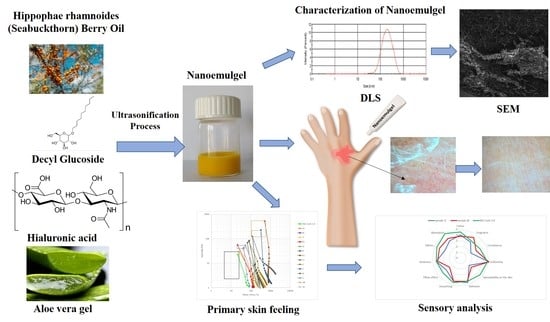
Nanoemulsion Gel Formulation Optimization for Burn Wounds: Analysis of Rheological and Sensory Properties
Abstract
1. Introduction
1.1. Emulsions
1.2. Gels
1.3. Ointments
1.4. Aerosols
1.5. Nanostructured Systems
2. Results
2.1. Characterization of the Obtained Nanoemulsions
2.2. Nanoemulgel Characteristics
2.3. Rheological Properties of the Preparations Supporting Burn Healing
- τ—shear stress, Pa
- τy—yield stress, Pa
- k—consistency parameter, Pa·sn
- γ—shear rate, s−1
- n—flow behavior index, -.
2.4. Sensory Analysis of the Preparations Supporting Burned Skin Regeneration
2.4.1. The Correlation of Feelings on the Skin after the Application of Preparations and Rheological Measurements
2.4.2. Sensory Assessment Profile of Selected Preparations
2.5. Effect of the Gel Nanoemulsion and Market Preparations on the Skin after Burns
3. Discussion
3.1. Discussion of the Rheological Results
Correlation Relating Rheological and Sensory Measurements
3.2. Effect of the Gel Nanoemulsion and Market Preparations on the Skin after Burns
3.2.1. The Active Components of the Nanoemulgel Supporting Burned Skin Regeneration
3.2.2. Effectiveness of Skin Regeneration after Burns–Analysis of Functional Parameters of the Skin
4. Materials and Methods
4.1. Materials
4.2. Nanoemulsions Gel Preparation and Characterization
4.2.1. Determination of Nanoemulsion Droplet Size
4.2.2. pH Measurements
4.2.3. SEM Analysis
4.2.4. Rheological Studies
4.2.5. Sensory Analysis
4.2.6. Test of the Effect of the Nanoemulsion and Market Preparations on the Skin Condition after Burns
4.2.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
Abbreviations
| NEGs | Nanoemulgels |
| PDI | Polydispersity index |
| SEM | Scanning electron microscope |
| DLS | Dynamic light scattering |
References
- D’Arpa, P.; Leung, K.P. Toll-Like Receptor Signaling in Burn Wound Healing and Scarring. Adv. Wound Care 2017, 6, 330–343. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prele, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Djemaa, F.G.B.; Bellassoued, K.; Zouari, S.; El Feki, A.; Ammar, E. Antioxidant and wound healing activity of Lavandula aspic L. ointment. J. Tissue Viability 2016, 25, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.A.; Zangabad, P.S.; Basri, S.M.; Zangabad, K.S.; Ghamarypou, A.; Aref, A.R.; Karimi, M.; Hamblinn, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Hoffmann, J.; Gendrisch, F.; Schempp, C.M.; Wolfle, U. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E. Cosmetic Emulsions, 1st ed.; Politechnika Krakowska: Kraków, Poland, 2019; pp. 10–13. [Google Scholar]
- Moghbela, A.; Ghalamborb, A.; Allipanaha, S. Wound Healing and Toxicity Evaluation of Aloe vera Cream on Outpatients with Second Degree Burns. Iran. J. Pharm. Sci. 2007, 3, 157–160. [Google Scholar]
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440. [Google Scholar] [CrossRef]
- McLaughlin, E.S.; Paterson, A.O. BURNS, Prevention, Causes and Treatment, 1st ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Yosipovitch, G.; Szolar, C.; Hui, X.Y. Effect of topically applied menthol on thermal, pain and itch sensations and biophysical properties of the skin. Arch. Dermatol. Res. 1996, 288, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R.; Li, X.; Jasti, B.R. Semi-solid dosagaes: Ointments, creams, and gels. In Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing, 1st ed.; Gad, S.C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–46. [Google Scholar] [CrossRef]
