Effect of Ionic Strength and Coexisting Ions on the Biosorption of Divalent Nickel by the Acorn Shell of the Oak Quercus crassipes Humb. & Bonpl.
Abstract
1. Introduction
2. Materials and Methods
2.1. Biosorbent
2.2. Stock and Test Solutions
2.3. Biosorption Experiments
2.4. Determination of the Ni2+ Concentration
2.5. Biosorption Kinetic Modeling
2.6. Determination of the Parameters of the Kinetic Models and Statistical Analysis
3. Results and Discussion
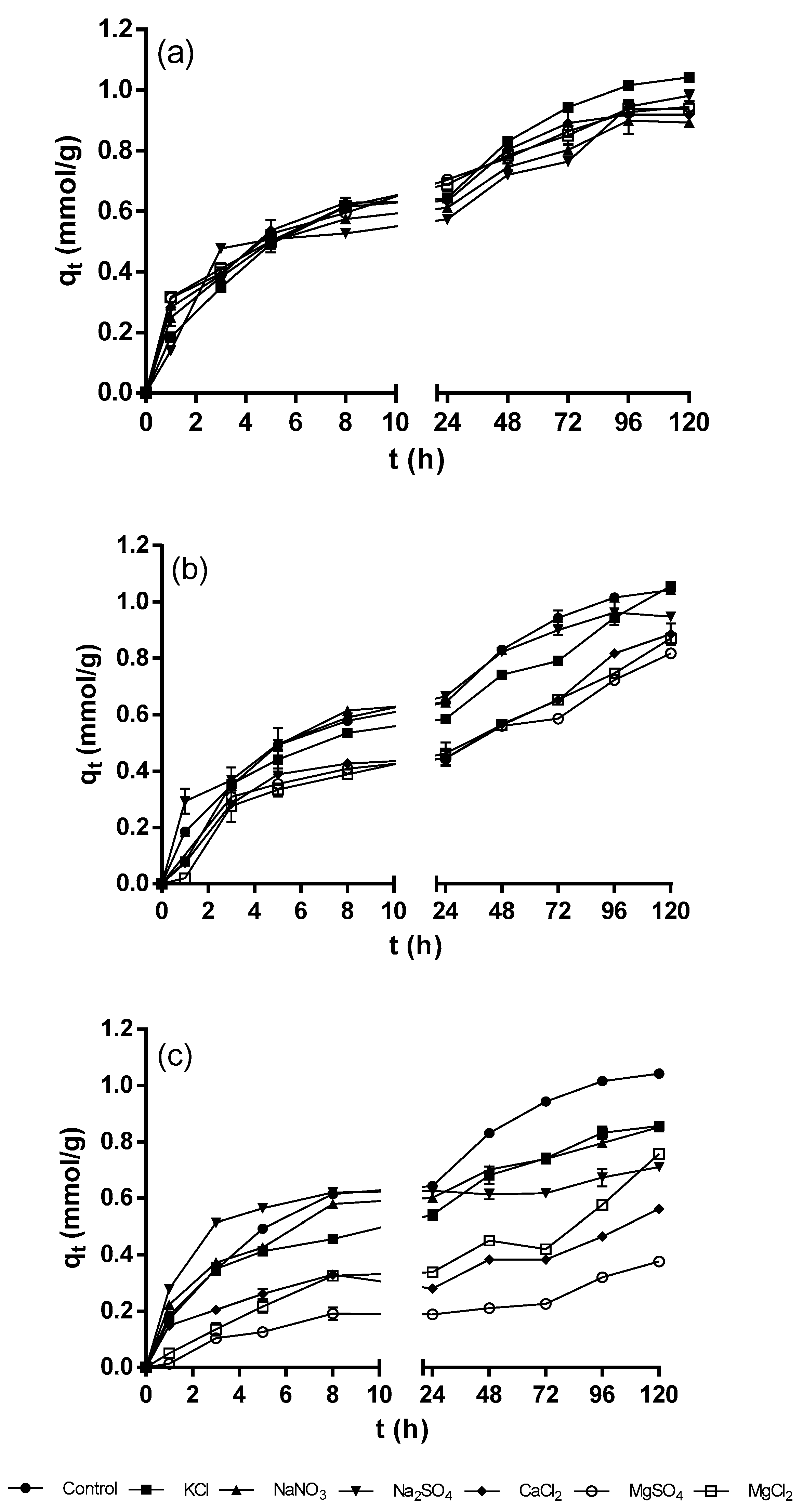
3.1. Influence of Ionic Strength on the Biosorption of Ni2+ by Acorn Shell of Quercus Crassipes Humb. & Bonpl. (QCS)
3.2. Influence of Coexisting Ionic Species on the Biosorption of Ni2+ by Acorn Shell of Quercus Crassipes Humb. & Bonpl. (QCS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barquilha, C.E.; Cossich, E.S.; Tavares, C.R.; Da Silva, E.A. Biosorption of nickel and copper ions from synthetic solution and electroplating effluent using fixed bed column of immobilized brown algae. J. Water Process. Eng. 2019, 32, 100904. [Google Scholar] [CrossRef]
- Lopez-Nuñez, P.V.; Aranda-Garcia, E.; Cristiani-Urbina, M.D.C.; Morales-Barrera, L.; Cristiani-Urbina, E. Removal of hexavalent and total chromium from aqueous solutions by Plum (P. domestica L.) tree bark. Environ. Eng. Manag. J. 2014, 13, 1927–1938. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, B. Removal of Ni (II) ions from aqueous solutions using modified rice straw in a fixed bed column. Bioresour. Technol. 2013, 146, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Altino, H.O.N.; Costa, B.E.; Da Cunha, R.N. Biosorption optimization of Ni(II) ions on Macauba (Acrocomia aculeata) oil extraction residue using fixed-bed column. J. Environ. Chem. Eng. 2017, 5, 4895–4905. [Google Scholar] [CrossRef]
- Orhan, Y.; Hrenovič, J.; Büyükgüngör, H. Biosorption of heavy metals from wastewater by biosolids. Eng. Life Sci. 2006, 6, 399–402. [Google Scholar] [CrossRef]
- Coman, V.; Robotin, B.; Ilea, P. Nickel recovery/removal from industrial wastes: A review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Flores-Garnica, J.G.; Morales-Barrera, L.; Pineda-Camacho, G.; Cristiani-Urbina, E. Biosorption of Ni(II) from aqueous solutions by Litchi chinensis seeds. Bioresour. Technol. 2013, 136, 635–643. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; Lostado-Lorza, R.; Gómez, F.S.; Escribano-Garcia, R. Adsorptive of Nickel in wastewater by olive stone waste: Optimization through multi-response surface methodology using desirability functions. Water 2020, 12, 1320. [Google Scholar] [CrossRef]
- Aranda-García, E.; Cristiani-Urbina, E. Kinetic, equilibrium, and thermodynamic analyses of Ni(II) biosorption from aqueous solution by acorn shell of Quercus crassipes. Water Air Soil Pollut. 2018, 229, 119. [Google Scholar] [CrossRef]
