The Removal of Silicate(IV) by Adsorption onto Hydrocalumite from the Sodium Hydroxide Leaching Solution of Black Dross
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silicate(IV) Removal Experiment
2.3. Preparation of Hydrocalumite
3. Results and Discussion
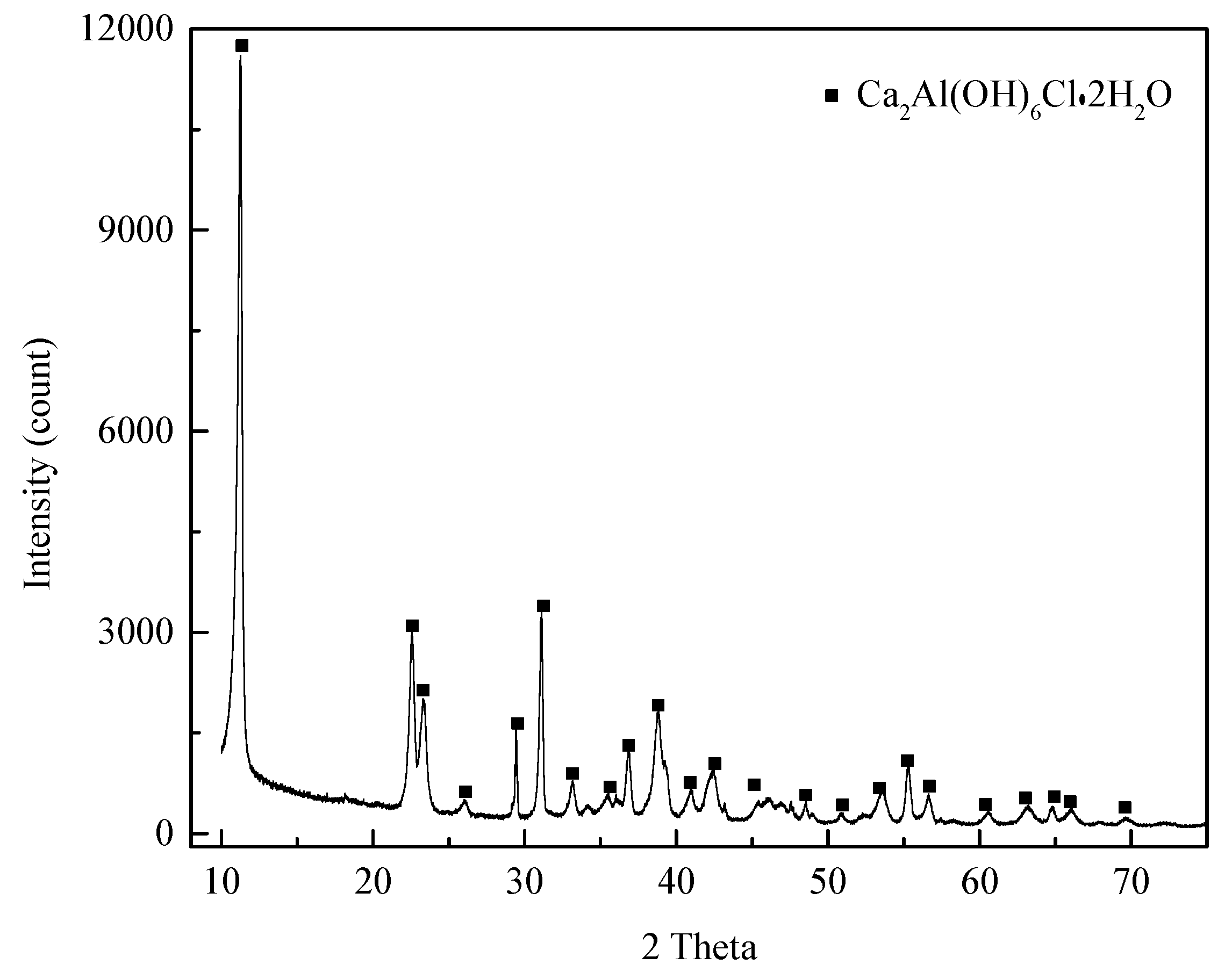
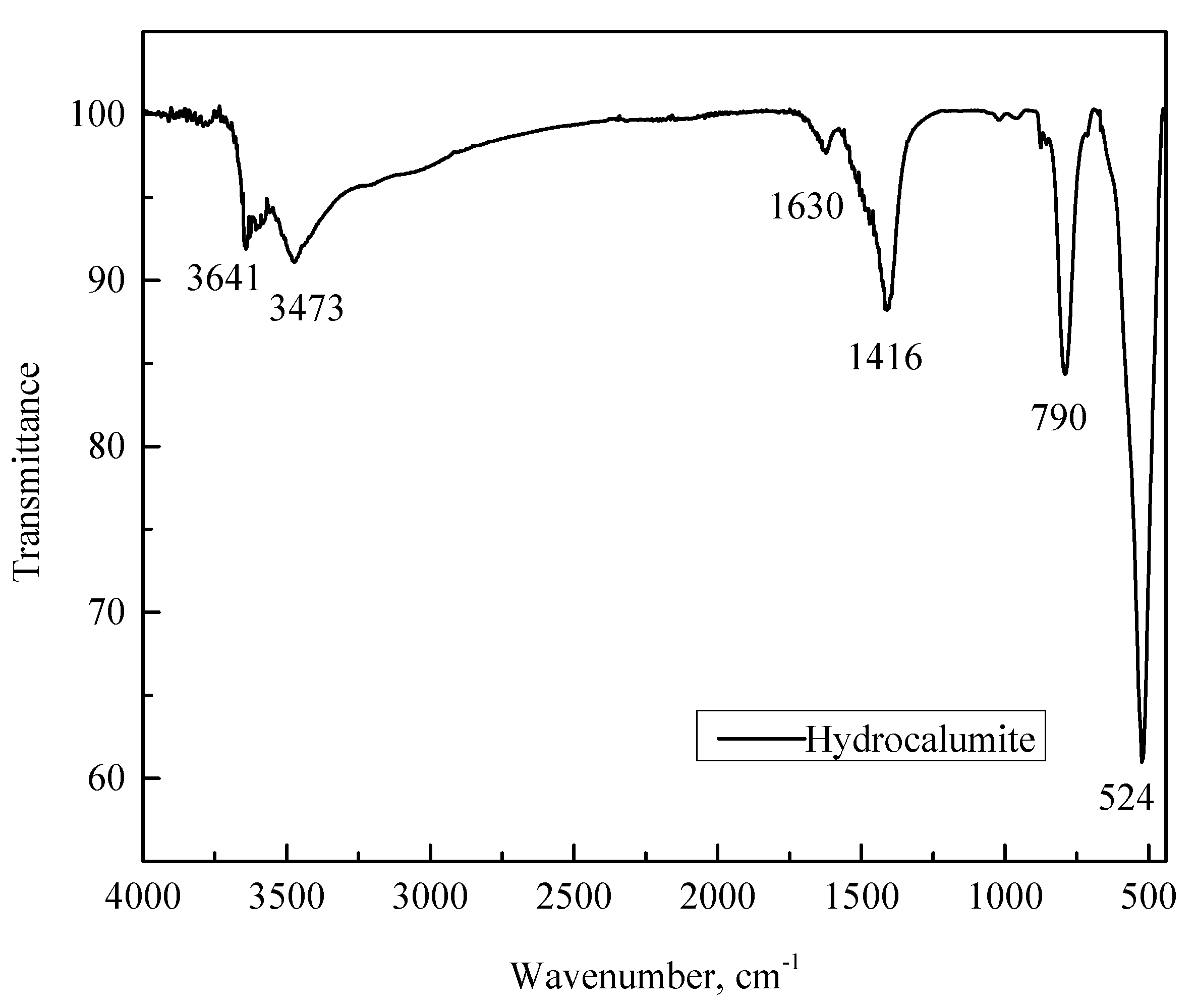
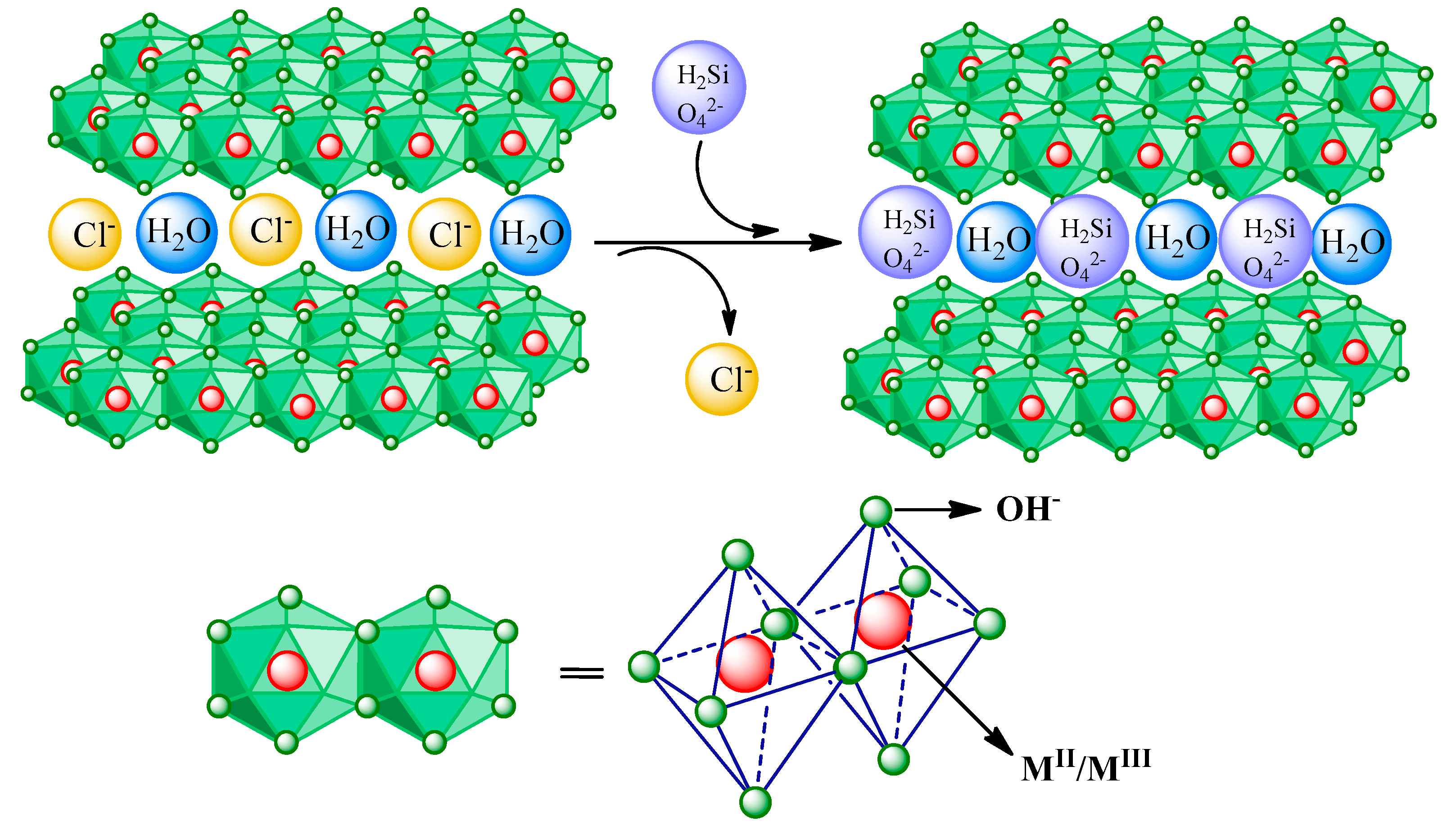
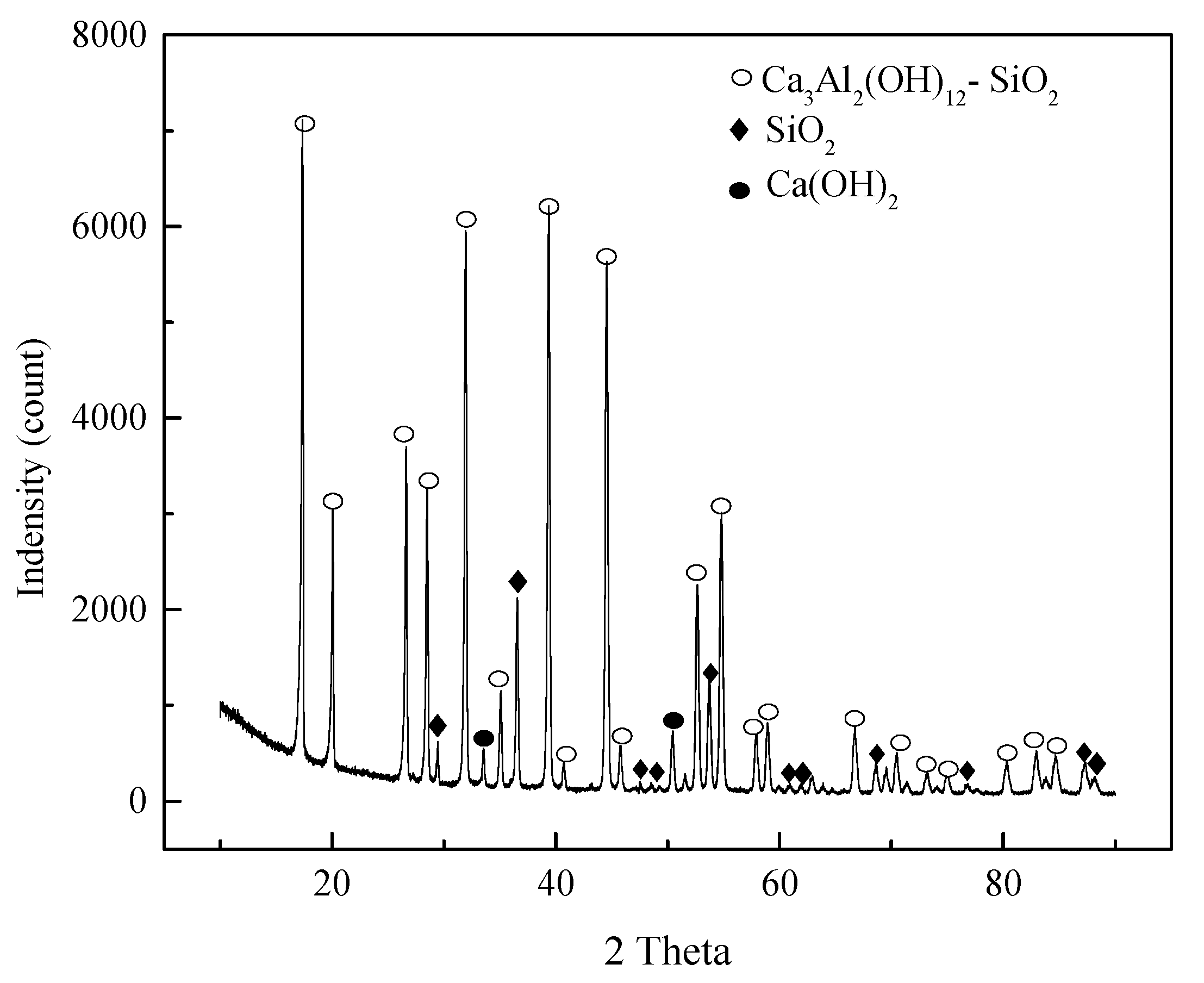
3.1. Characterization of Synthesized Hydrocalumite
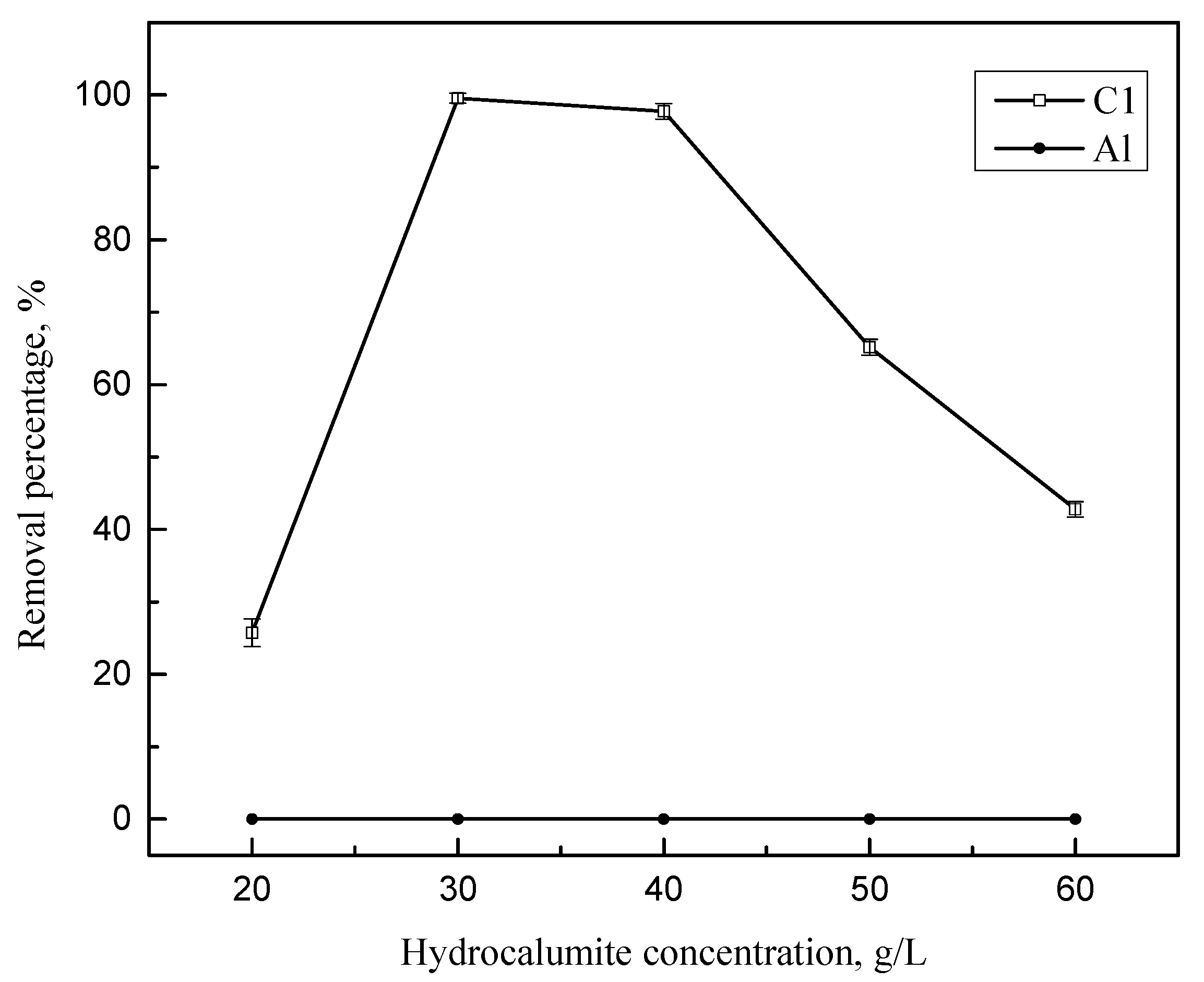
3.2. Effect of Hydrocalumite Dosage on the Removal of the Silicate(IV) from Leaching Solution
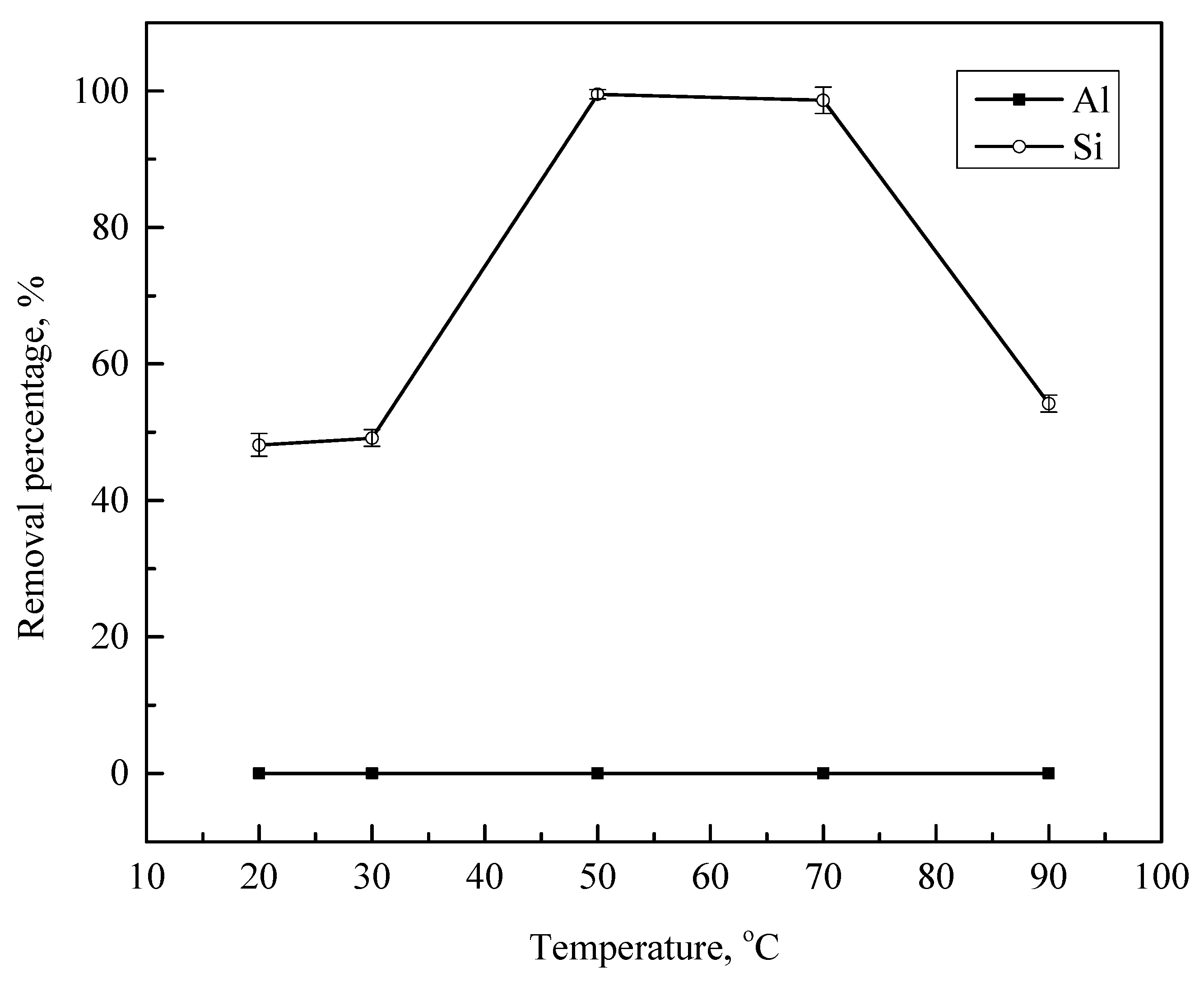
3.3. Effect of Temperature
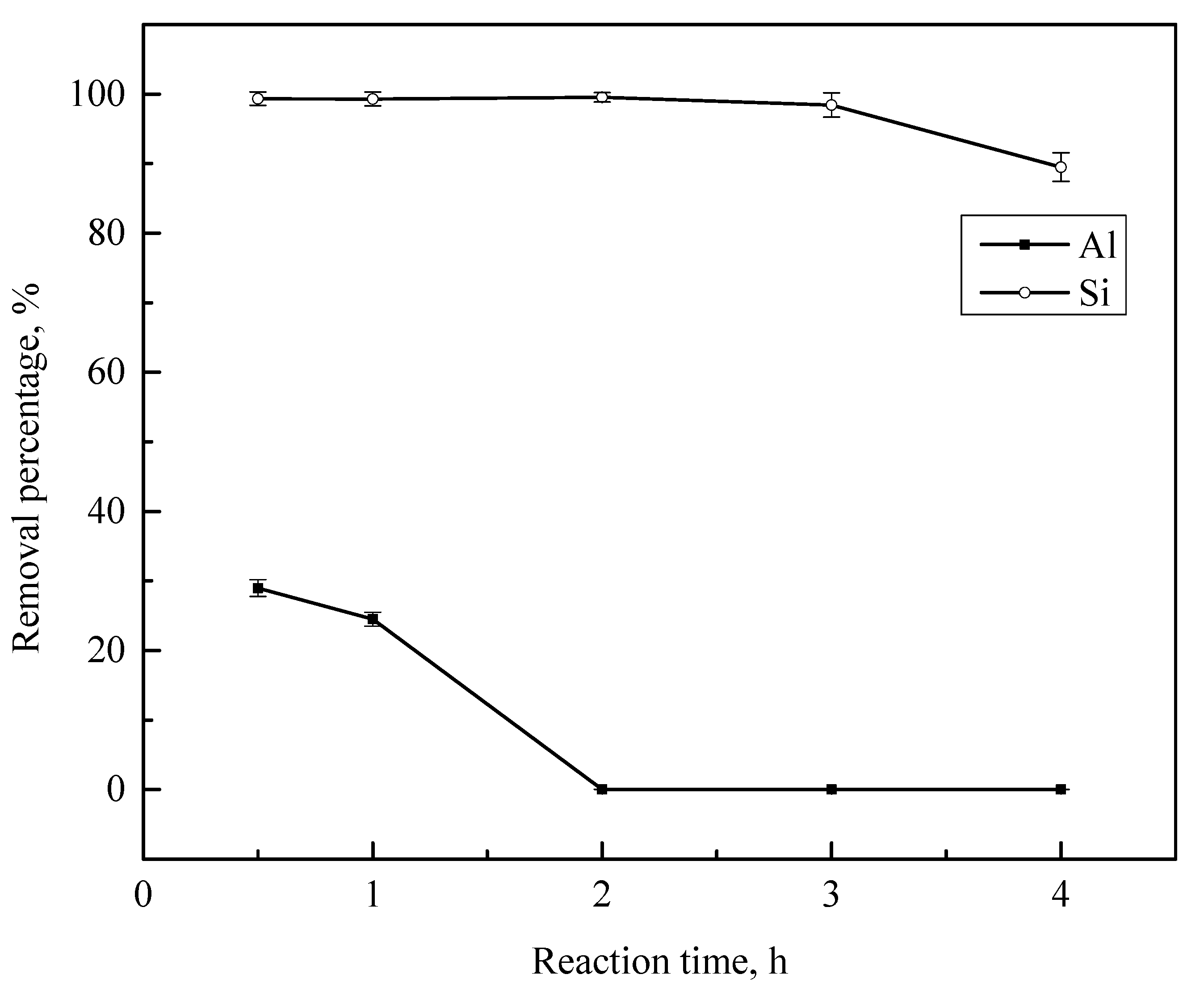
3.4. Effect of the Reaction Time
3.5. Effect of Silicate(IV) Concentration
3.6. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manfredi, O.; Wuth, W.; Bohlinger, I. Characterizing the physical and chemical properties of aluminum dross. JOM 1997, 49, 48–51. [Google Scholar] [CrossRef]