- Chang, R.; Raw, A.; Lionberger, R.; Yu, L. Generic Development of Topical Dermatologic Products: Formulation Development, Process Development, and Testing of Topical Dermatologic Products. AAPS J. 2013, 15. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, F.; Chen, L.; Shu, B.; Zhai, Q.; Wu, J.; Liu, X.; Qi, S.; Xu, Y. Negatively-charged aerosol improves burn wound healing by promoting eNOS-dependent angiogenesis. Am. J. Transl. 2018, 10, 246–255. [Google Scholar]
- Kane, A.A.; Rudolph, I.; Saydjari, S.S.; Rosenhaus, H.; Fink, H. Open method treatment of burns with aerosol spray. Am. J. Surg. 1959, 97, 211–216. [Google Scholar] [CrossRef]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 2010, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Miastkowska, M.; Lasoń, E. Selected Skin Delivery Systems; Cracow University of Technology Wydawnictwo PK: Kraków, Poland, 2020; pp. 36–53. [Google Scholar]
- Mao, Y.; Chen, X.; Xu, B.; Shen, Y.; Ye, Z.; Chaurasiya, B.; Liu, L.; Li, Y.; Xinga, X.; Chend, D. Eprinomectin nanoemulgel for transdermal delivery against endoparasites and ectoparasites: Preparation, in vitro and in vivo evaluation. Drug Deliv. 2019, 26, 1104–1114. [Google Scholar] [CrossRef]
- Aithal, G.C.; Nayak, U.Y.; Mehta, C.; Narayan, R.; Gopalkrishna, P.; Pandiyan, S.; Garg, S. Localized in Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations. Molecules 2018, 23, 1363. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions—Advances in formulation, characterization and applications in drug delivery. In Application of Nanotechnology in Drug Delivery, 1st ed.; Sezer, A.D., Ed.; IntechOpen: London, UK, 2014; pp. 77–126. [Google Scholar] [CrossRef]
- Miastkowska, M.; Sikora, E.; Ogonowski, J.; Zielina, M.; Łudzik, A. The kinetic study of isotretinoin release from nanoemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 63–68. [Google Scholar] [CrossRef]
- Akhtar, N.; Verma, A.; Pathak, K. Exploring preclinical and clinical effectiveness of nanoformulations in the treatment of atopic dermatitis: Safety aspects and patent reviews. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B.J. Production of sub-micron emulsions by ultrasound and microfluidization techniques. Food Eng. 2007, 82, 478–488. [Google Scholar] [CrossRef]
- Li, P.; Chiang, B. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason. Sonochem. 2012, 19, 192–197. [Google Scholar] [CrossRef]
- Tang, S.Y.; Shridharan, P.; Sivakumar, M. Impact of process parameters in the generation of novel aspirin nanoemulsions—Comparative studies between ultrasound cavitation and microfluidiser. Ultrason. Sonochem. 2013, 20, 485–497. [Google Scholar] [CrossRef]
- Sivakumar, M.; Tang, S.Y.; Tan, K.W. Cavitation technology—A greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason. Sonochem. 2014, 21, 2069–2083. [Google Scholar] [CrossRef]
- Rebolleda, S.; Sanz, M.T.; Benito, J.M.; Beltrán, S.; Escudero, I.; González San-José, M.L. Formulation and characterisation of wheat bran oil-in-water nanoemulsions. Food Chem. 2015, 167, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Wooster, T.J.; Ashokkumar, M.; Balachandran, S.; Mawson, R.; Simons, L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. 2008, 9, 170–175. [Google Scholar] [CrossRef]
