Understanding TiN Precipitation Behavior during Solidification of SWRH 92A Tire Cord Steel by Selected Thermodynamic Models
Abstract
1. Introduction
2. Material and Equilibrium Solubility Product
3. Thermodynamic Analysis
3.1. Segregation Models
3.2. Usage of the LRSM Model
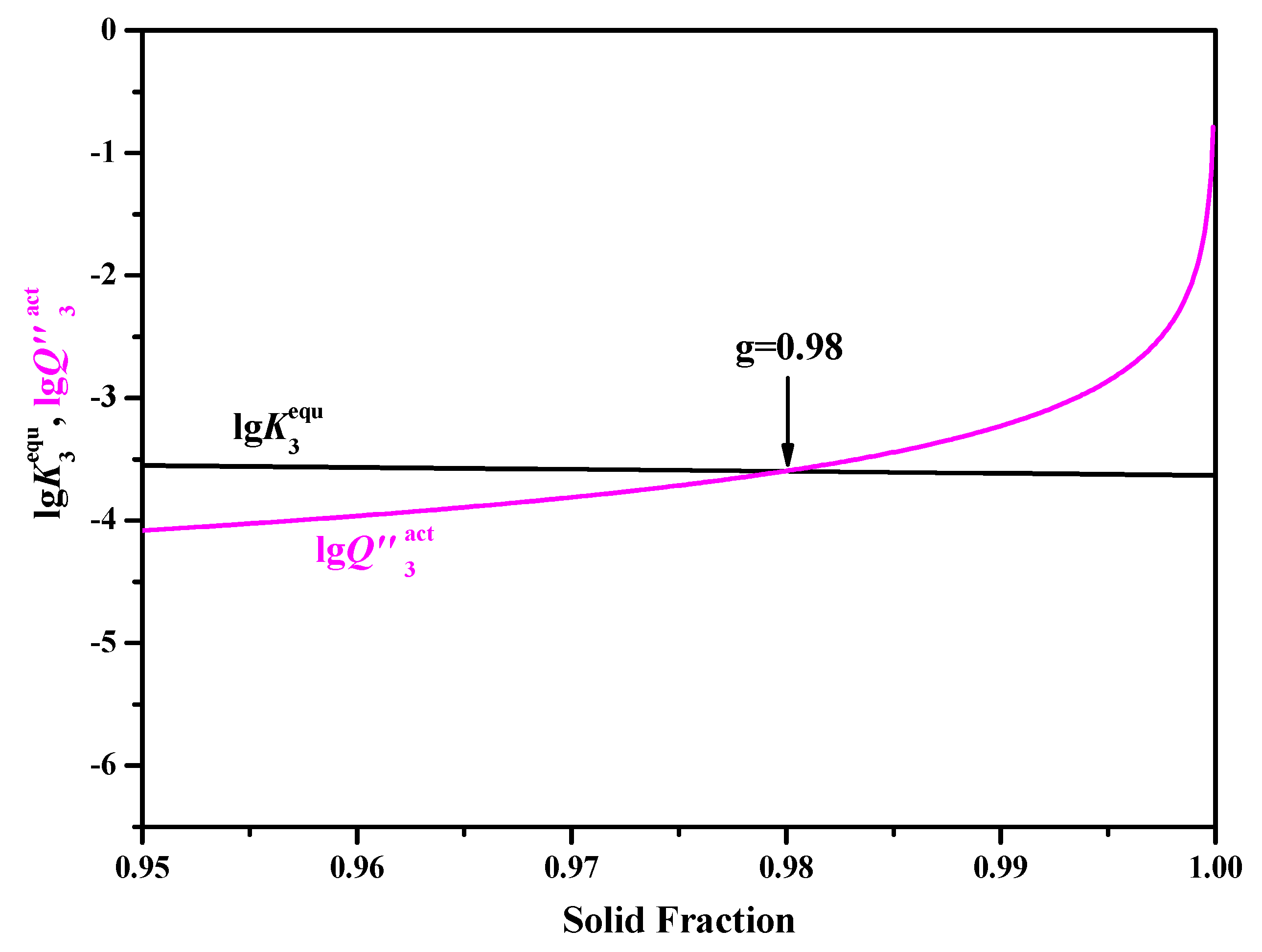
