A New Low-Cost and Reliable Method to Evaluate the Release of Hg0 from Synthetic Materials
Abstract
:1. Introduction
2. Mercury Pollution in the Former Mining Area of Abbadia San Salvatore
3. Materials and Methods
4. Results
4.1. Total and Leached Hg
4.2. Release of Hg0 from Anthropic Materials
4.3. Comparing the Hg0 Concentrations between “Painted” and “Raw” Materials
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harada, M. Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H. Mercury Pollution in Minamata; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Hong, Y.-S.; Kim, Y.-M.; Lee, K.E. Methylmercury Exposure and Health Effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeld, P.E.; Feng, L.G. Mercury, BPA, and Pesticides in Food. In Risks of Hazardous Wastes; Elsevier: Amsterdam, The Netherlands, 2011; pp. 223–235. [Google Scholar]
- Chan, H.M. Advances in Methylmercury Toxicology and Risk Assessment. Toxics 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broussard, L.A.; Hammett-Stabler, C.A.; Winecker, R.E.; Ropero-Miller, J.D. The Toxicology of Mercury. Lab. Med. 2002, 33, 614–625. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2011, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W. Mercury: Major issues in environmental health. Environ. Health Perspect. 1993, 100, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Pirrone, N. Mercury Fate and Transport in the Global Atmosphere: Emissions, Measurements and Models; Springer: New York, NY, USA, 2009; p. 627. [Google Scholar]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Spitz, K.; Trudinger, J. Mining and the Environment; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Vouk, V.B.; Fugaš, M.; Topolnik, Z. Environmental