- Tang, S.Y.; Manickam, S.; Wei, T.K.; Nashiru, B. Formulation development and optimization of a novel cremophor EL-based nanoemulsion using ultrasound cavitation. Ultrason. Sonochem. 2012, 19, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Peshkovsky, A.S.; Peshkovsky, S.L.; Bystryak, S. Scalable high-power ultrasonic technology for the production of translucent nanoemulsions. Chem. Eng. Process 2013, 69, 77–82. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Barreto, S.M.; Ostrosky, E.A.; da Rocha-Filho, P.A.; Veríssimo, L.M.; Ferrari, M. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) mill extract as moisturizing agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Brummer, R.; Godersky, S. Rheological studies to objectify sensations occurring when cosmetic emulsion are applied to the skin. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 89–94. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Ptaszek, A.; Kruk, J. Effective tool for assessment of the quality of barrier creams—Relationships between rheological, textural and sensory properties. Regul. Toxicol. Pharmacol. 2019, 103, 113–123. [Google Scholar] [CrossRef]
- Bekker, M.; Webber, G.V.; Louw, N.R. Relating rheological measurements to primary and secondary skin feeling when mineral-based and Fischer-Tropsch wax-based cosmetic emulsion and jellies are applied to the skin. Int. J. Cosm. Sci. 2013, 35, 354–361. [Google Scholar] [CrossRef]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Surf. B Biointerfaces 2013, 102, 371–378. [Google Scholar] [CrossRef]
- Barnes, H.A. Rheology of emulsions, In Emulsions: Structure, Stability and Interactions, 1st ed.; Petsev, D., Ed.; Elsevier: Albuquerque, NM, USA, 2004; pp. 721–760. [Google Scholar]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing natural and synthetic polymers: Relationships between both data. Colloids Surf. A Physicochem. Eng. Asp. 2013, 150–163. [Google Scholar] [CrossRef]
- Lemaitre-Aghazarian, V.; Piccerelle, P.; Reynier, J.P.; Joachim, J.; Phan-Tan-Luu, R.; Sergent, M. Texture optimization of water-in-oil emulsions. Pharm. Dev. Technol. 2004, 9, 125–134. [Google Scholar] [CrossRef]
- Barnes, H.A. Rheology of emulsions—A review. Colloids Surf. A Physicochem. Eng. Asp. 1994, 91, 89–95. [Google Scholar] [CrossRef]
- Miastkowska, M.; Banach, M.; Pulit-Prociak, J.; Sikora, E.; Głogowska, A.; Zielina, M. Statistical analysis of optimal ultrasound emulsification parameters in thistle-oil nanoemulsions. J. Surfactants Deterg. 2017, 20, 233–246. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem. Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.J.; King, K.C. Acute and Chronic Thermal Burn Evaluation and Management; StatPearls: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430730/ (accessed on 18 September 2020).
- Haj-Shafiei, S.; Ghosh, S.; Rousseau, D. Kinetic stability and rheology of wax-stabilized water-in-oil emulsions at different water cuts. J. Colloid Interface Sci. 2013, 410, 11–20. [Google Scholar] [CrossRef]
- Hodge, S.M.; Rousseau, D. Flocculation and coalescence in water-in-oil emulsions stabilized by paraffin wax crystals. Food Res. 2003, 36, 695–702. [Google Scholar] [CrossRef]