3.3. Usage of Ohnaka Model on Considering the Effect of Carbon on SDAS L
3.4. Use of the Ohnaka Model without Considering the Effect of Carbon on SDAS L
4. Conclusions
- (1)
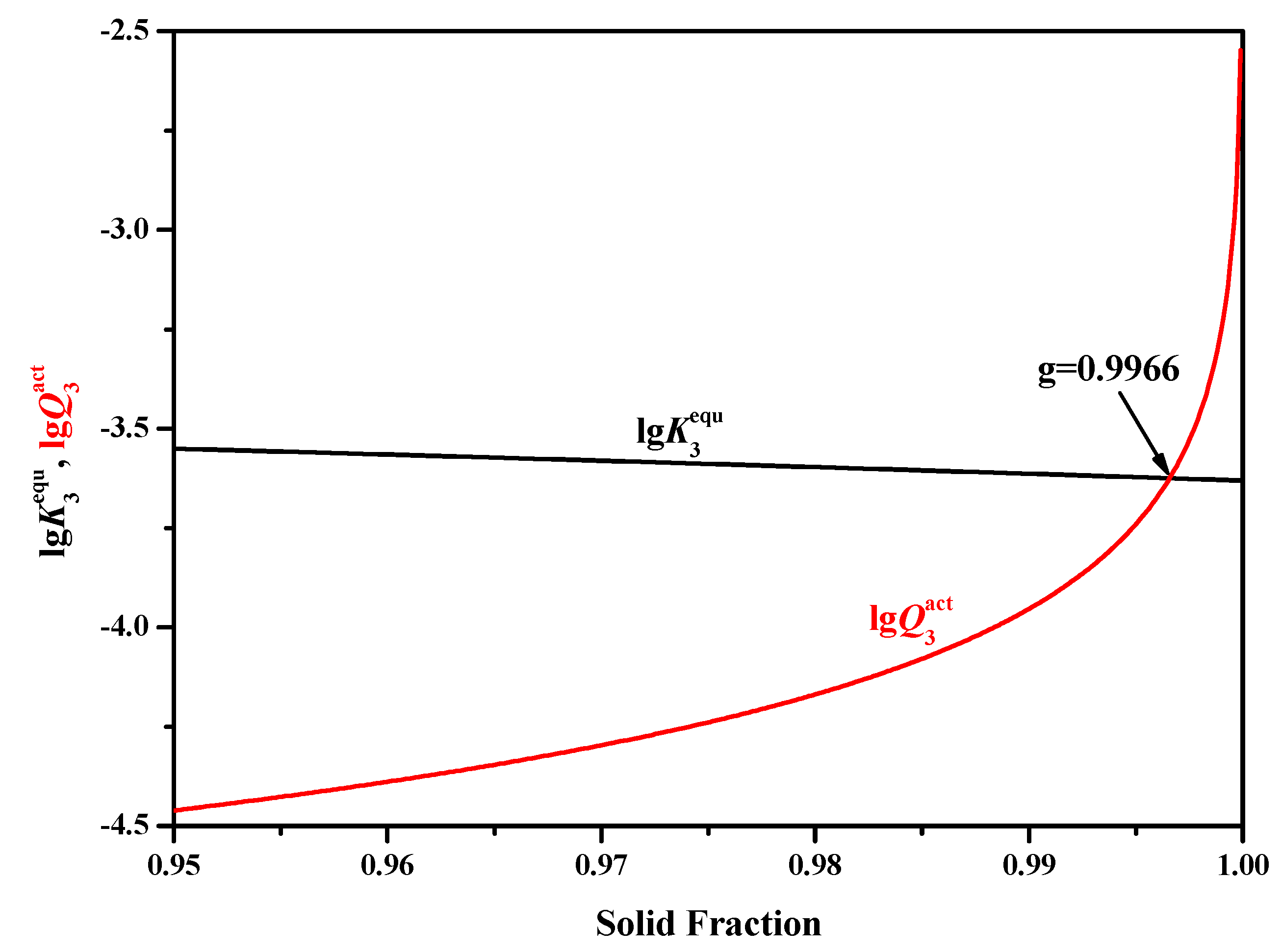
- Precipitation of TiN will not occur in the liquid phase region regardless of the selected micro- segregation models.