- Pandey, P.K.; Choubey, S.; Verma, Y.; Pandey, M.; Kamal, S.S.K.; Chandrashekhar, K. Biosorptive removal of Ni(II) from wastewater and industral effluent. Int. J. Environ. Res. Public Health 2007, 4, 332–339. [Google Scholar] [CrossRef]
- Suazo-Madrid, A.; Morales-Barrera, L.; Aranda-García, E.; Cristiani-Urbina, E. Ni(II) biosorption by Rhodotorula glutinis. J. Ind. Microbiol. Biotechnol. 2011, 38, 51–64. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Villen-Guzman, M.; Gutierrez-Pinilla, D.; Gomez-Lahoz, C.; Vereda-Alonso, C.; Rodriguez-Maroto, J.; Arhoun, B. Optimization of Ni (II) biosorption from aqueous solution on modified lemon peel. Environ. Res. 2019, 179, 108849. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Ali, I.H.; Al Mesfer, M.K.; Khan, M.I.; Danish, M.; Alghamdi, M.M. Exploring adsorption process of Lead (II) and Chromium (VI) ions from aqueous solutions on acid activated carbon prepared from Juniperus procera leaves. Processes 2019, 7, 217. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Kumar, N.S.; Asif, M.; Poulose, A.M.; Suguna, M.; Al-Hazza, M.I. Equilibrium and kinetic studies of biosorptive removal of 2,4,6-trichlorophenol from aqueous solutions using untreated agro-waste pine cone biomass. Processes 2019, 7, 757. [Google Scholar] [CrossRef]
- Aranda-García, E.; Cristiani-Urbina, E. Effect of pH on hexavalent and total chromium removal from aqueous solutions by avocado shell using batch and continuous systems. Environ. Sci. Pollut. Res. 2017, 26, 3157–3173. [Google Scholar] [CrossRef]
- Netzahuatl-Muñoz, A.R.; Cristiani-Urbina, M.D.C.; Cristiani-Urbina, E. Chromium biosorption from Cr(VI) aqueous solutions by Cupressus lusitanica bark: Kinetics, equilibrium and thermodynamic studies. PLoS ONE 2015, 10, e0137086. [Google Scholar] [CrossRef]
- Aranda-García, E.; Morales-Barrera, L.; Pineda-Camacho, G.; Cristiani-Urbina, E. Effect of pH, ionic strength, and background electrolytes on Cr(VI) and total chromium removal by acorn shell of Quercus crassipes Humb. & Bonpl. Environ. Monit. Assess. 2014, 186, 6207–6221. [Google Scholar] [CrossRef]
- Aranda-García, E.; Netzahuatl-Muñoz, A.R.; Cristiani-Urbina, M.; Morales-Barrera, L.; Pineda-Camacho, G.; Cristiani-Urbina, E. Bioreduction of Cr(VI) and chromium biosorption by acorn shell of Quercus crassipes Humb. & Bonpl. J. Biotechnol. 2010, 150, 228. [Google Scholar] [CrossRef]
- Aranda-García, E.; Cristiani-Urbina, E. Hexavalent chromium removal and total chromium biosorption from aqueous solution by Quercus crassipes acorn shell in a continuous up-flow fixed-bed column: Influencing parameters, kinetics, and mechanism. PLoS ONE 2020, 15, e0227953. [Google Scholar] [CrossRef]
- Mack, C.; Wilhelmi, B.; Duncan, J.; Burgess, J. Biosorption of precious metals. Biotechnol. Adv. 2007, 25, 264–271. [Google Scholar] [CrossRef]