- Samala, M.L.; Sridevi, G. Role of Polymers as Gelling Agents in the Formulation of Emulgels. Polym. Sci. 2016, 1, 2. [Google Scholar]
- Eid, A.M.; El-Enshasy, H.A.; Aziz, R.; Elmarzugi, N.A. Preparation, Characterization and Anti-Inflammatory Activity of Swietenia macrophylla Nanoemulgel. J. Nanomed. Nanotechnol. 2014, 5, 2. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Gibas, K.; Osak, E. Consistency as quality parameter of hydrophobic skin protection preparations. Pol. J. Cosmetol. 2020, 23, 27–34. [Google Scholar]
- Kurpiewska, J.; Liwkowicz, J. The composition of waterproof barrier creams’s ingredients and their barrier properties. Chemik 2012, 66, 991–996. [Google Scholar]
- Goik, U.; Goik, T.; Załęska, I. Beeswax properties and its use in cosmetics and cosmetology. Aesthetic Cosmetol. 2016, 5, 617–622. [Google Scholar]
- Moravkova, T.; Fillip, P. The influence of emulsifier on the rheological and sensory properties of cosmetic lotions. Adv. Mater Sci. Eng. 2013, 168503. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Kurpiewska, J.; Kułaszka, A. Rheological and sensory properties of hydrophilic skin protection gels based on polyacrylates. Int. J. Occup. Saf. Ergon. 2018, 24, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierski, M.; Puchała, J.; Chrapusta-Klimeczek, A.; Mańkowski, P.; Jankowski, A. The evaluation of efficiency of Hydrofiber® dressing with ionic silver Aquacel Ag® in the local treatment of burn. Klinika Zakażeń/Zakażenia 2005, 2, 108–113. [Google Scholar]
- Upadhyay, N.K.; Kumar, R.; Mandotra, S.K.; Meena, R.N.; Sidddiqui, M.S.; Sawhney, R.C.; Gupta, A. Safety and healing efficacy of Sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food Chem. Toxicol. 2009, 47, 1146–1153. [Google Scholar] [CrossRef]
- Koskovac, M.; Cupara, S.; Kipic, M. Sea Buckthorn Oil-A Valuable Source for Cosmeceuticals. Cosmetics 2017, 4, 40. [Google Scholar] [CrossRef]
- Cupara, S.M.; Ninkovic, M.B.; Knezevic, M.G.; Vuckovic, I.M.; Jankovic, S.M. Wound healing potential of liquid crystal structure emulsion with sea buckthorn oil. Health Med. 2011, 5, 1218–1223. [Google Scholar]
- Ebner, F.; Heller, A.; Rippke, F.; Tausch, I. Topical use of dexpanthenol in skin disorders. Am. J. Clin. Dermatol. 2002, 3, 427–433. [Google Scholar] [CrossRef]
- Araújo, L.U.; Grabe-Guimarães, A.; Mosqueira, V.C.; Carneiro, C.M.; Silva-Barcellos, N.M. Profile of wound healing process induced by allantoin. Acta Cir. Bras. 2010, 25, 460–466. [Google Scholar] [CrossRef]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The Effect of Aloe Vera Clinical Trials on Prevention and Healing of Skin Wound: A Systematic Review. Iran J. Med. Sci. 2019, 44, 1. [Google Scholar] [PubMed]
- Reynolds, T.; Dweck, A.C. Aloe vera leaf gel: A Review Update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Price, R.D.; Myers, S.; Leigh, I.M.; Navsaria, H.A. The Role of Hyaluronic Acid in Wound Healing. Assessment of Clinical Evidence. Am. J. Clin. Dermatol. 2005, 6, 393–402. [Google Scholar] [CrossRef]
- Islam, M.T.; Khan, S.H.; Hasan, M. Aloe vera gel: A new pigment printing thickener. Coloration Technol. 2016, 132, 255–264. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- Kong, M.; Park, H.J. Stability investigation of hyaluronic acid based nanoemulsion and its potential as transdermal carrier. Carbohydr. Polym. 2011, 83, 1303–1310. [Google Scholar] [CrossRef]