- (2)
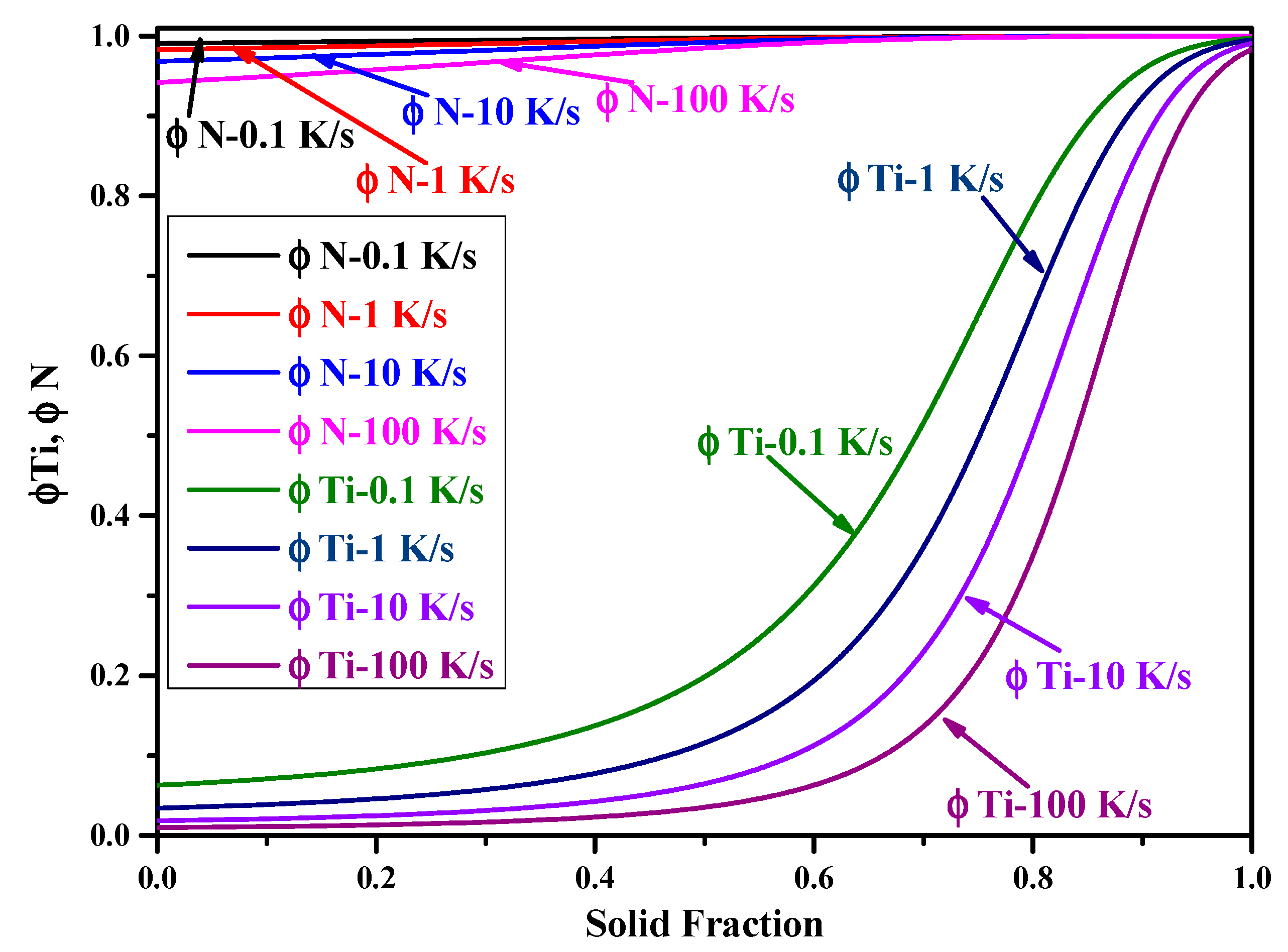
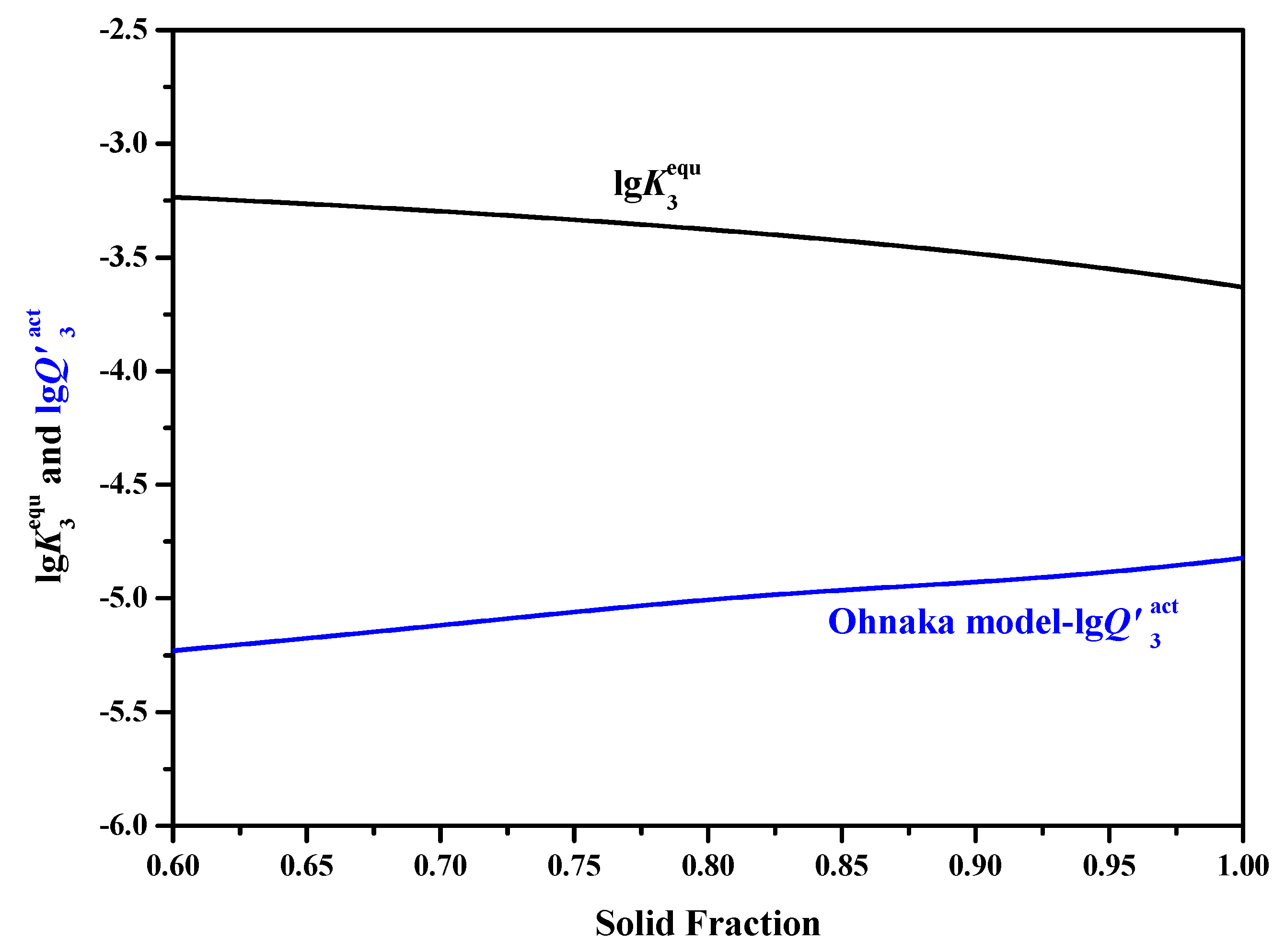
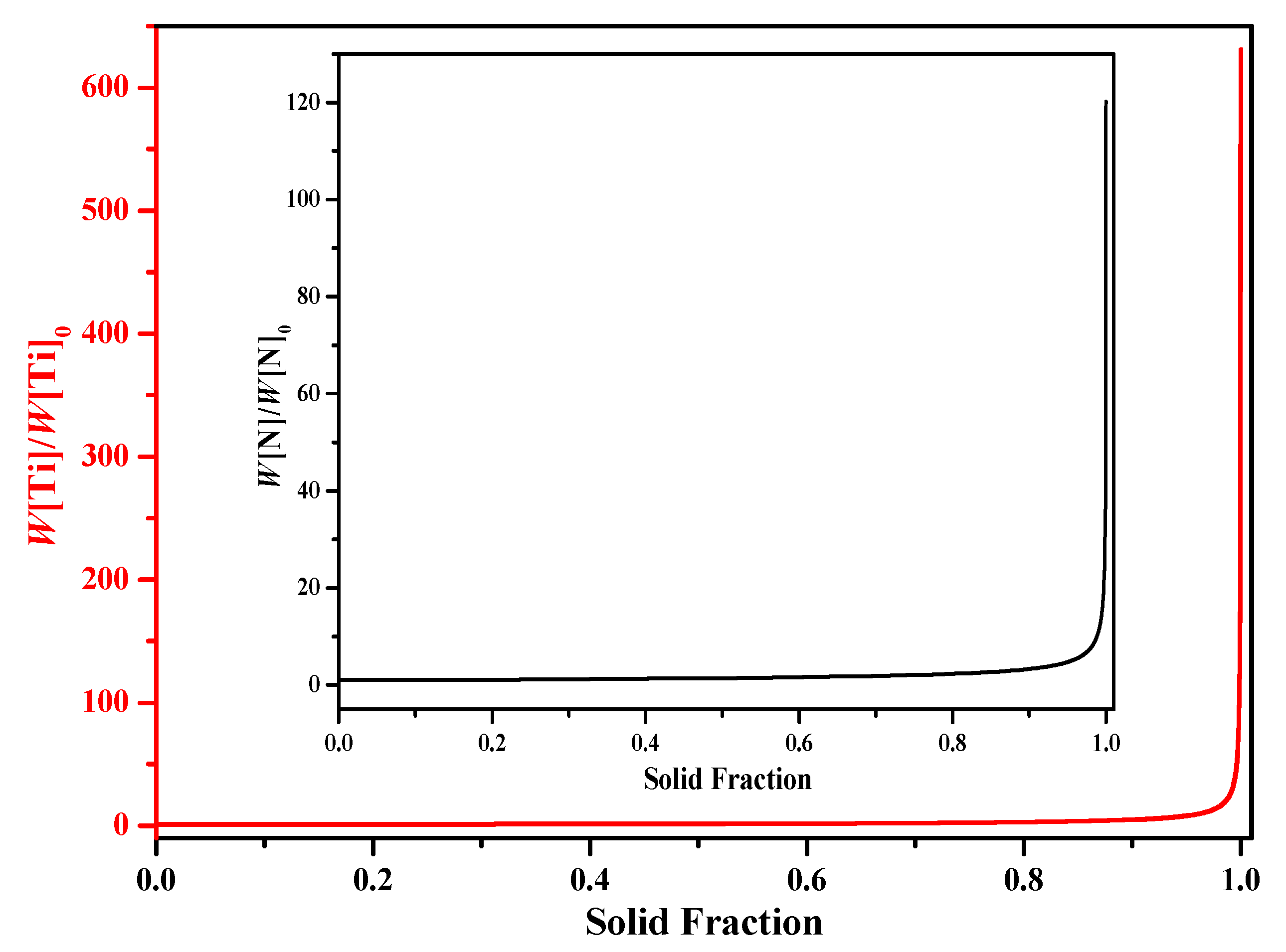
- When adopting the LRSM and Ohnaka (without considering the effect of carbon on secondary dendrite arm spacing (SDAS L)) models, TiN will precipitate in the mushy zone at the very late stage of the solidification process, with solid fractions larger than 0.9966 and 0.98, respectively. When considering the effect of carbon on SDAS L for the Ohnaka model, TiN will not precipitate in both the liquid phase and mushy zone.
