3.1. Influence of Ionic Strength on the Biosorption of Ni2+ by Acorn Shell of Quercus Crassipes Humb. & Bonpl. (QCS)
The ionic strength of an aqueous solution is an environmental parameter that significantly affects the biosorption of heavy metal ions at the interface between the solid biosorbent and the liquid phase [
24]. Thus, this study investigated its effect on the kinetics of biosorption of Ni
2+ by QCS.
Figure 1 shows the variation in Ni
2+ biosorption capacity as a function of the biosorption time at NaCl ionic strengths ranging from 0.2 to 2000 mM. The kinetic profile of the Ni
2+ biosorption at an ionic strength of 0.2 mM is similar to that of the control experiment (0 mM NaCl). There were no significant differences between the Ni
2+ biosorption capacities (
p > 0.05) for the control experiment and at an ionic strength of 0.2 mM at different experimental times, which indicates that the Ni
2+ biosorption is not affected by a NaCl ionic strength of 0.2 mM.
In contrary, the Ni2+ biosorption capacity decreased gradually as the ionic strength increased from 0.2 to 200 mM (p < 0.05). However, the Ni2+ biosorption capacities were very similar and there were no significant differences between them (p > 0.05) at different biosorption times and at very high ionic strengths of 200 and 2000 mM. These results indicate that the biosorption of Ni2+ is negatively affected by NaCl ionic strengths equal to or greater than 2 mM.
The global performance indexes for the biosorption of Ni
2+ at the different ionic strengths are summarized in
Table 1.
The global performance index revealed that a NaCl ionic strength of 0.2 mM had a negligible negative effect on the biosorption of the metal (ξ = −4%). However, as the ionic strength increased from 2 to 200 mM, the global performance index decreased from −17 to −55%, which indicates that the adverse effect of the ionic strength on the biosorption of Ni2+ increases with an increase in ionic strength (p < 0.05). Moreover, the global performance index for the biosorption of Ni2+ at NaCl ionic strengths of 200 and 2000 mM were similar, thus, the adverse effects on the biosorption of the metal were similar under these conditions (p > 0.05). These results are in agreement with the negative effects observed in the kinetic studies of the Ni2+ biosorption.
The decrease in Ni
2+ biosorption with increasing NaCl ionic strengths from 0.2 to 2000 mM can be attributed to the fact that the aqueous solution contain more positively charged Na
+ ions, which competed with the Ni
2+ cations for the available biosorption active sites on the QCS surface [
24,
33]. Moreover, changes in the ionic strength of a solution can make the reactive functional groups on the surface of the biosorbent less accessible to Ni
2+ ions [
20,
34]. Furthermore, Na
+ ions can reduce the concentration of other electrostatically bound counterions that balance the negative charge of the biomass, thus, Na
+ ions affect the intraparticle ion concentration and the binding of other ions, such as the Ni
2+ ions [
33]. Additionally, it has been reported that high Cl
− ion concentrations (present in NaCl) favor the formation of the nickel chloride (NiCl
−) complex, thus decreasing the number of free Ni
2+ ions in the aqueous solution, which results in a lower number of interactions between Ni
2+ ions and biosorption active sites and consequently, a decrease in the heavy metal biosorption capacity [
35].
Additionally, it has been reported that the NaCl ionic strength negatively affects the biosorption of Ni
2+ by grape stalks wastes [
35], filamentous fungi such as
Rhizopus sp.,
Mucor sp., and
Penicillium sp. [
36], and barley straw [
37]. Moreover, the NaCl ionic strength decreased the sorption capacity of other heavy metals, such as Cu
2+ by HNO
3-pretreated newspaper scraps, HNO
3-pretreated-maize spatha [
38], and by an exopolysaccharide of
Wangia profunda [
39]; also Cd
2+ by an exopolysaccharide of
Wangia profunda [
39] and by magnetic graphene oxide-supported sulfanilic acid [
24], Pb
2+ by
Sargassum filipendula [
40], and Cr
6+ by lignin [
28]. Likewise, the increase of NaCl ionic strength inhibited the biosorption of organic adsorbates, such as Methylene Blue and Rhodamine B dyes by
Phellinus igniarius and
Fomes fomentarius [
41], and benzene and toluene by
Macrocystis pyrifera [
42].