- UL Prospector. Available online: https://www.ulprospector.com/en/na/PersonalCare/Detail/100296/1003443/Decyl-Glucoside (accessed on 18 September 2020).
- Savic, S.; Pantelic, I.; Lukic, M.; Markovic, B.; Milic, J. Behind the alkyl polyglucoside-based structures: Lamellar liquid crystalline and lamellar gel phases in different emulsion systems. In Alkyl Polyglucosides: From Natural-Origin Surfactants to Prospective Delivery Systems; Pantelic, I., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 21–52. [Google Scholar] [CrossRef]
- Frew, Q.; Rennekampff, H.; Dziewulski, P.; Moiemen, N.; BBW-11 Study Group; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef]
- Al-Abboodi, A.; Zhang, S.; Al-Saady, M.; Ong, J.W.; Chan, P.P.Y.; Fu, J. Printing in situ tissue sealant with visible-light-crosslinked porous hydrogel. Biomed. Mater. 2019, 14, 45010. [Google Scholar] [CrossRef] [PubMed]
- ASTM E1490-19. Standard Guide for Two Sensory Descriptive Analysis Approaches for Skin Creams and Lotions; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Arct, J.; Ratz-Łyko, A.; Mieloch, M.; Witulska, M. Evaluation of Skin Colouring Properties of Curcuma Longa Extract. Indian J. Pharm. Sci. 2014, 76, 374–378. [Google Scholar]







| Phase | Raw Material Name | Composition (%mass) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | N10 | N11 | N12 | N13 | N14 | ||
| A | Decyl glucoside | 4.0 | 6.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 6.0 | 6.0 | 6.0 | 6.0 | 4.0 | 6.0 |
| Water | 86.0 | 84.0 | 91.0 | 86.0 | 91.0 | 91.0 | 91.0 | 91.0 | 89.0 | 89.0 | 89.0 | 89.0 | 93.0 | 91.0 | |
| B | Sea-buckthorn berry oil | 10.0 | 10.0 | 5.0 | 10.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 3.0 | 3.0 |
| Process parameters | |||||||||||||||
| Mixing speed (rpm) | 300 | 300 | 300 | 300 | 300 | 300 | 500 | 500 | 300 | 300 | 500 | 500 | 500 | 500 | |
| Pre-emulsification time (min) | 10 | 10 | 10 | 15 | 15 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Ultrasonic homogenization time (s) | 60 | 60 | 60 | 60 | 60 | 120 | 60 | 120 | 60 | 120 | 60 | 120 | 120 | 120 | |
| Stability | |||||||||||||||
| - creaming or phase separation +/− stable after 7 days + stable after 14 days | − | − | − | − | − | − | − | +/− | − | − | − | − | +/− | + | |
| Phase | Raw Material Name | Sample Name | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N14-A | N14-B | N14-C | N14-D | N14-E | N14-F | N14-G | NG-Carb-0.5 | NG-Carb-1.0 | ||
| A | Decyl glucoside | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Water | 79.0 | 83.8 | 90.0 | 90.0 | 90.0 | 90.0 | 88.8 | 86.8 | 86.3 | |
| Hyaluronic acid 1% | 5.0 | - | 1.0 | - | - | - | 1.0 | 1.0 | 1.0 | |
| Allantoin | 1.0 | 1.0 | - | 1.0 | - | - | - | - | - | |
| Aloe vera gel | 1.0 | 1.0 | - | - | 1.0 | - | 1.0 | 1.0 | 1.0 | |
| D-panthenol 75% | 4.0 | 4.0 | - | - | - | 1.0 | - | - | - | |
| Sodium benzoate | 0.1 | 0.1 | - | - | - | - | 0.1 | 0.1 | 0.1 | |
| B | Vitamin E | 0.1 | 0.1 | - | - | - | - | 0.1 | 0.1 | 0,1 |
| Sea-buckthorn berry oil | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | |
| C | Carbomer | - | - | - | - | - | - | - | 0.5 | 1.0 |
| 5% r-r citric acid | - | - | - | - | - | - | - | 0.5 | 0.5 | |
| Stability | - | - | + | - | + | - | + | + | + | |
| Nanoemulsion Name | Storage Time (Days) | Particle Diameter (nm) | PDI |
|---|---|---|---|