- Hu, X.-J.; Liu, Y.; Zeng, G.-M.; You, S.-H.; Wang, H.; Hu, X.; Guo, Y.-M.; Tan, X.; Guo, F.-Y. Effects of background electrolytes and ionic strength on enrichment of Cd(II) ions with magnetic graphene oxide–supported sulfanilic acid. J. Colloid Interface Sci. 2014, 435, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; De Nys, R.; Hu, Y.; Paul, N.A.; Roberts, D.A. Bioremediation of a complex industrial effluent by biosorbents derived from freshwater macroalgae. PLoS ONE 2014, 9, e94706. [Google Scholar] [CrossRef]
- Hernández-Estévez, A.; Cristiani-Urbina, E. Nickel(II) biosorption from aqueous solutions by shrimp head biomass. Environ. Monit. Assess. 2014, 186, 7987–7998. [Google Scholar] [CrossRef]
- Mitchell, A.; Mellon, M. Colorimetric determination of nickel with dimethylglyoxime. Ind. Eng. Chem. Anal. Ed. 1945, 17, 380–382. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Al-Muhtaseb, A.H.; Al-Laqtah, N.A.; Walker, G.; Allen, S.; Ahmad, M.N. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.-H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Basha, S.; Murthy, Z. Kinetic and equilibrium models for biosorption of Cr(VI) on chemically modified seaweed, Cystoseira indica. Process Biochem. 2007, 42, 1521–1529. [Google Scholar] [CrossRef]
- Subbaiah, M.V.; Vijaya, Y.; Kumar, N.S.; Reddy, A.S.; Abburi, K. Biosorption of nickel from aqueous solutions by Acacia leucocephala bark: Kinetics and equilibrium studies. Colloids Surfaces B Biointerfaces 2009, 74, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, S.; Volesky, B. Ionic strength and electrostatic effects in biosorption of divalent metal ions and protons. Environ. Sci. Technol. 1997, 31, 2478–2485. [Google Scholar] [CrossRef]
- Barnie, S.; Zhang, J.; Wang, H.; Yin, H.; Chen, H. The influence of pH, co-existing ions, ionic strength, and temperature on the adsorption and reduction of hexavalent chromium by undissolved humic acid. Chemosphere 2018, 212, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Villaescusa, I.; Fiol, N.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res. 2004, 38, 992–1002. [Google Scholar] [CrossRef]
- Mogollón, L.; Rodriguez, R.; Larrota, W.; Ramírez, N.; Torres, R. Biosorption of nickel using filamentous fungi. Appl. Biochem. Biotechnol. 1998, 70–72, 593–601. [Google Scholar] [CrossRef]
- Thevannan, A.; Mungroo, R.; Niu, C.H. Biosorption of nickel with barley straw. Bioresour. Technol. 2010, 101, 1776–1780. [Google Scholar] [CrossRef]
- Djemmoe, L.G.; Njanja, T.E.; Deussi, M.C.N.; Tonle, K.I. Assessment of copper(II) biosorption from aqueous solution by agricultural and industrial residues. Comptes Rendus Chim. 2016, 19, 841–849. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Shen, B.; Hou, W.; Zhang, Y. Biosorption of copper(II) and cadmium(II) by a novel exopolysaccharide secreted from deep-sea mesophilic bacterium. Colloids Surf. B Biointerfaces 2009, 72, 295–302. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Kumar, S. Biosorption of lead ions from the aqueous solution by Sargassum filipendula: Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2016, 4, 4587–4599. [Google Scholar] [CrossRef]