Conditions in the Mercury Mine of Idria. Occup. Environ. Med. 1950, 7, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Ladd, C.; Zusin, E.; Valic, F.; Almonte, J.B.; Gonzales, T.V. Adsorbtion and excretion of mercury in miners. J. Occup. Med. 1966, 3, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Bellander, T.; Merler, E.; Ceccarelli, F.; Boffetta, P. Historical Exposure to Inorganic Mercury at the smelter works of Abbadia San Salvatore, Italy. Am. Occup. Hyg. 1998, 42, 81–90. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013. [Google Scholar]
- Vaselli, O.; Nisi, B.; Rappuoli, D.; Cabassi, J.; Tassi, F. Gaseous Elemental Mercury and Total and Leached Mercury in Building Materials from the Former Hg-Mining Area of Abbadia San Salvatore (Central Italy). Int. J. Environ. Res. Public Health 2017, 14, 425. [Google Scholar] [CrossRef] [Green Version]
- Vaselli, O.; Rappuoli, D.; Bianchi, F.; Nisi, B.; Niccolini, M.; Esposito, A.; Cabassi, J.; Giannini, L.; Tassi, F. One hundred years of mercury exploitation at the mining area of Abbadia San Salvatore (Mt. Amiata, Central Italy): A methodological approach for a complex reclamation activity before the establishment of a new mining park. El patrimonio geològico y minero. Identidad y motor de desarrollo. Publicaciones del Instituto Geològico y minero de España Serie: Cuadernos del Museo Geominero, n. 29. In Proceedings of the XVII Congreso Internacional sobre Patrimonio Geológico y Minero, Almaden, Spain, 21–24 September 2017; pp. 1109–1126. [Google Scholar]
- Youcai, Z.; Sheng, H. Pollution Control and Resource Recovery: Industrial Construction and Demolition Wastes; Butterworth-Heinemann: Oxford, UK, 2016; p. 345. [Google Scholar]