- Youcai, Z.; Chenglong, Z. Pollution Control and Resource Reuse for Alkaline Hydrometallurgy of Amphoteric Metal Hazardous Wastes; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319551579. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: Hoboken, NJ, USA, 1996; ISBN 0471511846. [Google Scholar]
- Latour, I.; Miranda, R.; Blanco, A. Silica removal in industrial effluents with high silica content and low hardness. Environ. Sci. Pollut. Res. 2014, 21, 9832–9842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Chang, T.; Lin, C. Silica pretreatment for a RO brackish water source with high magnesium. Water Supply 2006, 4, 179–187. [Google Scholar] [CrossRef]
- Huuha, T.S.; Kurniawan, T.A.; Sillanpää, M.E.T. Removal of silicon from pulping whitewater using integrated treatment of chemical precipitation and evaporation. Chem. Eng. J. 2010, 158, 584–592. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Lee, M.S. Purification of the sodium hydroxide leaching solution of black dross by removal of silicate(IV) with polyacrylamide (PAM), under review. Min. Process. Extr. Met. Rev. 2019, 1–8. [Google Scholar] [CrossRef]
- Focke, W.W. A critical assessment of the methods for intercalating anionic surfactants in layered double hydroxides. J. Mater. Sci. 2008, 43, 6144–6158. [Google Scholar] [CrossRef] [Green Version]
- Pisson, J.; Briois, V.; Pascal, B. Staging of organic and inorganic anions in layered double hydroxides. J. Phys. Chem. B 2003, 107, 9243–9248. [Google Scholar] [CrossRef]
- Sasan, K.; Brady, P.V.; Krumhansl, J.L.; Nenoff, T.M. Removal of dissolved silica from industrial waters using inorganic ion exchangers. J. Water Process Eng. 2017, 17, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.N.; Lee, M.S.; Nguyen, T.H. Ball milling treatment of black dross for selective dissolution of alumina in sodium hydroxide leaching. Processes 2019, 6, 29. [Google Scholar] [CrossRef]
- Xing, W.D.; Ahn, B.D.; Lee, M.S. Treatment of black dross with water and NaOH solution. J. Kirr. 2017, 26, 53–60. [Google Scholar] [CrossRef]