Analysis of the kinetic model for biosorption study is crucial for understanding dynamics, mechanism, and reaction pathway of the biosorption process. It also helps in determining mass transfer, rate-controlling steps, and physicochemical interaction in the biosorption process. Additionally, knowledge about kinetics of metal biosorption is useful for determining optimum conditions for biosorption processes [
43]. In the present work, the experimental kinetic data of the biosorption of Ni
2+ by QCS at different ionic strengths were analyzed using the pseudo-first order, pseudo-second order, Elovich, fractional power, and intraparticle diffusion models. These models have been used previously to analyze and understand biosorption kinetics of Ni
2+ ions in single metal systems by different biosorbents [
7,
11,
26,
44] but to the best knowledge of the authors, the kinetic process of Ni
2+ biosorption from aqueous solutions containing NaCl or other background electrolytes has not been mathematically modeled. The parameters of the kinetic models are presented in
Table 2.
The highest r2 values and the lowest SSE, RMSE, and AIC values were obtained using the pseudo-second order, Elovich, and fractional power models. Therefore, these models represent the kinetic profiles of the biosorption of Ni2+ by QCS most adequately under the studied conditions.
The equilibrium Ni
2+ biosorption capacities obtained using the pseudo-second order model (q
e2) were similar to those obtained experimentally (q
e exp). Furthermore, as the NaCl ionic strength increased from 0 to 2000 mM, the rate constant of the pseudo-second order model (k
2) decreased from 0.166 to 0.133 g/mmol·h. A similar behavior was observed in the studies of the biosorption of total chromium by QCS at different ionic strengths [
20]. A previous study that analyzed the kinetics of the biosorption of Ni
2+ by QCS at different pH conditions, initial metal concentrations, and temperatures using the pseudo-first order and pseudo-second order models established that the pseudo-second order model described the biosorption kinetics of the heavy metal more satisfactorily than the pseudo-first order model [
9]. The present study demonstrates that the pseudo-second order model can also describe the kinetics of the biosorption of Ni
2+ by QCS at different ionic strengths.
Furthermore, it was observed that higher ionic strengths lead to higher rate constants of the fractional power model (v) and lower initial biosorption rates (Ae), as predicted by the Elovich model, as well as lower constants (kFP) and specific biosorption rates (kFP⋅v) of the fractional power model. The decrease in k2, Ae, and kFP⋅v with increasing ionic strengths indicates that the interactions between the QCS biomass and the Ni2+ cations decrease with increasing NaCl ionic strengths, which prevents the binding of Ni2+ ions to biosorption active sites and decreases the heavy metal biosorption capacity.
Notably, even though NaCl ionic strengths equal to and greater than 200 mM significantly affected the biosorption of Ni
2+ by QCS, this can be ignored in the present study since the ionic strength of industrial wastewater is lower than 100 mM [
45].
3.2. Influence of Coexisting Ionic Species on the Biosorption of Ni2+ by Acorn Shell of Quercus Crassipes Humb. & Bonpl. (QCS)