- Gao, X.; Gu, Y.; Xie, T.; Liu, Y.; Huang, S.; Zhao, Y. Simulation of Gaseous Mercury Adsorption of different building materials. J. Civ. Arch. Environ. Eng. 2014, 36, 6. [Google Scholar]
- Conticelli, S.; Boari, E.; Burlamacchi, L.; Cifelli, F.; Moscardi, F.; Laurenzi, M.A.; Pedraglio, L.F.; Francalanci, L.; Benvenuti, M.G.; Braschi, E.; et al. Geochemistry and Sr-Nd-Pb isotopes of Monte Amiata Volcano, Central Italy: Evidence for magma mixing between high-K calc-alkaline and leucititic mantle-derived magmas. Ital. J. Geosci. 2015, 134, 266–290. [Google Scholar] [CrossRef]
- Laurenzi, M.A.; Braschi, E.; Casalini, M.; Conticelli, S. New 40Ar-39Ar dating and revision of the geochronology of the Monte Amiata Volcano, Central Italy. Ital. J. Geosci. 2015, 134, 255–265. [Google Scholar] [CrossRef]
- Ferrara, R.; Mazzolai, B.; Edner, H.; Svanberg, S.; Wallinder, E. Atmospheric mercury sources in the Mt. Amiata area, Italy. Sci. Total. Environ. 1998, 213, 13–23. [Google Scholar] [CrossRef]
- Cipriani, C.; Tanelli, G. Risorse minerarie ed industria estrattiva in Toscana. Econ. Atti Mem. Accad. Toscana Sci. Lett. Colomb. 1983, 48, 241–282. [Google Scholar]
- Bargagli, R.; Iosco, F.P.; Barghigiani, C. Assessment of mercury dispersal in an abandoned mining area by soil and lichen analysis. Water Air Soil Pollut. 1987, 36, 219–225. [Google Scholar] [CrossRef]
- Ferrara, R.; Maserti, B.; Breder, R. Mercury in abiotic and biotic compartments of an area affected by a geochemical anomaly (Mt. Amiata, Italy). Water Air Soil Pollut. 1991, 56, 219–233. [Google Scholar] [CrossRef]