| N14 | 1 | 214.0 ± 0.7 | 0.214 ± 0.01 |
| 14 | 217.2 ± 1.1 | 0.197 ± 0.20 | |
| N14-C | 1 | 213.5 ± 1.6 | 0.208 ± 0.02 |
| 14 | 236.5 ± 2.3 | 0.173 ± 0.01 | |
| N14-E | 1 | 202.2 ± 2.6 | 0.212 ± 0.02 |
| 14 | 228.9 ± 1.5 | 0.185 ± 0.02 | |
| N14-G | 1 | 211.5 ± 1.4 | 0.205 ± 0.01 |
| 14 | 227.4 ± 1.8 | 0.288 ± 0.01 |
| Item | Preparation Name | Physicochemical Form | Scope of Application | Composition (acc. to the Information Provided by the Manufacturer) |
|---|---|---|---|---|
| 1 | A | balsam | 1st degree burns (sunburns) | Aqua, Panthenol, Cyclopentasiloxane, Cetearyl Ethylhexanoate, Glycerin, Aloe Barbadensis Leaf Juice Powder, Macadamia Ternifolia Seed Oil, Aleurites Moluccana Seed Oil, Sodium Hyaluronate, Tocopherol, Tocopheryl Acetate, Sodium Polyacrylate, Dimethicone, PEG-40 Hydrogenated Castor Oil, Polyacrylamide, C13-14 Isoparaffin/Laureth-7, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanoloamine, Phenoxyethanol, Ethylhexylglycerin, DMDM Hydantoin, Parfum, Butylphenyl Methylpropional, Citronellol, Hexyl Cinnamal, Limonene, Linalool |
| 2 | B | balsam | 1st degree burns (sunburns) | Aqua, Ethylhexyl Stearate, Glycerin, Caprylic/Capric Trigliceride, Polyglyceryl-3 Methylglucose Distearate, Prunus Amygdalus Dulcis Oil, Gossypium Herbaceum Seed Oil, Cetearyl Alcohol, Dimethicone, Poliacrylamide, C13-14 Isoparaffin, Laureth-7, Panthenol, Parfum, Allantoin, Tocopheryl Acetate, Soluble Collagen, Glycogen, Hydrolised Elastin, Sorbic Acid, Sodium Succinate, Bisabolol, Disodium EDTA, Citric Acid, Phenoxyethanol, DMDM Hydantoin, Methylparaben, Propylparaben, Coumarin, Eugenol |
| 3 | C | hydrogel | 1st and 2nd degree burns | Carbomer, Octylene Glycol, Glycerin, Polyethylene Glycol, Sodium Hydroxide, Aqua |
| 4 | D | hydrogel | 1st and 2nd degree burns (sunburns and thermal burns) | Aqua, Glycerin, Propylene Glycol, Ethanol, Triethanolamine, Carbomer, 1-Methylnicotinamide Chloride, Methylparaben, Eucalyptus Globulus Leaf Oil |
| 5 | E | cream | Scars after burns and surgery | Active substances: Allium Cepa Extract, Chamomilla Recutita Extract, Sodium Heparin, Allantoin Excipients: Cetearyl Alcohol (and) Sodium Lauryl Sulfate, Paraffinum Liquidum, Petrolatum, Mono- and Diglycerides (and) Potassium Stearate, Glycerin, Methyl Paraydroxybenzoate, Propyl Parahydroxybenzoate, Aqua |
| 6 | F | cream | 1st and 2nd degree burns (sunburns, thermal burns, after radiation therapy) | Aqua, Olea Europaea Fruit Oil, Sorbitan Olivate, Cetearyl Olivate, Glycerin, Panthenol 75%, Isostearyl Neopentanoate, Myreth-3 Myristate, Scutellaria Baicalensis Root Extract, Sorbeth-30, Squalane, Silybum Marianum Extract, Carbomer, Sodium Hydroxymethylglycinate 50%, Menthol, Sodium Hyaluronate |
| 7 | G | cream | Skin irritated after sunbathing, acceleration of wound healing, stimulation of regeneration and restoration of the epidermis | Aqua, Glycine Soja (Soybean) Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Cera Alba, Vitis Vinifera (Grape) Seed Oil, Hippophae Rhamnoides (Seabuckthorn) Berry Oil, Betulin, Sodium Stearate, Citric Acid |
| 8 | H | ointment | 1st degree burns (sunburns, after radiation and photo therapy) | Active substances: Allantoin, D-Panthenol Excipients: Lanolin, Parraffinum Liquidum, Petrolatum, Ethyl Parahydroxybenzoate, Aqua |
