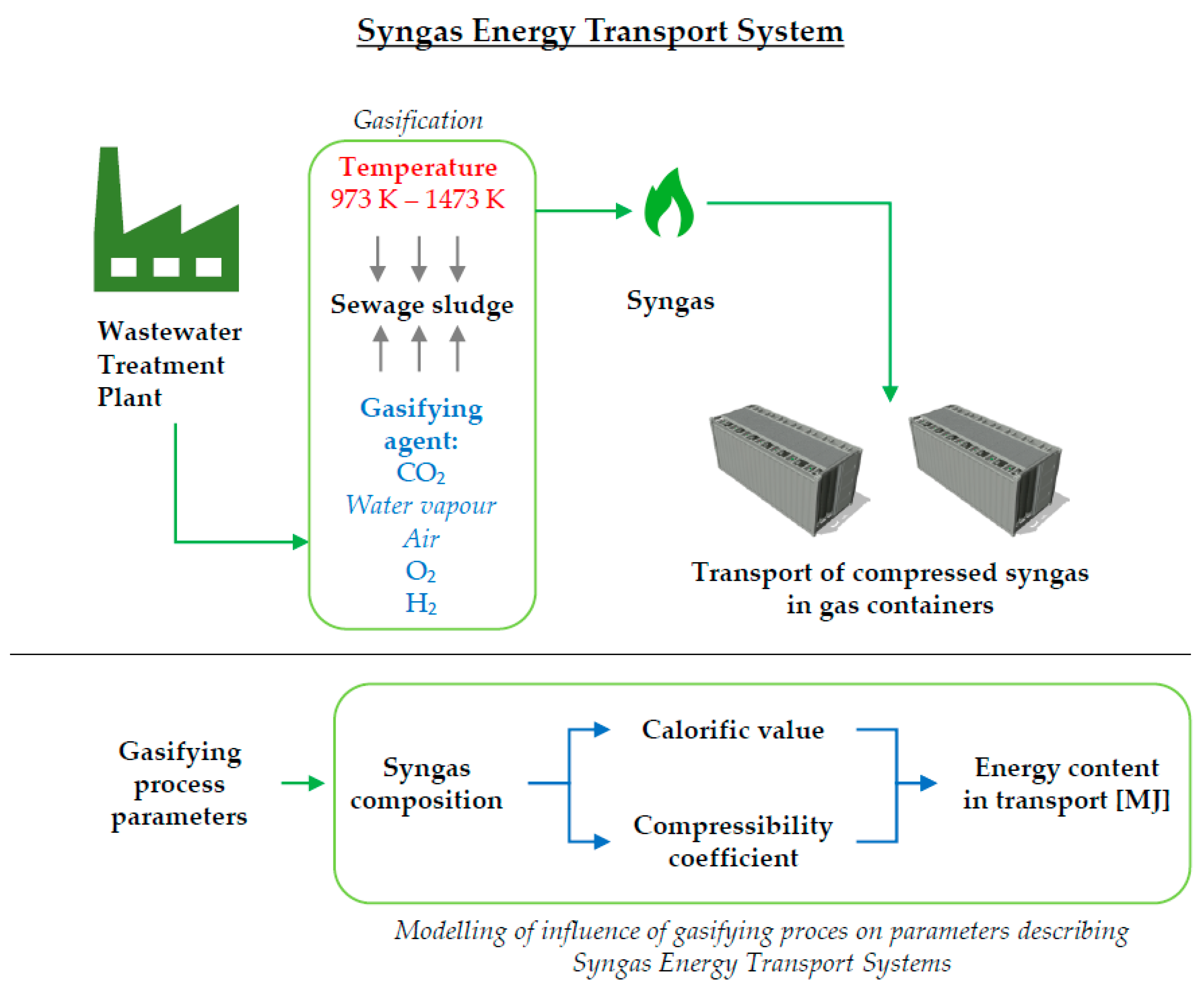
In this work, modeling of critical functionalities of the proposed SETS was performed. At first, theoretical modeling of the gasification process of SS was performed using the Gaseq Chemical Equilibrium Program 3.1 software. The novelty of the performed analysis is connected with the gasification of SS without any addition of other substrates such as wood or straw as well as with a range of performed analysis. The gasification process was analyzed for five different gasifying agents with variable molar numbers and 25 gasification temperatures. As a result, the chemical composition, and LHV of the syngas from SS were obtained. Results were compared with those available in the literature for verification of the proposed approach and validation of results.