- Vaselli, O.; Nisi, B.; Rappuoli, D.; Bianchi, F.; Cabassi, J.; Venturi, S.; Tassi, F.; Raco, B. Geochemical characterization of the ground waters from the former Hg-mining area of Abbadia San Salvatore (Mt. Amiata, central Italy): Criticalities and perspectives for the reclamation process. Ital. J. Geosci. 2015, 134, 304–322. [Google Scholar] [CrossRef]
- Gray, J.E.; Rimondi, V.; Costagliola, P.; Vaselli, O.; Lattanzi, P. Long-distance transport of Hg, Sb, and as from a mined area, conversion of Hg to methyl-Hg, and uptake of Hg by fish on the Tiber River basin, west-central Italy. Environ. Geochem. Health 2013, 36, 145–157. [Google Scholar] [CrossRef]
- Rimondi, V.; Chiarantini, L.; Lattanzi, P.; Benvenuti, M.; Beutel, M.; Venturi, S.; Colica, A.; Costagliola, P.; Gabbani, G.; Gray, J.E.; et al. Metallogeny, exploitation and environmental impact of the Mt. Amiata mercury ore district (Southern Tuscany, Italy). Ital. J. Geosci. 2015, 134, 323–336. [Google Scholar] [CrossRef]
- Chiarantini, L.; Rimondi, V.; Benvenuti, M.; Beutel, M.W.; Costagliola, P.; Gonnelli, C.; Lattanzi, P.; Paolieri, M. Black pine (Pinus nigra) barks as biomonitors of airborne mercury pollution. Sci. Total. Environ. 2016, 569, 105–113. [Google Scholar] [CrossRef]
- Chiarantini, L.; Rimondi, V.; Bardelli, F.; Benvenuti, M.; Cosio, C.; Costagliola, P.; Di Benedetto, F.; Lattanzi, P.; Sarret, G. Mercury speciation on Pinus Nigra barks from Monte Amiata (Italy): An X-ray absorption spectroscopy study. Environ. Poll. 2017, 227, 83–88. [Google Scholar] [CrossRef]
- Venturi, S.; Vaselli, O.; Tassi, F.; Nisi, B.; Pennisi, M.; Cabassi, J.; Bicocchi, G.; Rossato, L. Geochemical and isotopic evidences for a severe anthropogenic boron contamination: A case study from Castelluccio (Arezzo, central Italy). Appl. Geochem. 2015, 63, 146–157. [Google Scholar] [CrossRef]