Generally, there are various types of background electrolytes in industrial wastewater at varying concentrations, which can affect the biosorption of the heavy metal of interest. This study investigated the effect of coexisting ionic species on the biosorption of Ni2+ by QCS using different cations and anions at three different concentrations, namely 0.2, 2.0, and 20 mM.
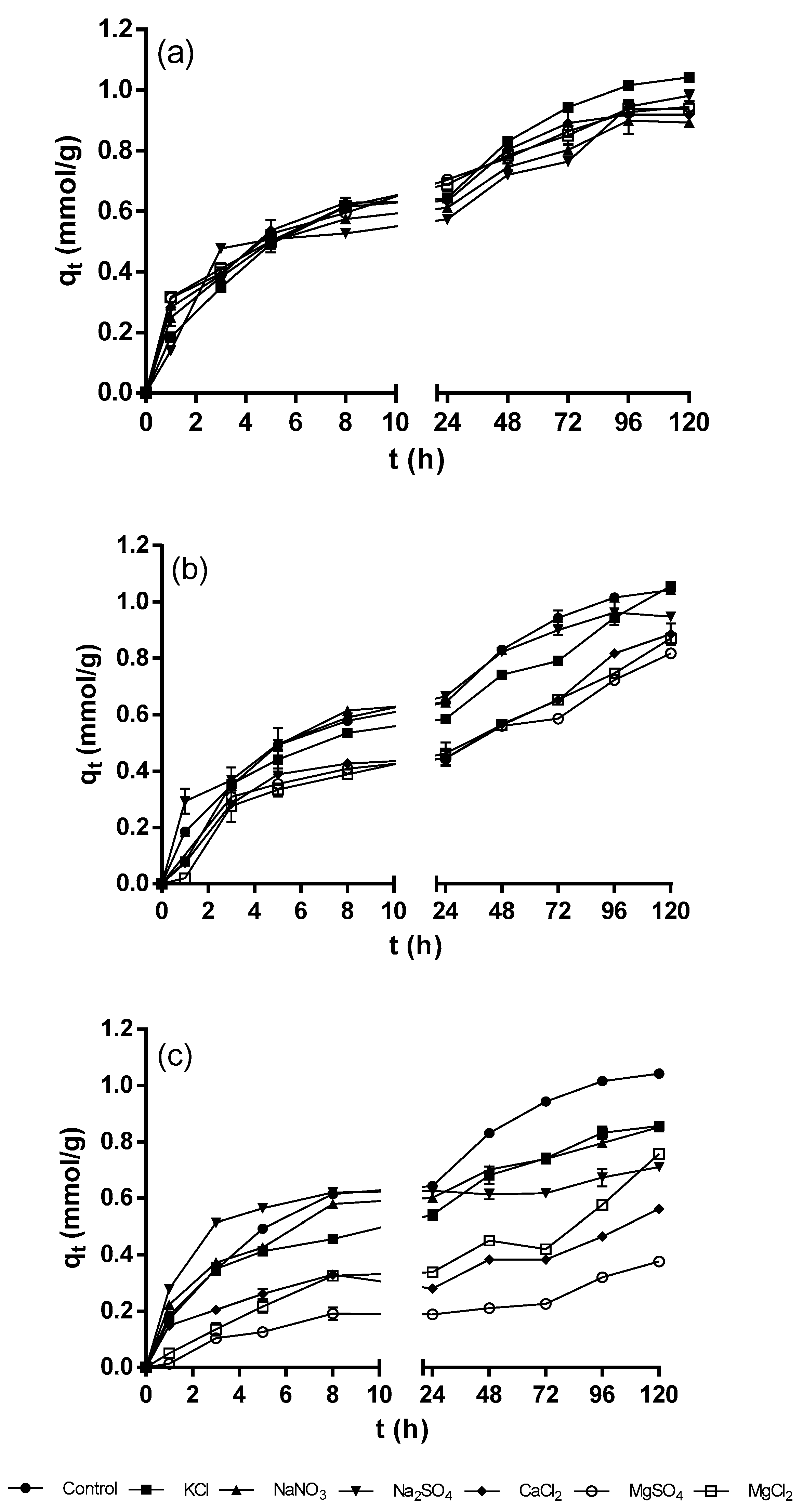
Figure 2 shows the kinetic profiles of the biosorption of Ni
2+ by QCS at the different concentrations of background electrolytes. There was a small variation in the Ni
2+ biosorption capacity over the course of the experiments for background electrolyte concentration of 0.2 mM compared to the control experiment (with no background electrolytes) (
Figure 2a). However, there were no statistically significant differences in all cases (
p > 0.05), thus, it is concluded that a background electrolyte concentration of 0.2 mM has no negative effect on the biosorption of Ni
2+. Moreover, the global performance indexes for the biosorption of Ni
2+ at a background electrolyte concentration of 0.2 mM were small and ranged from −7 to −5% (
Table 3), which confirms that background electrolytes at a concentration of 0.2 mM have a negligible effect on the biosorption of Ni
2+.
Increasing the concentration of background electrolytes to 2 mM led to a further decrease in the Ni
2+ biosorption capacity. However, as shown in
Figure 2b, the different background electrolytes influenced the biosorption of Ni
2+ at varying degrees. The decreases in Ni
2+ biosorption capacity were more significant in the presence of salts with divalent cations (CaCl
2, MgSO
4, and MgCl
2) and, to a lesser extent, with monovalent cations (KCl, NaNO
3, and Na
2SO
4). The statistical analysis revealed that there were significant differences in Ni
2+ biosorption capacities between the control and the experiments carried out in the presence of compounds with divalent cations (
p < 0.05). However, there were no statistically significant differences in the maximum Ni
2+ biosorption capacities between the control and the experiments performed with monovalent cation salts (KCl, NaNO
3, and Na
2SO
4) (
p > 0.05). The global performance indexes corroborated that the compounds containing divalent cations (CaCl
2, MgSO
4 and MgCl
2) affected the biosorption of Ni
2+ more negatively (from −24% to −20%) compared to the compounds containing monovalent cations (KCl, NaNO
3 and Na
2SO
4) (from −12% to −8%).
The background electrolytes at a concentration of 20 mM caused an even greater decrease in the Ni
2+ biosorption capacity compared to that obtained at 2 mM (
Figure 2c). The statistical analysis revealed that the differences between the Ni
2+ biosorption capacities of the control and the experiments carried out with all the background electrolytes were statistically significant (
p < 0.05). For all the experiments, the lowest Ni
2+ biosorption capacities were obtained when MgSO
4, CaCl
2, and MgCl
2 salts were present in the aqueous solutions (
Figure 2c). Additionally, the global performance indexes showed that the biosorption of Ni
2+ was more negatively affected at the highest concentration (20 mM) of background electrolytes and that the negative effect of the background electrolytes followed the order: MgSO
4 > CaCl
2 > MgCl
2 > Na
2SO
4 > NaNO
3 ≈ KCl.