- Maurya, N.S.; Mittal, A.K.; Cornel, P.; Rother, E. Biosorption of dyes using dead macro fungi: Effect of dye structure, ionic strength and pH. Bioresour. Technol. 2006, 97, 512–521. [Google Scholar] [CrossRef]
- Flores-Chaparro, C.E.; Ruiz, L.F.C.; De La Torre, M.C.A.; Huerta-Diaz, M.A.; Rangel-Mendez, J.R. Biosorption removal of benzene and toluene by three dried macroalgae at different ionic strength and temperatures: Algae biochemical composition and kinetics. J. Environ. Manag. 2017, 193, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M. A review on valorization of biomass in heavy metal removal from wastewater. J. Water Process. Eng. 2020, 38, 101602. [Google Scholar] [CrossRef]
- Vázquez-Palma, D.E.; Netzahuatl-Muñoz, A.R.; Pineda-Camacho, G.; Cristiani-Urbina, E. Biosorptive removal of nickel(II) ions from aqueous solutions by Hass avocado (Persea americana Mill. var. Hass) shell as an effective and low-cost biosorbent. Fresenius Environ. Bull. 2017, 26, 3501–3513. [Google Scholar]
- Park, D.; Yun, Y.S.; Jo, J.H.; Park, J.M. Effects of ionic strength, background electrolytes, heavy metals, and redox-active species on the reduction of hexavalent chromium by Ecklonia biomass. J. Microbiol. Biotechnol. 2005, 15, 780–786. [Google Scholar]
- Okoronkwo, A.E.; Aiyesanmi, A.F.; Olasehinde, E.F. Biosorption of nickel from aqueous solution by Tithonia diversifolia. Desalin. Water Treat. 2009, 12, 352–359. [Google Scholar] [CrossRef]
- Almohammadi, S.; Mirzaei, M. Removal of copper (II) from aqueous solutions by adsorption onto granular activated carbon in the presence of competitor ions. Adv. Environ. Technol. 2016, 2, 85–94. [Google Scholar]

| Ionic Strength (mM) | ξ (%) |
|---|---|
| 0.2 | −4 |
| 2 | −17 |
| 20 | −26 |
| 200 | −55 |
| 2000 | −58 |
| Pseudo-First Order Model | Pseudo-Second Order Model | |||||||||||||
| Ionic Strength (mM) | qe exp (mmol/g) | k1 (1/h) | qe1 (mmol/g) | r2 | SSE | RMSE | AIC | k2 (g/mmol·h) | qe2 (mmol/g) | r2 | SSE | RMSE | AIC | |
| Control | 1.078 | 0.127 ± 0.041 | 1.029 ± 0.057 | 0.9601 | 0.0710 | 0.0769 | −65.6 | 0.166 ± 0.054 | 1.118 ± 0.048 | 0.9872 | 0.0234 | 0.0441 | −81.1 | |
| 0.2 | 1.030 | 0.127 ± 0.035 | 0.998 ± 0.049 | 0.9771 | 0.0383 | 0.0619 | −60.0 | 0.158 ± 0.055 | 1.079 ± 0.048 | 0.9892 | 0.0181 | 0.0426 | −69.0 | |
| 2 | 0.910 | 0.102 ± 0.038 | 0.863 ± 0.061 | 0.9422 | 0.0751 | 0.0791 | −64.8 | 0.148 ± 0.061 | 0.953 ± 0.059 | 0.9775 | 0.0292 | 0.0494 | −78.0 | |
| 20 | 0.798 | 0.103 ± 0.025 | 0.776 ± 0.038 | 0.9772 | 0.0249 | 0.0476 | −72.7 | 0.142 ± 0.035 | 0.860 ± 0.034 | 0.9916 | 0.0092 | 0.0289 | −85.6 | |