Data obtained from the modeling of the SS gasification was used to develop and perform analytical modeling of the syngas compression to determine the energy content in a given volume of syngas as well as the compressibility coefficient that describes the state of obtained syngas at elevated pressure. For the scope of this work and the similarity of the application of the compressed syngas to CNG, the possibility of the application of models dedicated to determining the compressibility factor of NG was examined. Due to the obtained composition of syngas, there was a need to develop a model dedicated to the syngas based on several assumptions. This was done by the application of a real gas equation and method of weighting treatment to determine the syngas compressibility coefficient and syngas compression ratio describing the increase of volume under specific conditions.
As a result, the influence of the molar concentration of gasifying agents, and process temperature on the amount of energy possible to be stored and transported in the pressure vessel was obtained.
2.2. Sewage Sludge Gasification Modeling Procedure
The following assumptions were made based on [
32,
33]. Specifically, the boundary conditions were established in those experimental studies where the gasification of SS was carried out. Therefore, the assumptions of the model were taken from experimental research and were as follows:
gases are treated as semi-ideal,
all elemental C from the SS is converted to gaseous components,
process gases are limited to CO, CO2, water vapor, H2, N2, H2S, and CH4,
the total conversion of tar substances occurs in the oxidation zone,
the temperatures of the solid- and gas-phases are the same,
pressure losses are negligible (assumption of constant pressure).
The material was subjected to a thermal process at temperatures of 973, 998, 1023, 1048, 1073, 1098, 1123, 1148, 1173, 1198, 1223, 1248, 1273, 1298, 1323, 1348, 1373, 1398, 1423, 1448, and 1473 K. The equal incremental interval of 25 K was used. Such temperature selection is consistent with the model distribution of gasification temperatures, which occurs between 973 K and 1473 K [
33]. The process pressure was set to 1013 hPa according to experimental conditions described in [
33], and the authors wrote an assumption that the pressure is constant because there is a flow of gasifying agent and the resulting gas is withdrawn.
Five series of experiments were carried out for a different gasifying agent and its varying molar concentration. The following factors and their molar concentrations were used in the experiment:
oxygen, from 0.1 to 1.0 moles (interval 0.1 mole),
hydrogen, from 0.4 to 4.0 moles (interval 0.4 moles),
carbon dioxide, from 0.2 to 2.0 moles (interval 0.2 mole),
water vapor, from 0.2 to 2.0 moles (interval 0.2 m),
air, from 0.1 to 1.0 moles (interval 0.1 mole) for oxygen and from 0.367 to 3.760 moles for nitrogen (interval 0.367 moles).
The oxygen demand required for the gasification process was calculated based on the chemical reactions taking place in the process summarized in
Table 2.
Table 3 presents the comparison of initial and boundary conditions of simulations.
The concentration of the gasifying agent results from the reactions described in
Table 2. It presents the main reactions of the gasifying agent with the carbon element in equilibrium conditions. The number of moles in the equilibrium was divided into five. Additionally, the proportion of each factor was the same. Subsequently, values lower and higher than the equilibrium were determined.
The simulation of the SS gasification process was carried out using the Gaseq Chemical Equilibrium Program 3.1 software [
34]. Based on the obtained synthesis gas composition, the
LHV of 1 m
3 of gas was calculated for normal conditions [
35]:
where:
Vs—the percent component volume in the synthesis gas, %
LHVs—the theoretical calorific value of the synthesis gas component, MJ∙m3 (H2—10,748 MJ∙m3, CO—12,634 MJ∙m3, CH4—35,725 MJ∙m3, H2S—23,152 MJ∙m3).