These results clearly show that the Mg
2+ and Ca
2+ cations have a more pronounced effect on the biosorption of Ni
2+ from aqueous solutions by QCS compared to the Na
+ and K
+ cations. This could be attributed to the following reasons: First, the divalent Mg
2+ and Ca
2+ cations competed more efficiently with the Ni
2+ ions for the biosorption active sites present on the QCS surface compared to the monovalent Na
+ and K
+ cations. Second, the divalent Mg
2+ and Ca
2+ cations biosorb more easily on the QCS surface compared to the monovalent Na
+ and K
+ cations because of the higher electrostatic attraction. Finally, a Na
+ or K
+ ion biosorbed on the QCS surface occupies only one biosorption active site, whereas a Mg
2+ or Ca
2+ ion could occupy two sites, thus resulting in a greater decrease in the biosorption of Ni
2+ in the presence of MgCl
2, MgSO
4, and CaCl
2 [
24,
35,
46]. These reasons indicate that the biosorption active sites present on the surface of the QCS are not specific to Ni
2+ ions.
Additionally, the decrease in Ni
2+ biosorption can be owing to the presence of the Cl
- and SO
42- ions, which could increase the formation of nickel chloride and nickel sulfate complexes, respectively, thus decreasing the free Ni
2+ ions in solution [
46]. It was observed that the SO
42− ion had a more negative effect on the biosorption of Ni
2+ by QCS compared to the Cl
- and NO
3− ions.
Okoronkwo et al. [
46] reported that the Ca
2+ and Mg
2+ cations and the SO
42− anion cause a decrease in the ability of the Mexican sunflower (
Tithonia diversifolia) stems to bind the Ni
2+ ions in aqueous solutions. Ca
2+ and Mg
2+ cations also decreased the biosorption capacity of
Phellinus igniarius and
Fomes fomentarius for Methylene Blue and Rhodamine B dyes [
41]. Moreover, Mn
2+ and Ag
+ ions decreased the Cu
2+ removal efficiency of granular activated carbon [
47]. Likewise, an important decrease in biosorption capacity of
Macrocystis pyrifera for benzene and toluene was observed, when an artificial seawater solution (Instant Ocean®, Blacksburg, VA, USA) was used, probably because this solution contains different ions [
42].
Table 4 presents the equilibrium Ni
2+ biosorption capacity (q
e exp) for the control experiment (without background electrolytes) and at the different concentrations of background electrolytes. From
Table 4, it is evident that the highest biosorption capacity was obtained in the absence of background electrolytes. Moreover, the heavy metal biosorption capacity decreased as the concentration of background electrolytes increased, and the lowest equilibrium Ni
2+ biosorption capacities were obtained in the presence of MgSO
4, CaCl
2, and MgCl
2 at a concentration of 20 mM. The Elovich, fractional power, and pseudo-second order kinetic models described the kinetic profiles of the biosorption of Ni
2+ at the three different concentrations of background electrolytes more adequately, as evidenced by the lower SSE, RMSE, and AIC values and the higher r
2 values (
Table 4,
Table 5 and
Table 6).
The equilibrium Ni2+ biosorption capacities predicted by the pseudo-second order model were close to the values obtained experimentally, and they decreased as the concentration of the background electrolytes increased. It is also evident that background electrolytes affected the parameters of all the tested kinetic models, and this occurred to a greater extent at a concentration of 20 mM. Among the observed changes, the decrease in the specific biosorption rate of the fractional power model with increasing concentrations of background electrolytes should be noted.
The goodness of fit between Ni
2+ biosorption kinetics and pseudo-second order, Elovich, and fractional power models suggests that the biosorption process of Ni
2+ by QCS from aqueous solutions, containing different types and concentrations of background electrolytes, has a chemical process (chemisorption) as the rate-limiting step of the overall rate of heavy metal biosorption [
18,
29].
To our knowledge, the effect of ionic strength and background electrolytes on the parameters of kinetic models of the biosorption of Ni2+ has not been previously reported.
The results obtained in the present study clearly demonstrate the remarkable capacity of QCS to biosorb Ni2+ from aqueous solutions containing various types of impurities with different concentrations.
Additionally, the results of this study show that the impurities in the water greatly affect the performance of the biosorption of heavy metals. Furthermore, this study provides valuable information on the effect of ionic strength, the type, and concentration of background electrolytes on the biosorption of Ni2+, which is of great importance for the application of biosorption technology in the treatment of industrial wastewater contaminated with Ni2+.