| 200 | 0.510 | 0.044 ± 0.021 | 0.512 ± 0.059 | 0.9229 | 0.0372 | 0.0557 | −74.6 | 0.138 ± 0.104 | 0.565 ± 0.073 | 0.9521 | 0.0231 | 0.0439 | −81.3 | |
| 2000 | 0.491 | 0.070 ± 0.037 | 0.467 ± 0.047 | 0.9211 | 0.0339 | 0.0556 | −68.7 | 0.133 ± 0.084 | 0.547 ± 0.063 | 0.9275 | 0.0183 | 0.0408 | −76.7 | |
| Elovich | Intraparticle Diffusion | |||||||||||||
| Ae (mmol/g·h) | Be (g/mmol) | r2 | SSE | RMSE | AIC | kid (mmol/g·h0.5) | c (mmol/g) | r2 | SSE | RMSE | AIC | |||
| Control | 1.885 ± 1.095 | 6.577 ± 0.707 | 0.9964 | 0.0066 | 0.0234 | −98.85 | 0.090 ± 0.026 | 0.259 ± 0.190 | 0.8227 | 0.3231 | 0.1641 | −44.37 | ||
| 0.2 | 1.003 ± 0.531 | 6.216 ± 0.676 | 0.9969 | 0.0052 | 0.0229 | −83.86 | 0.090 ± 0.023 | 0.184 ± 0.173 | 0.8857 | 0.1910 | 0.1382 | −40.68 | ||
| 2 | 0.671 ± 0.396 | 6.728 ± 0.892 | 0.9931 | 0.0090 | 0.0273 | −94.56 | 0.078 ± 0.019 | 0.188 ± 0.139 | 0.8679 | 0.1714 | 0.1195 | −53.24 | ||
| 20 | 0.274 ± 0.072 | 6.391 ± 0.470 | 0.9972 | 0.0031 | 0.0167 | −99.94 | 0.072 ± 0.014 | 0.127 ± 0.099 | 0.9248 | 0.0824 | 0.0866 | −57.13 | ||
| 200 | 0.142 ± 0.088 | 9.761 ± 1.769 | 0.9841 | 0.0076 | 0.0253 | −96.77 | 0.047 ± 0.006 | 0.061 ± 0.047 | 0.9549 | 0.0217 | 0.0426 | −82.15 | ||
| 2000 | 0.071 ± 0.040 | 8.420 ± 1.729 | 0.9795 | 0.0088 | 0.0283 | −86.19 | 0.046 ± 0.005 | 0.046 ± 0.039 | 0.9697 | 0.0130 | 0.0344 | −81.10 | ||
| Fractional Power | ||||||||||||||
| kFP (mmol/g) | v (1/h) | kFP⋅v (mmol/g·h) | r2 | SSE | RMSE | AIC | ||||||||
| Control | 0.503 ± 0.040 | 0.168 ± 0.019 | 0.0842 | 0.9963 | 0.0067 | 0.0237 | −98.58 | |||||||
| 0.2 | 0.427 ± 0.038 | 0.193 ± 0.021 | 0.0826 | 0.9975 | 0.0042 | 0.0206 | −86.41 | |||||||
| 2 | 0.360 ± 0.036 | 0.202 ± 0.024 | 0.0729 | 0.9948 | 0.0068 | 0.0238 | −98.39 | |||||||
| 20 | 0.251 ± 0.028 | 0.256 ± 0.027 | 0.0641 | 0.9951 | 0.0054 | 0.0222 | −92.55 | |||||||
| 200 | 0.131 ± 0.020 | 0.299 ± 0.035 | 0.0390 | 0.9926 | 0.0036 | 0.0172 | −107.5 | |||||||
| 2000 | 0.102 ± 0.022 | 0.342 ± 0.049 | 0.0350 | 0.9890 | 0.0048 | 0.0208 | −94.22 | |||||||
| Background Electrolyte | ξ (%) | ||
|---|---|---|---|
| 0.2 mM | 2 mM | 20 mM | |
| KCl | −6 | −8 | −21 |
| NaNO3 | −6 | −12 | −20 |
| Na2SO4 | −5 | −10 | −27 |
| CaCl2 | −6 | −20 | −54 |
| MgSO4 | −7 | −23 | −77 |
| MgCl2 | −7 | −24 | −42 |
| Pseudo-First Order Model | Pseudo-Second Order Model | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mM) | Background Electrolyte | qe exp (mmol/g) | k1 (1/h) | qe1 (mmol/g) | r2 | SSE | RMSE | AIC | k2 (g/mmol·h) | qe2 (mmol/g) | r2 | SSE | RMSE | AIC |
| Control | 1.000 | 0.142 ± 0.026 | 0.957 ± 0.047 | 0.9724 | 0.0633 | 0.0629 | −94.0 | 0.170 ± 0.035 | 1.034 ± 0.041 | 0.9870 | 0.0299 | 0.0433 | −107.5 | |
| 0.2 | KCl | 0.929 | 0.180 ± 0.044 | 0.887 ± 0.058 | 0.9431 | 0.0983 | 0.0784 | −86.1 | 0.280 ± 0.077 | 0.936 ± 0.047 | 0.9734 | 0.0459 | 0.0536 | −99.8 |
| NaNO3 | 0.943 | 0.135 ± 0.042 | 0.901 ± 0.050 | 0.9274 | 0.1452 | 0.0953 | −91.6 | 0.176 ± 0.079 | 0.975 ± 0.041 | 0.9574 | 0.0852 | 0.0730 | −104.6 | |
