. Water Resour. Manag. 2011, 25, 1183–1193. [Google Scholar] [CrossRef]
- Lonappan, L.; Pulicharla, R.; Rouissi, T.; Brar, S.K.; Vermab, M.; Surampallic, R.Y.; Valero, J.R. Diclofenac in municipal wastewater treatment plant: Quantification using laser diode thermal desorption-atmospheric pressure chemicalionization-tandem mass spectrometry approach in comparison with an established liquid chromatography-electrosprayionization-tandem mass spectrometry method. J. Chromatogr. A 2016, 1433, 106–113. [Google Scholar]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Langenhoff, A.; Inderfurth, N.; Veuskens, T.; Schraa, G.; Blokland, M.; Kujawa-Roeleveld, K.; Rijnaarts, H. Microbial removal of the pharmaceutical compounds ibuprofen and diclofenac from wastewater. BioMed Res. Int. 2013, 2013, 325806. [Google Scholar] [CrossRef]
- Liu, Y.J.; Lo, S.L.; Liou, Y.H.; Hu, C.Y. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation–flotation with a cationic surfactant. Sep. Purif. Technol. 2015, 152, 148–154. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Meisel, L.M.; Lino, C.M.; Pena, A. Environmental impact of pharmaceuticals from Portuguese wastewaters: Geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015, 136, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kim, H.W.; Oh, J.-E.; Park, H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef] [PubMed]
- Gurung, K.; Chaker Ncibi, M.; Sillanpää, M. Assessing membrane fouling and the performance of pilot-scale membrane bioreactor (MBR) to treat real municipal wastewater during winter season in Nordic regions. Sci. Total Environ. 2017, 579, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Bessa, V.S.; Moreira, I.S.; Tiritan, M.E.; Castro, P.M.L. Enrichment of bacterial strains for the biodegradation of diclofenac and carbamazepine from activated sludge. Int. Biodeterior. Biodegrad. 2017, 120, 135–142. [Google Scholar] [CrossRef]
- Moreira, I.S.; Bessa, V.S.; Murgolo, S.; Piccirillo, C.; Mascolo, G.; Castro, P.M.L. Biodegradation of diclofenac by the bacterial strain Labrys portucalensis F11. Ecotoxicol. Environ. Saf. 2018, 152, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Biotransformation of three pharmaceutical active compounds by the fungus Phanerochaete chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation 2012, 23, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; p. 63. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA): Washington, DC, USA, 1998. [Google Scholar]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef] [PubMed]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for Illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Edgar, R.C. SEARCH_16S: A new algorithm for identifying 16S ribosomal RNA genes in contigs and chromosomes. bioRxiv 2017, 124131. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 2016, 081257. [Google Scholar] [CrossRef]
- Allard, G.; Ryan, F.J.; Jeffery, I.B.; Claesson, M.J. SPINGO: A rapid species-classifier for microbial amplicon sequences. BMC Bioinform. 2015, 16, 324. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, S.F.; Smith, B.J.; Hein, R.; Roller, B.R.K.; Schmidt, T.M. rrnDB: Improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res. 2015, 43, D593–D598. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Herdegen, M.; Migalska, M.; Radwan, J. AmpliSAS: A web server for multilocus genotyping using next-generation amplicon sequencing data. Mol. Ecol. Resour. 2016, 16, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø. PAST-Paleontological Statistics; Version 3.25; Natural History Museum, University of Oslo: Olso, Norway, 2019; Available online: https://folk.uio.no/ohammer/past/index.html (accessed on 6 August 2019).
- Trapido, M.; Epold, I.; Dulova, N. Degradation of diclofenac in aqueous solution by homogeneous and heterogeneous photolysis. J. Environ. Eng. Ecol. Sci. 2012, 1, 3. [Google Scholar]
- Princic, A.; Mahne, I.; Megusar, F.; Paul, E.A.; Tiedje, J.M. Effects of pH and oxygen and ammonium concentrations on the community structure of nitrifying bacteria from wastewater. Appl. Environ. Microbiol. 1998, 64, 3584–3590. [Google Scholar]
- Campbell, B.J. The family Acidobacteriaceae. In The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 405–415. [Google Scholar]
- de Castro, V.H.L.; Schroeder, L.F.; Quirino, B.F.; Kruger, R.H.; Barreto, C.C. Acidobacteria from oligotrophic soil from the Cerrado can grow in a wide range of carbon source concentrations. Can. J. Microbiol. 2013, 59, 746–753. [Google Scholar] [CrossRef]
- Aqeel, H.; Basuvaraj, M.; Hall, M.; Neufeld, J.D.; Liss, S.N. Microbial dynamics and properties of aerobic granules developed in a laboratory-scale sequencing batch reactor with an intermediate filamentous bulking stage. Appl. Microbiol. Biotechnol. 2016, 100, 447–460. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Zhang, M.; Kurisu, F.; Toyota, K. Bacterial degraders of coexisting dichloromethane, benzene, and toluene, identified by stable-isotope probing. Water Air Soil Pollut. 2017, 228, 418. [Google Scholar] [CrossRef]