- Sholupov, S.E.; Pogarev, S.E.; Ryzhov, V.V.; Mashyanov, N.R.; Stroganov, A. Zeeman atomic absorption spectrometer RA-915+ for direct determination of mercury in air and complex matrix samples. Fuel Process. Technol. 2004, 85, 473–485. [Google Scholar] [CrossRef]
- Glasser, F.P.; Marchand, J.; Samson, E. Durability of concrete—Degradation phenomena involving detrimental chemical reactions. Cem. Concr. Res. 2008, 38, 226–246. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mahmoud, E.; Yamin, M.; Patibandla, V.C. Experimental study on Portland cement pervious concrete mechanical and hydrological properties. Constr. Build. Mater. 2014, 50, 524–529. [Google Scholar] [CrossRef]
- Pepe, M.; Filho, R.D.T.; Koenders, E.A.; Martinelli, E. A novel mix design methodology for Recycled Aggregate Concrete. Constr. Build. Mater. 2016, 122, 362–372. [Google Scholar] [CrossRef]
- Ouyang, K.; Shi, C.; Chu, H.; Guo, H.; Song, B.; Ding, Y.; Guan, X.; Zhu, J.; Zhang, H.; Wang, Y.; et al. An overview on the efficiency of different pretreatment techniques for recycled concrete aggregate. J. Clean. Prod. 2020, 263, 121264. [Google Scholar] [CrossRef]
- Spedding, D.; Hamilton, R. Adsorption of mercury vapor by indoor surfaces. Environ. Res. 1982, 29, 30–41. [Google Scholar] [CrossRef]
- Bahadori, A. Essentials of Coating, Painting, and Lining for the Oil, Gas and Petrochemical Industries; Elsevier: Amsterdam, The Netherlands, 2015; p. 731. [Google Scholar]

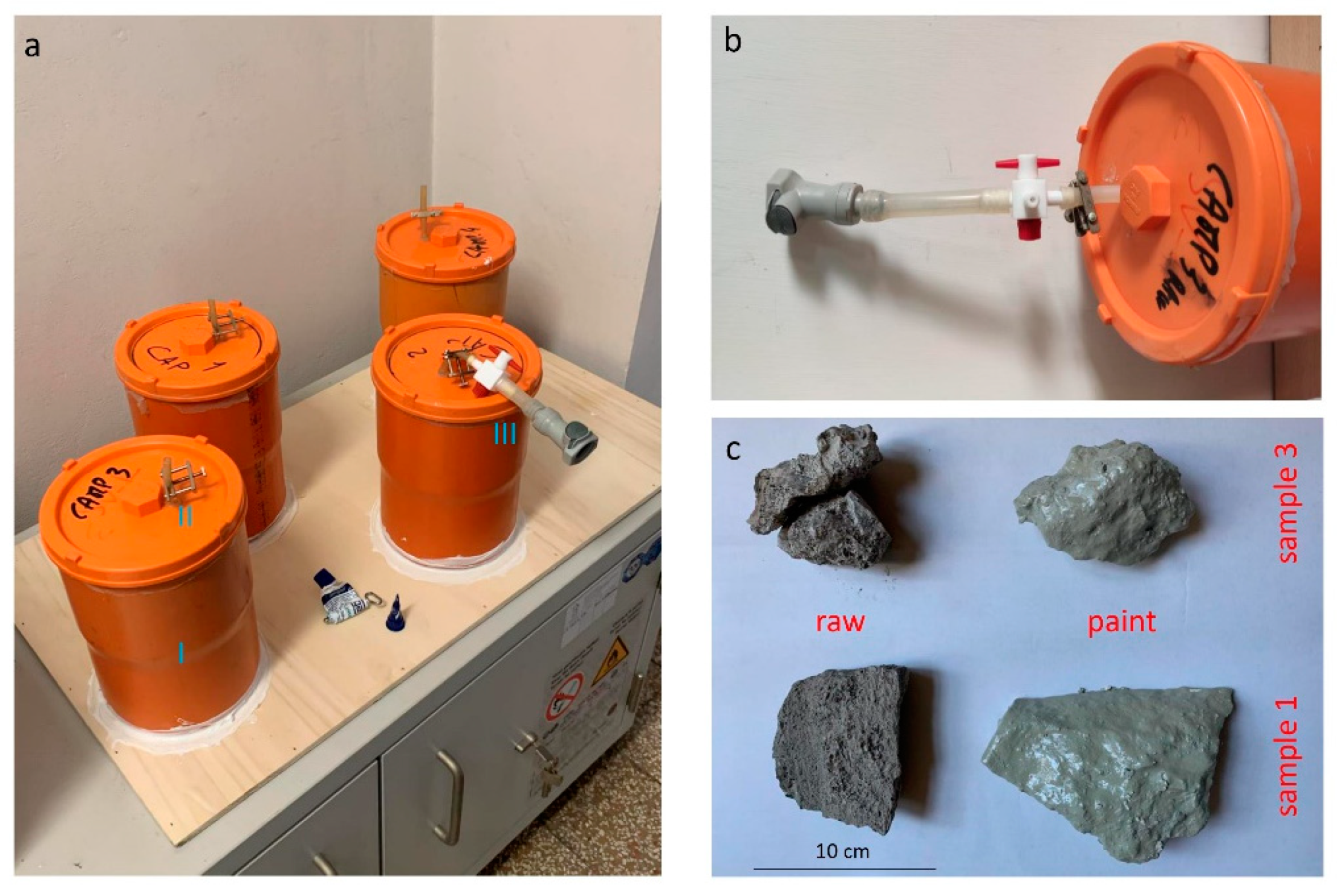
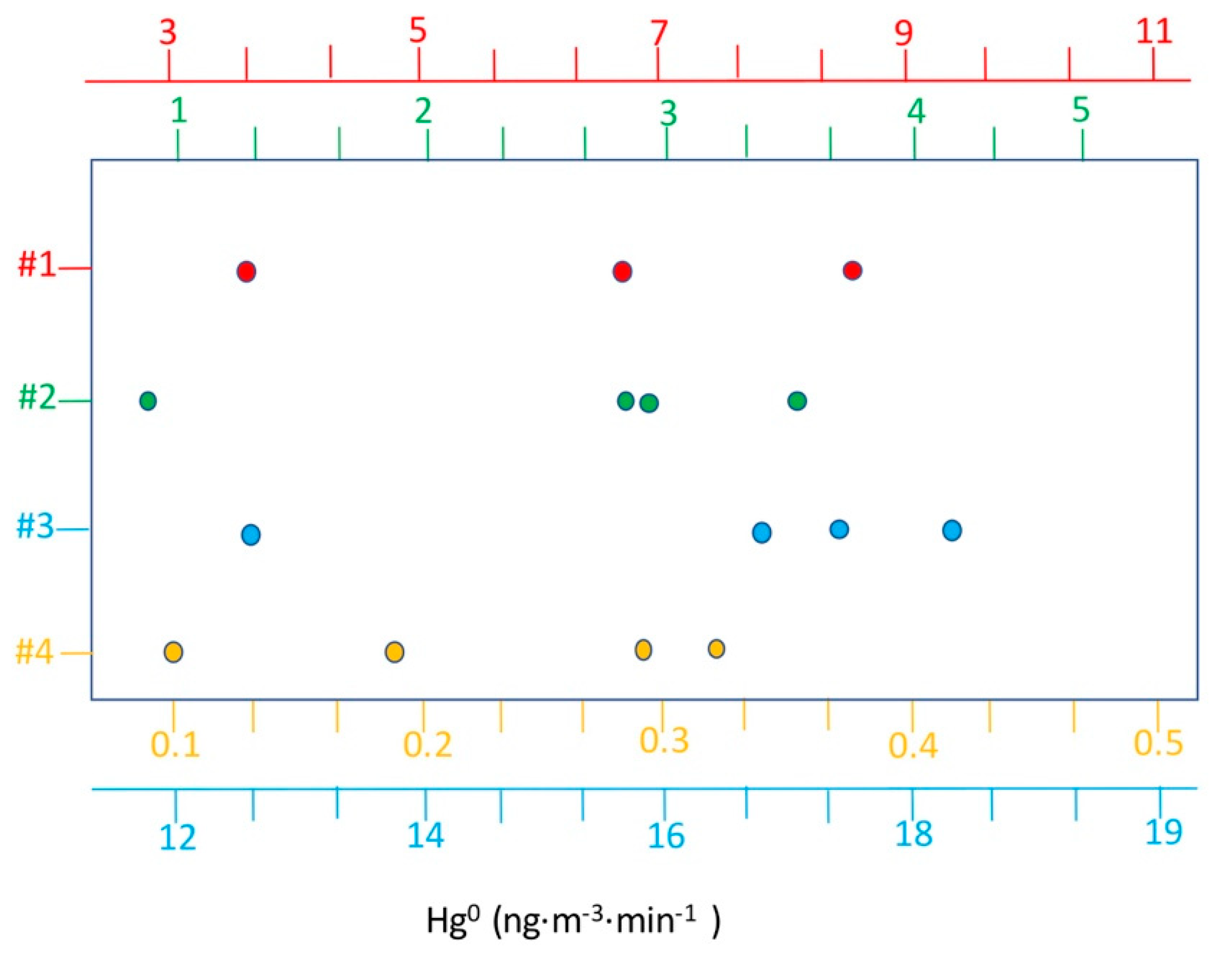

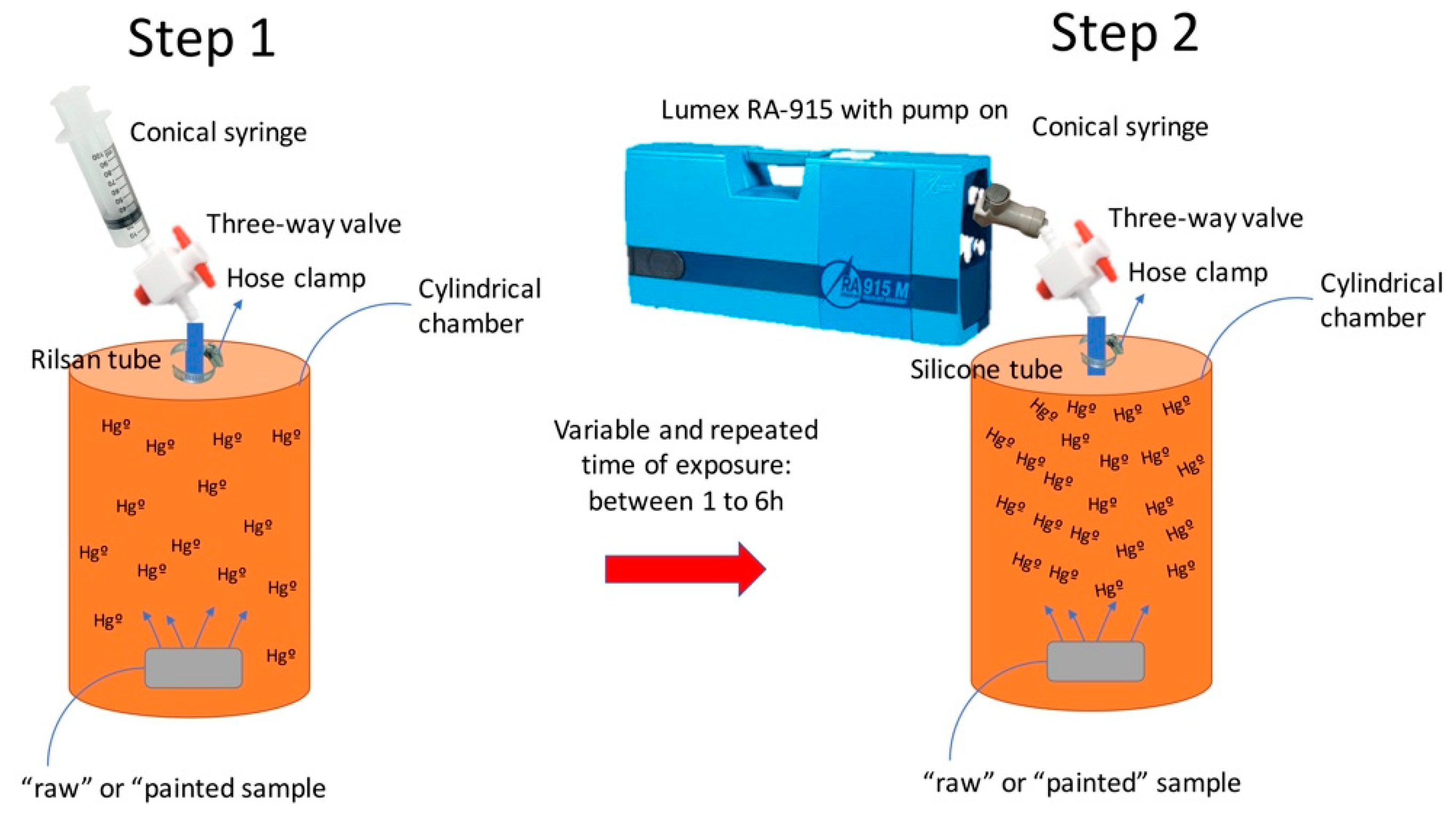
| ID | Material | Hgtot | Hgleachate | Hg tot/Hg Leachate |
|---|---|---|---|---|
| Type | (mg·kg−1) | (μg·L−1) | ||
| #1 | Concrete from the first pillar | 106 | 483 | 219 |