| Na2SO4 | 0.937 | 0.163 ± 0.038 | 0.902 ± 0.057 | 0.9493 | 0.0929 | 0.0762 | −87.1 | 0.239 ± 0.068 | 0.957 ± 0.051 | 0.9724 | 0.0506 | 0.0562 | −98.0 | |
| CaCl2 | 0.909 | 0.193 ± 0.038 | 0.876 ± 0.046 | 0.9628 | 0.0624 | 0.0624 | −94.3 | 0.308 ± 0.068 | 0.922 ± 0.036 | 0.9836 | 0.0276 | 0.0415 | −108.9 | |
| MgSO4 | 0.912 | 0.190 ± 0.049 | 0.869 ± 0.060 | 0.9355 | 0.1054 | 0.0812 | −84.8 | 0.309 ± 0.094 | 0.914 ± 0.051 | 0.9672 | 0.0537 | 0.0579 | −97.0 | |
| MgCl2 | 0.909 | 0.192 ± 0.049 | 0.870 ± 0.059 | 0.9371 | 0.1025 | 0.0800 | −85.3 | 0.315 ± 0.095 | 0.914 ± 0.050 | 0.9679 | 0.0523 | 0.0572 | −97.4 | |
| 2 | KCl | 0.931 | 0.133 ± 0.040 | 0.882 ± 0.070 | 0.9355 | 0.1397 | 0.0935 | −79.7 | 0.159 ± 0.065 | 0.967 ± 0.077 | 0.9553 | 0.0968 | 0.0778 | −86.4 |
| NaNO3 | 0.865 | 0.187 ± 0.040 | 0.829 ± 0.072 | 0.9529 | 0.0723 | 0.0672 | −79.1 | 0.308 ± 0.066 | 0.875 ± 0.070 | 0.9771 | 0.0352 | 0.0469 | −88.6 | |
| Na2SO4 | 0.897 | 0.181 ± 0.060 | 0.844 ± 0.075 | 0.9093 | 0.1625 | 0.1008 | −77.0 | 0.272 ± 0.121 | 0.899 ± 0.074 | 0.9383 | 0.1105 | 0.0831 | −84.0 | |
| CaCl2 | 0.785 | 0.121 ± 0.035 | 0.782 ± 0.066 | 0.9454 | 0.0732 | 0.0750 | −71.7 | 0.174 ± 0.071 | 0.845 ± 0.071 | 0.9602 | 0.0533 | 0.0641 | −76.4 | |
| MgSO4 | 0.709 | 0.119 ± 0.029 | 0.765 ± 0.062 | 0.9644 | 0.0316 | 0.0562 | −62.2 | 0.192 ± 0.059 | 0.812 ± 0.057 | 0.9795 | 0.0181 | 0.0426 | −68.9 | |
| MgCl2 | 0.757 | 0.108 ± 0.033 | 0.755 ± 0.065 | 0.9465 | 0.0696 | 0.0732 | −72.4 | 0.153 ± 0.065 | 0.824 ± 0.073 | 0.9602 | 0.0517 | 0.0631 | −76.9 | |
| 20 | KCl | 0.810 | 0.152 ± 0.052 | 0.737 ± 0.063 | 0.8968 | 0.1490 | 0.0910 | −90.5 | 0.230 ± 0.088 | 0.811 ± 0.058 | 0.9499 | 0.0724 | 0.0634 | −104.9 |
| NaNO3 | 0.795 | 0.204 ± 0.054 | 0.737 ± 0.048 | 0.9309 | 0.0921 | 0.0715 | −100.1 | 0.350 ± 0.104 | 0.793 ± 0.041 | 0.9666 | 0.0445 | 0.0497 | −114.6 | |
| Na2SO4 | 0.667 | 0.524 ± 0.090 | 0.642 ± 0.020 | 0.9779 | 0.0190 | 0.0325 | −131.7 | 1.325 ± 0.355 | 0.671 ± 0.023 | 0.9766 | 0.0201 | 0.0334 | −130.5 | |
| CaCl2 | 0.470 | 0.212 ± 0.072 | 0.427 ± 0.040 | 0.9031 | 0.0383 | 0.0505 | −95.8 | 0.698 ± 0.335 | 0.451 ± 0.040 | 0.9287 | 0.0282 | 0.0433 | −101.0 | |
| MgSO4 | 0.307 | 0.211 ± 0.054 | 0.211 ± 0.016 | 0.9576 | 0.0040 | 0.0175 | −115.4 | 1.158 ± 0.572 | 0.233 ± 0.023 | 0.9486 | 0.0048 | 0.0192 | −112.5 | |
| MgCl2 | 0.584 | 0.047 ± 0.024 | 0.627 ± 0.098 | 0.8653 | 0.1335 | 0.0913 | −80.6 | 0.089 ± 0.063 | 0.715 ± 0.117 | 0.9117 | 0.0875 | 0.0739 | −88.1 | |
| Elovich | Intraparticle Diffusion | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mM) | Background Electrolyte | Ae (mmol/g·h) | Be (g/mmol) | r2 | SSE | RMSE | AIC | kid (mmol/g·h0.5) | c (mmol/g) | r2 | SSE | RMSE | AIC |
| Control | 0.418 ± 0.110 | 5.369 ± 0.418 | 0.9916 | 0.0193 | 0.0348 | −115.3 | 0.087 ± 0.014 | 0.183 ± 0.089 | 0.9126 | 0.2006 | 0.1120 | −73.22 | |