- (3)
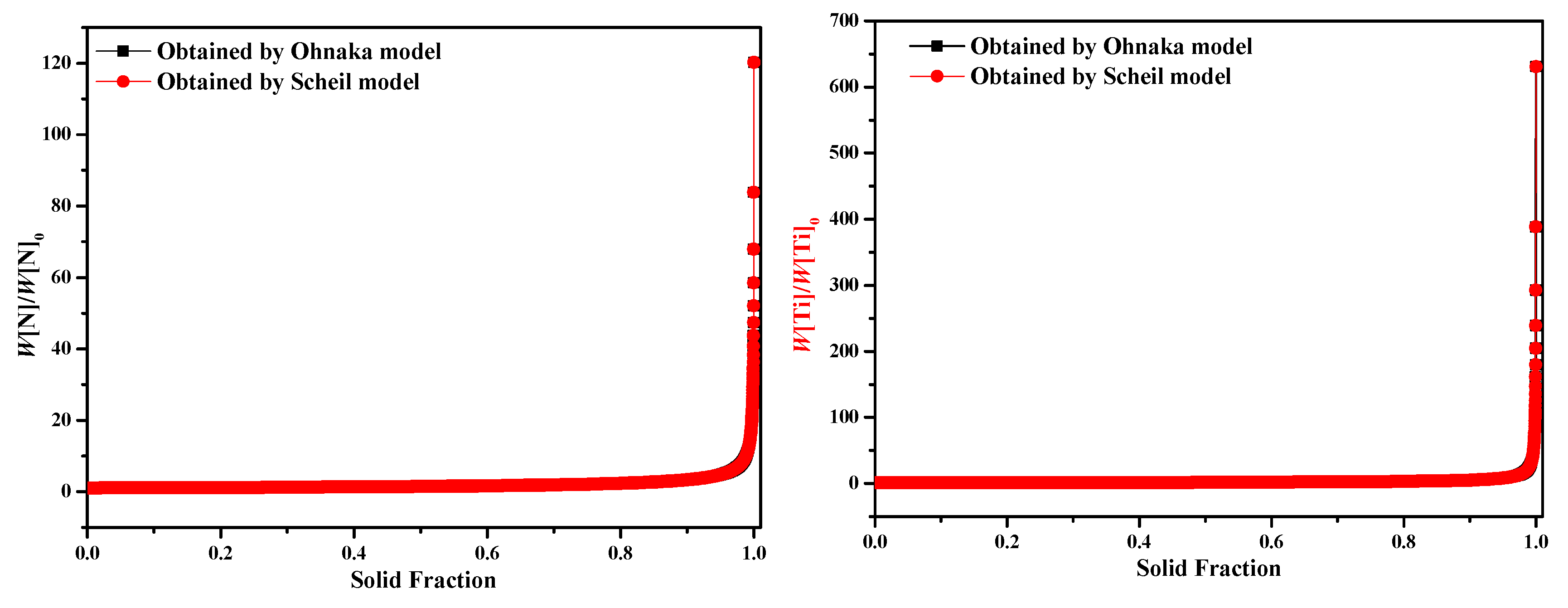
- Results of different segregation models show that the Ohnaka model (considering the effect of carbon on SDAS L) is similar to the Lever-rule model at the very late stage during the solidification process; however, for the case without considering the effect of carbon on SDAS L, the result is similar to the Scheil model.
- (4)
- Due to the fact that different segregation models may lead to different results, more attention should be paid to selecting the appropriate model or developing new models to analyze the actual segregation phenomena. Besides, further experimental work and theoretical analysis to understand in depth the precipitation behavior of TiN in SWRH 92A tire cord steel will be required in the future.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, C.F.; Xue, Z.L.; Jin, W.T. Precipitation and Solid Solution of Titanium Carbonitride Inclusions in Hypereutectoid Tire Cord Steel. J. Iron Steel Res. Int. 2016, 23, 338–343. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Lei, J.L.; Zhang, J.; Xiong, R.; Zou, F.; Xue, Z.L. Effect of carbon content on Ti inclusion precipitated in tire cord steel. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 283–286. [Google Scholar] [CrossRef][Green Version]
- Lei, J.L.; Xue, Z.L.; Jiang, Y.D.; Zhang, J.; Zhu, T. Study on TiN precipitation during solidification for hypereutectoid tire crod steel. Metal. Int. 2012, 17, 10–16. [Google Scholar]
- Jiang, Y.D.; Xue, Z.L.; Zhang, J. Genetic analysis for large TiN inclusions in wire rod for tire cord steel of SWRH82A. J. Iron Steel Res. Int. 2014, 21, 91–94. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, D.; Sun, J.; Wang, C.; Matsuura, H. An integrated study on the evolution of inclusions in EH36 shipbuilding steel with Mg addition: From casting to welding. Metall. Mater. Trans. B 2018, 49, 481–489. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.; Yang, Y.; Deng, X.; Barati, M.; McLean, A. Effect of alloy addition on inclusion evolution in stainless steels. Ironmak. Steelmak. 2017, 44, 152–158. [Google Scholar] [CrossRef]
- Ma, W.J.; Bao, Y.P.; Zhao, L.H.; Wang, M. Control of the precipitation of TiN inclusions in gear steels. Int. J. Miner. Metal. Mater. 2014, 21, 234–239. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Z.L.; Zhu, H.Y.; Lei, J.L. Thermodynamic analysis of precipitation behavior of Ti-bearing inclusions in SWRH 92A tire cord steel. Results Phys. 2019. [Google Scholar] [CrossRef]
- Cai, X.F.; Bao, Y.P.; Wang, M.; Lin, L.; Dai, D.C.; Gu, C. Investigation of precipitation and growth behavior of Ti inclusions in tire cord steel. Metall. Res. Technol. 2015, 112, 407–418. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Z.L.; Jiang, Y.D.; Lei, J.L.; Zou, F.; Xiong, R. Research on precipitation of Ti inclusions during solidification of tire cord steel SWRH82A. Metal. Int. 2014, 19, 34–38. [Google Scholar]