- Navrozidou, E.; Melidis, P.; Ntougias, S. Biodegradation aspects of ibuprofen and identification of ibuprofen-degrading microbiota in an immobilized cell bioreactor. Environ. Sci. Pollut. Res. 2019, 26, 14238–14249. [Google Scholar] [CrossRef]
- Green, S.J.; Prakash, O.; Jasrotia, P.; Overholt, W.A.; Cardenas, E.; Hubbard, D.; Tiedje, J.M.; Watson, D.B.; Schadt, C.W.; Brooks, S.C.; et al. Denitrifying bacteria from the genus Rhodanobacter dominate bacterial communities in the highly contaminated subsurface of a nuclear legacy waste site. Appl. Environ. Microbiol. 2012, 78, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Jiang, L.; Zhang, D.; Luo, C.; Wang, Y.; Yu, Z.; Yin, H.; Zhang, G. Bacteria capable of degrading anthracene, phenanthrene, and fluoranthene as revealed by DNA based stable-isotope probing in a forest soil. J. Hazard. Mater. 2016, 308, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratzke, C.; Gore, J. Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 2018, 16, e2004248. [Google Scholar] [CrossRef] [PubMed]
- Wegrzy, A.; Felis, E. Isolation of bacterial endophytes from Phalaris arundinacea and their potential in diclofenac and sulfamethoxazole degradation. Pol. J. Microbiol. 2018, 67, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Palyzová, A.; Zahradník, J.; Marešová, H.; Sokolová, L.; Kyslíková, E.; Grulich, M.; Štěpánek, V.; Řezanka, T.; Kyslík, P. Potential of the strain Raoultella sp. KDF8 for removal of analgesics. Folia Microbiol. 2018, 63, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, K.; Hapeshi, E.; Vasquez, M.I.; Fatta-Kassinos, D.; Vyrides, I. Diclofenac biodegradation by newly isolated Klebsiella sp. KSC: Microbial intermediates and ecotoxicological assessment. J. Environ. Chem. Eng. 2018, 6, 3242–3248. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Stolz, A.; Bürger, S.; Kuhm, A.; Kämpfer, P.; Busse, H.-J. Pusillimonas noertemannii gen. nov., sp. nov., a new member of the family Alcaligenaceae that degrades substituted salicylates. Int. J. Syst. Evol. Microbiol. 2005, 55, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Castellet-Rovira, F.; Lucas, D.; Villagrasa, M.; Rodríguez-Mozaz, S.; Barceló, D.; Sarrà, M. Stropharia rugosoannulata and Gymnopilus luteofolius: Promising fungal species for pharmaceutical biodegradation in contaminated water. J. Environ. Manag. 2018, 207, 396–404. [Google Scholar] [CrossRef]
- Cruz-Morató, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barceló, D.; Vicent, T.; Sarrà, M.; Marco-Urrea, E. Hospital wastewater treatment by fungal bioreactor: Removal efficiency for pharmaceuticals and endocrine disruptor compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef]
- Aracagök, Y.D.; Göker, H.; Cihangir, N. Biodegradation of diclofenac with fungal strains. Arch. Environ. Prot. 2018, 44, 55–62. [Google Scholar]
- Niu, L.; Li, Y.; Xu, L.; Wang, P.; Zhang, W.; Wang, C.; Cai, W.; Wang, L. Ignored fungal community in activated sludge wastewater treatment plants: Diversity and altitudinal characteristics. Environ. Sci. Pollut. Res. 2017, 24, 4185–4193. [Google Scholar] [CrossRef] [PubMed]






| Other Physicochemical Parameters | Mean ± SE (n = 6) |
|---|---|
| NH4+-Nin (mg/L) | 44.80 ± 6.62 |
| NH4+-Nef (mg/L) | 21.00 ± 6.93 |
| TKNin (mg/L) | 61.60 ± 2.42 |
| TKNef (mg/L) | 21.56 ± 3.77 |
| PO43−-Pin (mg/L) | 13.17 ± 1.36 |
| PO43−-Pef (mg/L) | 7.07 ± 1.75 |
| NO3−-Nef (mg/L) | 1.81 ± 0.85 |
| NO2−-Nef (mg/L) | 0.21 ±0.12 |
| BODef (mg/L) | 8.00 ± 1.22 |
| DO (mg/L) | 4.32 ± 0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navrozidou, E.; Remmas, N.; Melidis, P.; Karpouzas, D.G.; Tsiamis, G.; Ntougias, S. Biodegradation Potential and Diversity of Diclofenac-degrading Microbiota in an Immobilized Cell Biofilter. Processes 2019, 7, 554. https://doi.org/10.3390/pr7090554
Navrozidou E, Remmas N, Melidis P, Karpouzas DG, Tsiamis G, Ntougias S. Biodegradation Potential and Diversity of Diclofenac-degrading Microbiota in an Immobilized Cell Biofilter. Processes. 2019; 7(9):554. https://doi.org/10.3390/pr7090554
Chicago/Turabian StyleNavrozidou, Efstathia, Nikolaos Remmas, Paraschos Melidis, Dimitrios G. Karpouzas, George Tsiamis, and Spyridon Ntougias. 2019. "Biodegradation Potential and Diversity of Diclofenac-degrading Microbiota in an Immobilized Cell Biofilter" Processes 7, no. 9: 554. https://doi.org/10.3390/pr7090554
APA StyleNavrozidou, E., Remmas, N., Melidis, P., Karpouzas, D. G., Tsiamis, G., & Ntougias, S. (2019). Biodegradation Potential and Diversity of Diclofenac-degrading Microbiota in an Immobilized Cell Biofilter. Processes, 7(9), 554. https://doi.org/10.3390/pr7090554