| #2 | Concrete from the second pillar | 79 | 19.9 | 3969 |
| #3 | Wall rock from the fume tubing | 242 | 1350 | 179 |
| #4 | Roof tile | 16.9 | 0.5 | 33,800 |
| Exposure Time | Hg0 | ||
|---|---|---|---|
| ID | Date | (h) | (in ng·m−3) |
| #1 | 24 December 2019 | 6.15 | 3221 |
| #2 | 24 December 2019 | 5.22 | 923 |
| #3 | 24 December 2019 | 6.20 | 6621 |
| #4 | 24 December 2019 | 5.51 | 115 |
| Exposure Time | Hg0 | ||
| ID | Date | (h) | (in ng·m−3) |
| #1 | 3 January 2020 | 1.48 | n.d. |
| #2 | 3 January 2020 | 1.41 | 358 |
| #3 | 3 January 2020 | 2.03 | 1570 |
| #4 | 3 January 2020 | 1.40 | 29 |
| Exposure Time | Hg0 | ||
| ID | Date | (h) | (in ng·m−3) |
| #1 | 15 January 2020 | 6.45 | 2625 |
| #2 | 15 January 2020 | 6.32 | 323 |
| #3 | 15 January 2020 | 6.41 | 7563 |
| #4 | 15 January 2020 | 6.35 | 74 |
| Exposure Time | Hg0 | ||
| ID | Date | (h) | (in ng·m−3) |
| #1 | 22 January 2020 | 6.34 | 1380 |
| #2 | 22 January 2020 | 6.47 | 1086 |
| #3 | 22 January 2020 | 6.37 | 6670 |
| #4 | 22 January 2020 | 7.00 | 45 |
| ID | Starting Day | Exposure Time (h) | Hg0 (ng·m−3) | Starting Day | Exposure Time (h) | Hg0 (ng·m−3) | Starting Day | Exposure Time (h) | Hg0 (ng·m−3) |
|---|---|---|---|---|---|---|---|---|---|
| #1 raw | 5 March 2020 | 2.06 | 278 | 5 March 2020 | 6.17 | 357 | 5 March 2020 | 38.22 | 521 |
| #1 painted | 5 March 2020 | 2.04 | 774 | 5 March 2020 | 6.17 | 1938 | 5 March 2020 | 38.2 | 6706 |
| #3 raw | 5 March 2020 | 2.02 | 1496 | 5 March 2020 | 6.17 | 2228 | 5 March 2020 | 38.18 | 5373 |
| #3 painted | 5 March 2020 | 2.00 | 1240 | 5 March 2020 | 6.17 | 3131 | 5 March 2020 | 38.16 | 11,446 |
| ID | Starting Day | Exposure Time (h) | Hg0 (ng·m−3) | Starting Day | Exposure Time (h) | Hg0 (ng·m−3) | Starting Day | Exposure Time (h) | Hg0 (ng·m−3) |