- Das, B.R.; Dash, B.; Tripathy, B.C.; Bhattacharya, I.N.; Das, S.C. Production of g -alumina from waste aluminium dross. Miner. Process. Extr. Metall. 2007, 20, 252–258. [Google Scholar] [CrossRef]
- Grishchenko, R.O.; Emelina, A.L.; Makarov, P.Y. Thermodynamic properties and thermal behavior of Friedel’s salt. Thermochimica Acta 2013, 570, 74–79. [Google Scholar] [CrossRef]
- Wu, Y.; Chi, Y.; Bai, H.; Qian, G.; Cao, Y.; Zhou, J.; Xu, Y.; Liu, Q.; Ping, Z.; Qiao, S. Effective removal of selenate from aqueous solutions by the Friedel phase. J. Hazard. Mater. 2010, 176, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Qian, G.; Cao, Y.; Chi, Y.; Xu, Y.; Zhou, J.; Liu, Q.; Ping, Z.; Qiao, S. Effective removal and fixation of Cr (VI) from aqueous solution with Friedel’s salt. J. Hazard. Mater. 2009, 170, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jia, Y.; Ma, J.; Li, Z. Removal of arsenic from water by Friedel’s salt (FS: 3CaO Al2O3 CaCl2 10H2O). J. Hazard. Mater. 