2.3. Modeling of Gas Transport Volume of Syngas as a Function of Its Variable Composition
As a result of the SS gasification modeling, chemical compositions and
LHVs were obtained for different gases as a result of the conducted analysis. Those values are required to determine the volume of gas in the transport and energy content of compressed syngas under specific storage conditions in a SETS. Those conditions were assumed based on the described syngas transport unit in the form of an ISO container with 68 pressure vessels, all connected into one storage volume of 14,960 m
3. This volume is referred to as a ‘water’ volume of the storage unit and represents the amount of space gas can occupy when stored in the storage unit. The increase of gas volume is achieved by increased gas pressure in the compression process. This, in turn, makes it possible to store and transport more gas, which increases the energy content. Analytical calculations were carried out to determine the gas volume in the transport unit. These calculations were based on the results of the SS gasification modeling and resulting chemical composition of syngas obtained for given gasification factors and the temperature of the gasification process. For this work, the real gas equation was used [
36], which is based on the introduction of an additional coefficient to the ideal gas equation, the so-called ‘gas compressibility coefficient’ Z, which takes into account intermolecular interactions in the analyzed gas. In standard temperature and pressure (STP) conditions (273.15 K and absolute pressure of 100 kPa), the compressibility coefficient of real gases is assumed to be equal to one, which means gas under STP conditions may be treated as an ideal gas [
36]. For most gases, with a pressure increase, the gas compressibility coefficient decreases, which means that real gas occupies a smaller volume as compared to an ideal gas at the same pressure and temperature [
9]. The real gas equation constituting the basis for theoretical calculations is shown below (based on [
36]):
where:
P—gas pressure, Pa,
V—gas volume, m3,
Z—gas compressibility coefficient,
n—the number of moles of gas,
R—universal gas constant, J∙mol−1∙k−1,
T—gas temperature, K.
Equation (2) allows the determination of gas parameters at any temperature and volume, assuming the knowledge of gas compressibility coefficient. The
Z value can be determined using a series of equations and methods, depending on the composition of the analyzed gas. For natural gas, the most commonly used methods are GERG-2008, SGERG-88, and AGA-8 [
37,
38,
39]. Authors have analyzed the possibility to apply methods mentioned above because there are many programs in which these three models are used for the determination of the natural gas compressibility factor. If the composition of syngas makes it suitable to use one of the above methods to determine the Z factor, it will make it possible to use already known solutions to determine the compressibility factor of syngas. Uncertainty of the compressibility factor using the GERG-2008 for single-phase mixtures is less than 0.1% in the normal range and 0.5% in the extended range of application [
39]. The normal and extended range is related to the percentage of gas constituents, which represents applicability of this calculation method. However, due to the strictly defined scope of application, these methods cannot be directly applied to the syngas analysis due to the excessive amount of CO, H
2O, H
2, and N
2 in the obtained syngas.
Figure 2 presents the list of modeled syngas compositions (minimum and maximum values) with the scope of application of the GERG-2008 method. This creates a need to determine a new approach in the determination of the syngas compressibility coefficient that will make it possible to calculate this value for the syngas for the obtained ranges of constituents (those are values obtained for the gasification of SS under conditions mentioned in
Table 3 and are the result of this work):
CO2—up to 0.41 molar fraction,
CO—up to 0.60 molar fraction,
H2O—up to 0.39 molar fraction,
H2—up to 0.78 molar fraction,
N2—up to 0.59 molar fraction,
H2S—up to 0.02 molar fraction,
CH4—up to 0.37 molar fraction.
In addition to the methods mentioned above dedicated to NG, other methods for determining the gas state, including syngas (e.g., the Redlich-Kwong equation, the Peng-Robinson equation, and others) [
37,
40] are known. Those methods rely on values of critical properties (critical temperature and critical pressure) of analyzed gas, which often requires to apply several mixing rules based on the gas composition. There is also an additional difficulty in finding cubic roots of those equations of state in order to obtain the compressibility factor. For this work, the simplified model [
36] was used that was confirmed to be effective in gases composed mostly of non-hydrocarbon components. This approach, (a.k.a. the method of weighting treatment), is based on the extraction of the
Z-factors for both the hydrocarbon and non-hydrocarbon components. This is followed by adding them up, through a weighting treatment, to estimate the final value of the
Z-factor for the gas mixture. Since the compressibility factor of all constituents of the obtained syngas is known, this method was chosen to be used for determination of the
Z-factor of the obtained syngas. The equation used in this study is presented below (based on [
36]):
where:
Zs—compression factor syngas with a given chemical composition,
yi—mole fraction of the syngas component,
Zi—compressibility factor of syngas component (mono-component gas).