| 0.2 | KCl | 0.918 ± 0.196 | 6.937 ± 0.357 | 0.9960 | 0.0069 | 0.0208 | −133.9 | 0.074 ± 0.014 | 0.236 ± 0.091 | 0.8803 | 0.2067 | 0.1137 | −72.69 |
| NaNO3 | 0.856 ± 0.304 | 7.391 ± 0.632 | 0.9891 | 0.0168 | 0.0324 | −117.9 | 0.069 ± 0.015 | 0.222 ± 0.091 | 0.8641 | 0.2088 | 0.1142 | −72.51 | |
| Na2SO4 | 0.738 ± 0.248 | 6.552 ± 0.560 | 0.9890 | 0.0201 | 0.0354 | −114.7 | 0.077 ± 0.015 | 0.224 ± 0.092 | 0.8840 | 0.2124 | 0.1152 | −72.20 | |
| CaCl2 | 1.051 ± 0.392 | 7.210 ± 0.625 | 0.9888 | 0.0188 | 0.0343 | −115.8 | 0.072 ± 0.016 | 0.245 ± 0.102 | 0.8437 | 0.2623 | 0.1280 | −68.40 | |
| MgSO4 | 1.078 ± 0.331 | 7.306 ± 0.518 | 0.9925 | 0.0123 | 0.0278 | −123.4 | 0.072 ± 0.015 | 0.243 ± 0.092 | 0.8690 | 0.2141 | 0.1157 | −72.06 | |
| MgCl2 | 1.128 ± 0.355 | 7.362 ± 0.529 | 0.9922 | 0.0126 | 0.0281 | −123.0 | 0.071 ± 0.015 | 0.247 ± 0.094 | 0.8643 | 0.2211 | 0.1175 | −71.48 | |
| 2 | KCl | 0.284 ± 0.139 | 5.350 ± 0.845 | 0.9689 | 0.0674 | 0.0649 | −92.85 | 0.085 ± 0.013 | 0.135 ± 0.084 | 0.9187 | 0.1760 | 0.1049 | −75.58 |
| NaNO3 | 0.409 ± 0.137 | 5.765 ± 0.568 | 0.9863 | 0.0275 | 0.0414 | −109.0 | 0.082 ± 0.011 | 0.172 ± 0.071 | 0.9372 | 0.1257 | 0.0886 | −81.65 | |
| Na2SO4 | 0.600 ± 0.407 | 6.655 ± 1.190 | 0.9548 | 0.0810 | 0.0712 | −89.55 | 0.075 ± 0.016 | 0.197 ± 0.098 | 0.8653 | 0.2412 | 0.1228 | −69.21 | |
| CaCl2 | 0.257 ± 0.135 | 6.352 ± 1.061 | 0.9715 | 0.0382 | 0.0542 | −81.42 | 0.070 ± 0.012 | 0.125 ± 0.077 | 0.9223 | 0.1041 | 0.0895 | −66.38 | |
| MgSO4 | 0.342 ± 0.117 | 7.261 ± 0.740 | 0.9920 | 0.0071 | 0.0266 | −80.22 | 0.062 ± 0.014 | 0.151 ± 0.084 | 0.9126 | 0.0774 | 0.0880 | −51.52 | |
| MgCl2 | 0.191 ± 0.098 | 6.178 ± 1.074 | 0.9709 | 0.0379 | 0.0540 | −81.55 | 0.069 ± 0.011 | 0.100 ± 0.071 | 0.9329 | 0.0872 | 0.0819 | −69.02 | |
| 20 | KCl | 0.399 ± 0.128 | 6.997 ± 0.418 | 0.9865 | 0.0196 | 0.0330 | −131.1 | 0.069 ± 0.010 | 0.167 ± 0.064 | 0.9142 | 0.1238 | 0.0830 | −94.19 |
| NaNO3 | 0.815 ± 0.334 | 8.064 ± 0.639 | 0.9839 | 0.0214 | 0.0345 | −129.3 | 0.064 ± 0.014 | 0.217 ± 0.083 | 0.8440 | 0.2077 | 0.1074 | −83.84 | |
| Na2SO4 | 0.425 ± 0.587 | 15.69 ± 0.790 | 0.9219 | 0.0675 | 0.0612 | −106.3 | 0.041 ± 0.019 | 0.323 ± 0.116 | 0.5324 | 0.4025 | 0.1495 | −70.61 | |
| CaCl2 | 0.576 ± 0.479 | 14.73 ± 1.665 | 0.9505 | 0.0195 | 0.0361 | −107.2 | 0.037 ± 0.009 | 0.120 ± 0.054 | 0.8315 | 0.0665 | 0.0666 | −86.41 | |
| MgSO4 | 0.163 ± 0.170 | 24.01 ± 7.875 | 0.9113 | 0.0082 | 0.0253 | −104.3 | 0.024 ± 0.009 | 0.050 ± 0.039 | 0.7443 | 0.0239 | 0.0429 | −88.45 | |
| MgCl2 | 0.074 ± 0.041 | 6.100 ± 1.519 | 0.9453 | 0.0542 | 0.0582 | −96.78 | 0.062 ± 0.009 | 0.040 ± 0.042 | 0.9486 | 0.0509 | 0.0564 | −97.91 | |
| Fractional Power | ||||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mM) | Background Electrolyte | kFP (mmol/g) | v (1/h) | kFPv (mmol/g·h) | r2 | SSE | RMSE | AIC |