2011, 195, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, S.; Tavakoli, O.; Khoobi, M.; Ansari, A.; Ali, M. Application of novel magnetic β -cyclodextrin-anhydride polymer nano-adsorbent in cationic dye removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 80, 452–463. [Google Scholar] [CrossRef]
- Shu, Y.; Shao, Y.; Wei, X.; Wang, X.; Sun, Q.; Zhang, Q.; Li, L. Synthesis and characterization of Ni-MCM-41 for methyl blue adsorption. Microporous Mesoporous Mater. 2015, 214, 88–94. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Z. The effect of sodium stearate—Modified hydrocalumite on the thermal stability of polyvinyl chloride The effect of sodium stearate-modified hydrocalumite on the thermal stability of polyvinyl chloride. J. Appl. Polym. Sci. 2018, 135, 45758. [Google Scholar] [CrossRef]
- Gevers, B.R.; Labuschagn, F.J.W.J. Temperature effects on the dissolution-precipitation synthesis of hydrocalumite. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019; Volume 2055, p. 050010. [Google Scholar]
- Sio, A.O.; Caco, C.; Matschei, T.; Lothenbach, B.; Glasser, F.P. Thermodynamic properties of Portland cement hydrates in the system CaO–Al2O3–SiO2–CaSO4–CaCO3–H2O. Cem. Concr. Res. 2007, 37, 1379–1410. [Google Scholar] [CrossRef]
- Alagha, L.; Wang, S.; Xu, Z.; Masliyah, J. Adsorption kinetics of a novel organic–inorganic hybrid polymer on silica and alumina studied by quartz crystal microbalance. J. Phys. Chem. C 2011, 115, 15390–15402. [Google Scholar] [CrossRef]
- Pavlovic, M.; Huber, R.; Adok-Sipiczki, M. Ion specific effects on the stability of layered double hydroxide colloids. Soft Matter 2016, 12, 4024–4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner-Canham, G. Descriptive Inorganic Chemistry; Macmillan: New York, NY, USA, 2009; ISBN 9781464125577. [Google Scholar]
- Rehman, R.; Anwar, J.; Mahmud, T.; Salman, M. Removal of murexide (Dye) from aqueous media using rice husk as an adsorbent removal of murexide (Dye) from aqueous media using rice husk as an adsorbent. J. Chem. Soc. Pak. 2011, 33, 598. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 345, 1361–1403. [Google Scholar] [CrossRef]