- Jin, Y.; Du, S. Precipitation behaviour and control of TiN inclusions in rail steels. Ironmak. Steelmak. 2018, 45, 224–229. [Google Scholar] [CrossRef]
- Liu, T.; Chen, D.; He, W.; Long, M.; Gui, L.; Duan, H.; Cao, J. Study on the Formation and Control of TiN Inclusion in Mushy Zone for High Ti Microalloyed Steel. In TMS 2018 147th Annual Meeting & Exhibition Supplemetal Proceedings; Springer: Cham, Switzerland, 2018; pp. 605–615. [Google Scholar]
- Liu, H.; Wang, H.; Li, L.; Zheng, J.; Li, Y.; Zeng, X. Investigation of Ti inclusions in wire cord steel. Ironmak. Steelmak. 2011, 38, 53–58. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.F.; Duan, H.; Zhang, Y.; Luo, Y.; Conejo, A.N. Extraction, thermodynamic analysis, and precipitation mechanism of MnS-TiN complex inclusions in low-sulfur steels. Metall. Mater. Trans. A 2016, 47, 3015–3025. [Google Scholar] [CrossRef]
- Chen, J. Manual of Chart and Data in Common Use of Steel Making; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Fu, J.; Zhu, J.; Di, L.; Tong, F.S.; Liu, D.L.; Wang, L.Y. Study on the precipitation behavior of TiN in the microalloyed steels. Acta Metall. Sin. 2000, 36, 801–804. [Google Scholar]
- Tian, Q.; Wang, G.C.; Zhao, Y.; Li, J.; Wang, Q. Precipitation Behaviors of TiN Inclusion in GCr15 Bearing Steel Billet. Metall. Mater. Trans. B 2018, 49, 1149–1164. [Google Scholar] [CrossRef]
- Pa, M.; Dp, D.; Chandra, T.; Cr, K. Grain growth predictions in microalloyed steels. ISIJ. Int. 1996, 36, 194–200. [Google Scholar]
- Luo, S.; Wang, B.; Wang, Z.; Jiang, D.; Wang, W.; Zhu, M. Morphology of Solidification Structure and MnS Inclusion in High Carbon Steel Continuously Cast Bloom. ISIJ Int. 2017, 57, 2000–2009. [Google Scholar] [CrossRef]
- Suzuki, S.; Weatherly, G.; Houghton, D. The response of carbo-nitride particles in hsla steels to weld thermal cycles. Acta Metall. 1987, 35, 341–352. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, G.C.; Shang, D.; Lei, H.; Yuan, X.; Wang, Q.; Li, J. In Situ Observation of the Precipitation, Aggregation, and Dissolution Behaviors of TiN Inclusion on the Surface of Liquid GCr15 Bearing Steel. Metall. Mater. Trans. B 2018, 49, 3137–3150. [Google Scholar] [CrossRef]
- Ma, Z.; Janke, D. Characteristics of oxide precipitation and growth during solidification of deoxidized steel. ISIJ Int. 1998, 38, 46–52. [Google Scholar] [CrossRef]
- Bower, T.F.; Brody, H.; Flemings, M.C. Measurements of solute redistribution in dendritic solidification. Trans. Metall. Soc. AIME 1966, 236, 624–634. [Google Scholar]
- Clyne, T.; Kurz, W. Solute redistribution during solidification with rapid solid state diffusion. Metall. Trans. A 1981, 12, 965–971. [Google Scholar] [CrossRef]
- Ohnaka, I. Mathematical analysis of solute redistribution during solidification with diffusion in solid phase. Trans. Iron Steel Inst. Jpn. 1986, 26, 1045–1051. [Google Scholar] [CrossRef]