| 9 | I | ointment | 1st degree burns (thermal) | Petrolatum, Lanolin, Linum Usitatissimum Oil, Propolis, Parfum, Linalool, Citronellol, Limonene, Coumarin, Geraniol |
| 10 | J | ointment | 1st degree burns Skin sanitizing after burn | Zinci Oxydum, Tormentillae Extractum Fluidum, Ammonii Bituminosulfonas, Natrii Tetraboras, Vaselinum Flavum, Lanolinum, Aqua, Vanilinum |
| 11 | K | lotion | 1st degree burns (sunburns) | Aqua, Hexyl Laurate, PPG-15 Stearyl Ether, Butyrospermum Parkii (Shea Butter), Glycerin, Steareth-2, Steareth-21, Dimethicone, Panthenol, Menthyl Lactate, Tocopheryl Acetate, Calcium Gluconate, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Polysorbate 80, Cetearyl Alcohol, Carbomer, Phenoxyethanol, Methylparaben, Ethylparaben, Propylene Glycol, Diazolidinyl Urea, Parfum (Fragrance), Benzyl Salicylate, Butylphenyl Methylpropional, Citronellol, Limonene, Linalool, Benzyl Benzoate, Geraniol, Benzyl Alcohol, Sodium Hydroxide |
| 12 | L | lotion | Skin irritated after sunbathing, epidermis regeneration | Water, Paraffinum Liquidum, Dimethicone, Propylene Glycol, Cetyl Alcohol, Cetearyl Alcohol, Cetearyl Ethylhexanoate, Isopropyl Myristate, PEG-8 Distearate, Glyceryl Stearate, PEG-100 Stearate, Parfum, Caprylyl Glycol, Hydrolized Collagen, Allantoin, Imidazolidinyl Urea, Triethanoloamine, Carbomer, Glycerin, Tetrasodium EDTA, Niacinamide, Carrageenan (Chondrus Crispus), Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Phenoxyethanol, Xanthan Gum, Polyisobutene, Helix Aspersa, Ethylhexylglycerin, Avena Sativa Kernel Extract, PEG-7 Trimethylolpropane Coconut Ether, Glucose |
| 13 | M | gel | 1st degree burns (sunburns, thermal burns) | Aqua (modified solution of medicinal water: bicarbonate-chloride-sodium-bromide-iodide-boron from Uzdrowisko Rabka S.A.), Aloe Vera (Aloe Barbadiensis) Extract, Propylene Glycol, Glycerin, Symphytum Officinale Extract, Panthenol, Triethanoloamine, Allantoin, Carbomer, DMDM Hydantoin |
| 14 | N | gel | 1st degree burns (sunburns) | Aqua, Panthenol, PEG-40 Hydrogenated Castor Oil, Phenoxyethanol, Methylparaben, Ethylparaben, Propylene Glycol, Allantoin, Retinyl Palmitate, Calcium Gluconate, Carbomer, 2-Bromo-2-Nitropropane-1,3-Diol, Parfum (Fragrance), Sodium Hydroxide |
| Sample | Physicochemical Form | Apparent Viscosity, Pas at γ = 1–500 s−1 | Yield Stress τ0, Pa | |||
|---|---|---|---|---|---|---|
| 1.0 s−1 | 10.0 s−1 | 100.0 s−1 | 500 s−1 | |||
| A | balsam | 81.86 | 8.385 | 2.670 | 1.079 | 81.80 |
| B | balsam | 68.45 | 5.910 | 1.330 | 0.335 | 67.47 |
| C | hydrogel | 30.45 | 4.810 | 1.445 | 0.390 | 24.94 |
| D | hydrogel | 55.58 | 4.740 | 1.263 | 0.439 | 21.42 |
| E | cream | 52.89 | 7.425 | 1.710 | 0.712 | 52.77 |
| F | cream | 123.4 | 9.215 | 2.255 | 0.686 | 121.6 |
| G | cream | 530.5 | 44.40 | 7.670 | 1.610 | 505.8 |
| H | ointment | 123.6 | 16.10 | 7.491 | 3.623 | 119.7 |
| I | ointment | 78.97 | 11.94 | 3.330 | 1.319 | 77.14 |
| J | ointment | 229.7 | 14.72 | 3.805 | 1.494 | 327.8 |
| K | lotion | 84.39 | 8.230 | 2.490 | 0.928 | 83.20 |
| L | lotion | 43.71 | 3.495 | 0.880 | 0.282 | 43.18 |
| M | gel | 40.16 | 5.330 | 2.102 | 0.604 | 39.28 |
| N | gel | 55.93 | 7.197 | 2.110 | 0.757 | 54.32 |
| NG-Carb-1.0 | nanoemulgel | 22.43 | 2.863 | 0.836 | 0.277 | 21.75 |
| Name of the Sample | Physicochemical Form | τy (Pa) | k (Pa·sn) | n (-) | R2 |
|---|---|---|---|---|---|