- Zul, M.; Chowdhury, S.; Su, S.; Aziz, A. Enhanced photocatalytic activity of Orange II in aqueous solution using solvent-based TiO2 nanotubes: Kinetic, equilibrium and thermodynamic studies. J. Clean. Prod. 2018, 203, 848–859. [Google Scholar]
- Haghseresht, F.; Lu, G.Q. Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]




| Conditions | Al(III), ppm | Si(IV), ppm |
|---|---|---|
| Effect of Hydrocalumite concentration | ||
| 20 g/L | 15,094 | 111.4 |
| 30 g/L | 15,028 | 0.6 |
| 40 g/L | 15,291.4 | 3.4 |
| 50 g/L | 15,590.8 | 52.2 |
| 60 g/L | 15,690.9 | 85.9 |
| Effect of temperature | ||
| 20°C | 15,690.8 | 77.8 |
| 30°C | 15,594.1 | 76.3 |
| 50°C | 15,028 | 0.6 |
| 70°C | 15,291.4 | 2.1 |
| 90°C | 15,299.8 | 68.7 |
| Effect of reaction time | ||
| 0.5 h | 10,622.1 | 1.0 |
| 1 h | 11,291.6 | 1.1 |
| 2 h | 15,028.0 | 0.6 |
| 3 h | 15,253.4 | 2.4 |
| 4 h | 15,356.5 | 15.8 |
| Composition | Al(III) | Si(IV) | Purity of Al(III) (%) |
|---|---|---|---|
| Before adsorption (ppm) | 14,954.8 | 150 | 99 |
| After adsorption (ppm) | 15,028.0 | 0.6 | 99.99 |
| Ion | Freundlich | ||
|---|---|---|---|
| 1/nF | KF | R2 | |
| Si | 0.29 | 10.23 | 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.N.; Lee, M.S. The Removal of Silicate(IV) by Adsorption onto Hydrocalumite from the Sodium Hydroxide Leaching Solution of Black Dross. Processes 2019, 7, 612. https://doi.org/10.3390/pr7090612
Nguyen TTN, Lee MS. The Removal of Silicate(IV) by Adsorption onto Hydrocalumite from the Sodium Hydroxide Leaching Solution of Black Dross. Processes. 2019; 7(9):612. https://doi.org/10.3390/pr7090612
Chicago/Turabian StyleNguyen, Thi Thuy Nhi, and Man Seung Lee. 2019. "The Removal of Silicate(IV) by Adsorption onto Hydrocalumite from the Sodium Hydroxide Leaching Solution of Black Dross" Processes 7, no. 9: 612. https://doi.org/10.3390/pr7090612
APA StyleNguyen, T. T. N., & Lee, M. S. (2019). The Removal of Silicate(IV) by Adsorption onto Hydrocalumite from the Sodium Hydroxide Leaching Solution of Black Dross. Processes, 7(9), 612. https://doi.org/10.3390/pr7090612