In the case of syngas resulting from the modeling of the SS gasification process, Equation (3) takes the form:
The underlying assumptions of the above model are:
the analyzed gas is treated as a mixture of one-component real gases,
this model does not take into account phase changes occurring as a result of gas heating or compression,
it is assumed that in the tested range the mixture is in a gaseous or supercritical state,
compressibility factor values for mono-component gases are determined for a specific gas state, which corresponds to the modeled state of syngas,
the gas analyzed under STP conditions behaves like an ideal gas.
Values of compressibility of the mono-constituent gases for specific conditions were determined by the use of the Coolprop software [
41], which is used to determine the state of mono-constituent gases. Resulting compressibility values are presented in
Table 4.
For modeling of the syngas parameters in a compressed form, the following parameters were assumed. The syngas transport will take place in a mobile storage unit, equipped with tanks with a total capacity of 14,960 m
3, with a working pressure of 20 MPa. To determine the gas volume in the transport container, the following Formula (5) was used that was derived from Equation (2) assuming that there is no change in the number of moles of gas under compression:
where:
V2—the volume of gas in a transport unit, nm3,
P1—working pressure of the transport unit, MPa,
Z1—compressibility coefficient of gas at temperature T1, pressure P1 and volume V1,
V1—water volume of the transport unit, m3,
P2—gas pressure under STP conditions, MPa,
Z2—gas compressibility coefficient under STP conditions,
T1, T2—gas temperatures in state 1 and 2.
Assuming that the real gas could be considered as ideal under STP conditions (273.15 K and absolute pressure of 100 kPa) and taking into account boundary conditions, Equation (5) may have a form (where t denotes the temperature of the gas in transport, in K):
Assumption of the ideality of the gas under STP conditions is in agreement with work performed by Werle [
32] who used similar assumptions for the modeling of sewage sludge gasification. This made it possible to refer results obtained in this manuscript to other researches. After the simplification of (6),
V2 may be calculated as follows:
In the case of natural gas, the ratio of the water volume of the transport unit to the gas volume under STP conditions is designated as
Bg (FVF) (formation volume factor) [
38]. In this paper, the authors defined a similar coefficient for the syngas, which was defined as the inverse of FVF and referred to as the syngas compression ratio:
The resulting volume of the syngas in the transport can be determined using a simplified form of Equation (8):
The value of the Bsyn coefficient describes, how many times the volume of the syngas will increase when compressed to a given pressure, here equal to 20 MPa.
For the assumed boundary conditions of the analysis (
P1 = 20 MPa,
T = 293 K), Equation (10) may be written:
From Equation (10), it follows that as the gas compressibility factor increases, the value of the syngas compression coefficient decreases. The amount of energy in the transport unit was determined by the formula:
For the assumed volume of the water in the transport unit and taking Equation (11) into account, Equation (12) has a form:
From formula (12), it follows that with the increase of the calorific value of the gas obtained and as the gas compressibility coefficient decreases, the energy content in a given volume of a mobile storage unit under defined pressure increases.
To sum up, the modeling technique in this study utilizes a real gas equation to calculate the volume of gas, that will occupy space in STP conditions after its decompression from a pressure vessel in which this gas occupies 14,960 m3 of volume under pressure of 20 MPa. The calculation scheme involved implementation of the given syngas composition in Equation (4) to determine the Z factor and then use this Z factor to determine the volume of gas in the transport, Bsyn, and energy content from Equations (7), (9) and (11), respectively. These calculations were repeated five times for five different syngas compositions.