| Control | 0.289 ± 0.043 | 0.275 ± 0.036 | 0.0793 | 0.9768 | 0.0534 | 0.0578 | −97.02 | |
| 0.2 | KCl | 0.340 ± 0.025 | 0.221 ± 0.018 | 0.0753 | 0.9906 | 0.0162 | 0.0318 | −118.6 |
| NaNO3 | 0.320 ± 0.036 | 0.220 ± 0.028 | 0.0705 | 0.9786 | 0.0329 | 0.0453 | −105.8 | |
| Na2SO4 | 0.327 ± 0.035 | 0.233 ± 0.027 | 0.0761 | 0.9825 | 0.0321 | 0.0448 | −106.2 | |
| CaCl2 | 0.352 ± 0.041 | 0.210 ± 0.029 | 0.0741 | 0.9755 | 0.0411 | 0.0507 | −101.8 | |
| MgSO4 | 0.347 ± 0.027 | 0.213 ± 0.020 | 0.0738 | 0.9887 | 0.0185 | 0.0340 | −116.1 | |
| MgCl2 | 0.351 ± 0.028 | 0.210 ± 0.020 | 0.0738 | 0.9879 | 0.0197 | 0.0351 | −115.0 | |
| 2 | KCl | 0.232 ± 0.053 | 0.308 ± 0.055 | 0.0714 | 0.9579 | 0.0911 | 0.0755 | −87.43 |
| NaNO3 | 0.269 ± 0.028 | 0.278 ± 0.026 | 0.0747 | 0.9881 | 0.0237 | 0.0385 | −111.6 | |
| Na2SO4 | 0.295 ± 0.061 | 0.246 ± 0.051 | 0.0724 | 0.9442 | 0.0999 | 0.0790 | −85.77 | |
| CaCl2 | 0.209 ± 0.047 | 0.294 ± 0.053 | 0.0614 | 0.9659 | 0.0457 | 0.0593 | −78.71 | |
| MgSO4 | 0.236 ± 0.021 | 0.254 ± 0.022 | 0.0598 | 0.9942 | 0.0051 | 0.0226 | −84.11 | |
| MgCl2 | 0.179 ± 0.045 | 0.319 ± 0.059 | 0.0572 | 0.9646 | 0.0461 | 0.0595 | −78.6 | |
| 20 | KCl | 0.245 ± 0.025 | 0.264 ± 0.026 | 0.0645 | 0.9845 | 0.0224 | 0.0351 | −128.4 |
| NaNO3 | 0.301 ± 0.035 | 0.216 ± 0.030 | 0.0652 | 0.9719 | 0.0374 | 0.0456 | −118.1 | |
| Na2SO4 | 0.423 ± 0.059 | 0.107 ± 0.038 | 0.0451 | 0.9078 | 0.0794 | 0.0664 | −103.1 | |
| CaCl2 | 0.175 ± 0.031 | 0.213 ± 0.045 | 0.0372 | 0.9472 | 0.0208 | 0.0373 | −106.1 | |
| MgSO4 | 0.085 ± 0.027 | 0.239 ± 0.093 | 0.0204 | 0.8746 | 0.0117 | 0.0300 | −99.14 | |
| MgCl2 | 0.101 ± 0.029 | 0.404 ± 0.069 | 0.0406 | 0.9564 | 0.0432 | 0.0520 | −100.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda-García, E.; Chávez-Camarillo, G.M.; Cristiani-Urbina, E. Effect of Ionic Strength and Coexisting Ions on the Biosorption of Divalent Nickel by the Acorn Shell of the Oak Quercus crassipes Humb. & Bonpl. Processes 2020, 8, 1229. https://doi.org/10.3390/pr8101229
Aranda-García E, Chávez-Camarillo GM, Cristiani-Urbina E. Effect of Ionic Strength and Coexisting Ions on the Biosorption of Divalent Nickel by the Acorn Shell of the Oak Quercus crassipes Humb. & Bonpl. Processes. 2020; 8(10):1229. https://doi.org/10.3390/pr8101229
Chicago/Turabian StyleAranda-García, Erick, Griselda Ma. Chávez-Camarillo, and Eliseo Cristiani-Urbina. 2020. "Effect of Ionic Strength and Coexisting Ions on the Biosorption of Divalent Nickel by the Acorn Shell of the Oak Quercus crassipes Humb. & Bonpl." Processes 8, no. 10: 1229. https://doi.org/10.3390/pr8101229
APA StyleAranda-García, E., Chávez-Camarillo, G. M., & Cristiani-Urbina, E. (2020). Effect of Ionic Strength and Coexisting Ions on the Biosorption of Divalent Nickel by the Acorn Shell of the Oak Quercus crassipes Humb. & Bonpl. Processes, 8(10), 1229. https://doi.org/10.3390/pr8101229