- Matsumiya, T.; Kajioka, H.; Mizoguchi, S.; Ueshima, Y.; Esaka, H. Mathematical analysis of segregations in continuously-cast slabs. Trans. Iron Steel Inst. Jpn. 1984, 24, 873–882. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, J.H. Synergistic effect of nitrogen and refractory material on TiN formation and equiaxed grain structure of ferritic stainless steel. Metall. Mater. Trans. B 2018, 49, 877–893. [Google Scholar] [CrossRef]
- Won, Y.M.; Thomas, B.G. Simple model of microsegregation during solidification of steels. Metall. Mater. Trans. A 2001, 32, 1755–1767. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.J.; Liu, M.K. Micro-segregation model calculation of residual tin in boiler and pressure vessel steel. Philos. Mag. 2019, 99, 1041–1056. [Google Scholar] [CrossRef]
- Goto, H.; Miyazawa, K.I.; Yamada, W.; Tanaka, K. Effect of cooling rate on composition of oxides precipitated during solidification of steels. ISIJ Int. 1995, 35, 708–714. [Google Scholar] [CrossRef]




| Elements | Si | P | S | O | Mn | N | Ti |
|---|---|---|---|---|---|---|---|
| Content/mass% | 0.18 | 0.018 | 0.0064 | 0.0018 | 0.51 | 0.0043 | 0.0005 |
| Elements | C | Si | P | S | O | Mn | N | Ti |
|---|---|---|---|---|---|---|---|---|
| 65 | 8 | 30 | 25 | 80 | 5 | 90 | 20 | |
| 175 | 20 | 280 | 575 | 160 | 30 | - | 40 |
| C | Si | P | S | O | N | Mn | Ti | |
|---|---|---|---|---|---|---|---|---|
| −0.165 | 0.05 | −0.0064 | −0.11 | −1.8 | −1.8 | 0.0043 | 0.013 | |
| 0.13 | 0.047 | 0.045 | 0.007 | 0.05 | 0 | −0.021 | −0.53 |
| Models | Equation | Conditions | No |
|---|---|---|---|
| Lever-rule | Complete diffusion both in liquid and γ-Fe phase | (1) | |
| Scheil model | Complete diffusion in liquid and no diffusion in γ-Fe phase | (2) | |
| Basic equations | Complete diffusion in liquid and finite diffusion in γ-Fe phase | (3) | |
| Brody–Fleming model | (4) | ||
| Ohnaka model | (5) | ||
| Clyne–Kurz model | (6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xue, Z.-L.; Chen, Y.-L.; Bi, X.-G. Understanding TiN Precipitation Behavior during Solidification of SWRH 92A Tire Cord Steel by Selected Thermodynamic Models. Processes 2020, 8, 10. https://doi.org/10.3390/pr8010010
Wang L, Xue Z-L, Chen Y-L, Bi X-G. Understanding TiN Precipitation Behavior during Solidification of SWRH 92A Tire Cord Steel by Selected Thermodynamic Models. Processes. 2020; 8(1):10. https://doi.org/10.3390/pr8010010
Chicago/Turabian StyleWang, Lu, Zheng-Liang Xue, Yi-Liang Chen, and Xue-Gong Bi. 2020. "Understanding TiN Precipitation Behavior during Solidification of SWRH 92A Tire Cord Steel by Selected Thermodynamic Models" Processes 8, no. 1: 10. https://doi.org/10.3390/pr8010010
APA StyleWang, L., Xue, Z.-L., Chen, Y.-L., & Bi, X.-G. (2020). Understanding TiN Precipitation Behavior during Solidification of SWRH 92A Tire Cord Steel by Selected Thermodynamic Models. Processes, 8(1), 10. https://doi.org/10.3390/pr8010010