| A | balsam | 61.860 | 19.796 | 0.506 | 0.999 |
| B | balsam | 4.936 | 35.240 | 0.236 | 0.991 |
| C | hydrogel | 12.650 | 16.456 | 0.391 | 0.999 |
| D | hydrogel | 4.942 | 23.328 | 0.323 | 0.997 |
| E | cream | 53.839 | 8.723 | 0.5573 | 0.993 |
| F | cream | 61.931 | 40.001 | 0.306 | 0.994 |
| G | cream | 513.4 | 35.12 | 0.34 | 0.990 |
| H | ointment | 20.258 | 51.872 | 0.570 | 0.998 |
| I | ointment | 19.973 | 62.201 | 0.380 | 0.998 |
| J | ointment | 304.048 | 17.493 | 0.629 | 0.999 |
| K | lotion | 60.798 | 18.658 | 0.491 | 0.999 |
| L | lotion | 40.510 | 9.232 | 0.389 | 0.996 |
| M | gel | 85.435 | 4.211 | 0.605 | 0.993 |
| N | gel | 41.108 | 20.170 | 0.445 | 0.998 |
| NG-Carb-1.0 | nanoemulgel | 14.938 | 7.371 | 0.436 | 0.997 |
| Groups | N | Sum | Average | Variance | Post-Hoc |
|---|---|---|---|---|---|
| Preparation G | 4 | 8 | 2 | 2 | b |
| Preparation M | 4 | 10 | 2,5 | 3 | ab |
| NG-Carb-1.0 | 4 | 18 | 4,5 | 1 | a |
| Results | Parameter Value (%) | ||
|---|---|---|---|
| Hydration | Elasticity | Smoothness | |
| Preparation G (emulsion) | |||
| Assessor 1 | 16 ± 1.24 | 22 ± 1.24 | 3 ± 0.94 |
| Assessor 2 | 15 ± 1.41 | 26 ± 0.47 | 8 ± 0.47 |
| Preparation M (gel) | |||
| Assessor 1 | 25 ± 0.94 | 11 ± 0.47 | 2 ± 0.94 |
| Assessor 2 | 28 ± 1.24 | 13 ± 1.69 | 8 ± 0.47 |
| NG-Carb-1.0 (nanoemulgel) | |||
| Assessor 1 | 22 ± 1.69 | 15 ± 0.47 | 3 ± 0.47 |
| Assessor 2 | 18 ± 1.24 | 20 ± 1.24 | 7 ± 0.47 |
| Control test (without the use of the preparation) | |||
| Assessor 1 | 5 ± 0.94 | 5 ± 0.94 | −4 ± 0.47 |
| Assessor 2 | 3 ± 0.47 | 8 ± 0.94 | −3 ± 0.47 |
| Results | Parameter Value (%) | ||
|---|---|---|---|
| Hydration | Elasticity | Smoothness | |
| Preparation G (emulsion) | |||
| Assessor 3 | 10 ± 0.81 | 19 ± 0.47 | 5 ± 0.47 |
| Assessor 4 | 6 ± 0.47 | 10 ± 0.47 | 13 ± 0.47 |
| Preparation M (gel) | |||
| Assessor 3 | 11 ± 0.47 | 12 ± 0.47 | 9 ± 0.47 |
| Assessor 4 | 8 ± 0.47 | 7 ± 0.47 | 17 ± 0.94 |
| NG-Carb-1.0 (nanoemulgel) | |||
| Assessor 3 | 16 ± 0.94 | 15 ± 0.94 | 2 ± 0.47 |
| Assessor 4 | 15 ± 0.47 | 18 ± 0.47 | 16 ± 0.47 |
| Control test | |||
| Assessor 3 | 11 ± 0.47 | −3 ± 0.47 | 2 ± 0.47 |
| Assessor 4 | 8 ± 0.47 | −1 ± 0.47 | 1 ± 0.94 |
| Property | k | n |
|---|---|---|
| consistency | 0.546 | 0.424 |
| spreadability | 0.895 | 0.814 |
| adhesion | 0.140 | 0.070 |
| stickiness | 0.378 | 0.493 |
| absorption | 0.793 | 0.692 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miastkowska, M.; Kulawik-Pióro, A.; Szczurek, M. Nanoemulsion Gel Formulation Optimization for Burn Wounds: Analysis of Rheological and Sensory Properties. Processes 2020, 8, 1416. https://doi.org/10.3390/pr8111416
Miastkowska M, Kulawik-Pióro A, Szczurek M. Nanoemulsion Gel Formulation Optimization for Burn Wounds: Analysis of Rheological and Sensory Properties. Processes. 2020; 8(11):1416. https://doi.org/10.3390/pr8111416
Chicago/Turabian StyleMiastkowska, Małgorzata, Agnieszka Kulawik-Pióro, and Mariola Szczurek. 2020. "Nanoemulsion Gel Formulation Optimization for Burn Wounds: Analysis of Rheological and Sensory Properties" Processes 8, no. 11: 1416. https://doi.org/10.3390/pr8111416
APA StyleMiastkowska, M., Kulawik-Pióro, A., & Szczurek, M. (2020). Nanoemulsion Gel Formulation Optimization for Burn Wounds: Analysis of Rheological and Sensory Properties. Processes, 8(11), 1416. https://doi.org/10.3390/pr8111416