| #1 raw | 7 March 2020 | 171.30 | n.d. | 14 March 2020 | 69.00 | 1035 | 17 March 2020 | 95.40 | 2271 |
| #1 painted | 7 March 2020 | 171.28 | 6470 | 14 March 2020 | 68.58 | 3129 | 17 March 2020 | 95.40 | 3630 |
| #3 raw | 7 March 2020 | 171.26 | 7387 | 14 March 2020 | 68.56 | 8202 | 17 March 2020 | 95.40 | 8838 |
| #3 painted | 7 March 2020 | 171.24 | 12,587 | 14 March 2020 | 68.54 | 11,472 | 17 March 2020 | 95.40 | 12,632 |
| ID | Starting day | Exposure time (h) | Hg0 (ng·m−3) | ||||||
| #1 raw | 21 March 2020 | 47.20 | 420 | ||||||
| #1 painted | 21 March 2020 | 47.18 | 3098 | ||||||
| #3 raw | 21 March 2020 | 47.16 | 7524 | ||||||
| #3 painted | 21 March 2020 | 47.14 | 11,125 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzaroni, M.; Nisi, B.; Rappuoli, D.; Cabassi, J.; Vaselli, O. A New Low-Cost and Reliable Method to Evaluate the Release of Hg0 from Synthetic Materials. Processes 2020, 8, 1282. https://doi.org/10.3390/pr8101282
Lazzaroni M, Nisi B, Rappuoli D, Cabassi J, Vaselli O. A New Low-Cost and Reliable Method to Evaluate the Release of Hg0 from Synthetic Materials. Processes. 2020; 8(10):1282. https://doi.org/10.3390/pr8101282
Chicago/Turabian StyleLazzaroni, Marta, Barbara Nisi, Daniele Rappuoli, Jacopo Cabassi, and Orlando Vaselli. 2020. "A New Low-Cost and Reliable Method to Evaluate the Release of Hg0 from Synthetic Materials" Processes 8, no. 10: 1282. https://doi.org/10.3390/pr8101282
APA StyleLazzaroni, M., Nisi, B., Rappuoli, D., Cabassi, J., & Vaselli, O. (2020). A New Low-Cost and Reliable Method to Evaluate the Release of Hg0 from Synthetic Materials. Processes, 8(10), 1282. https://doi.org/10.3390/pr8101282








