In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids
Abstract
:1. Introduction
2. Materials and Methods
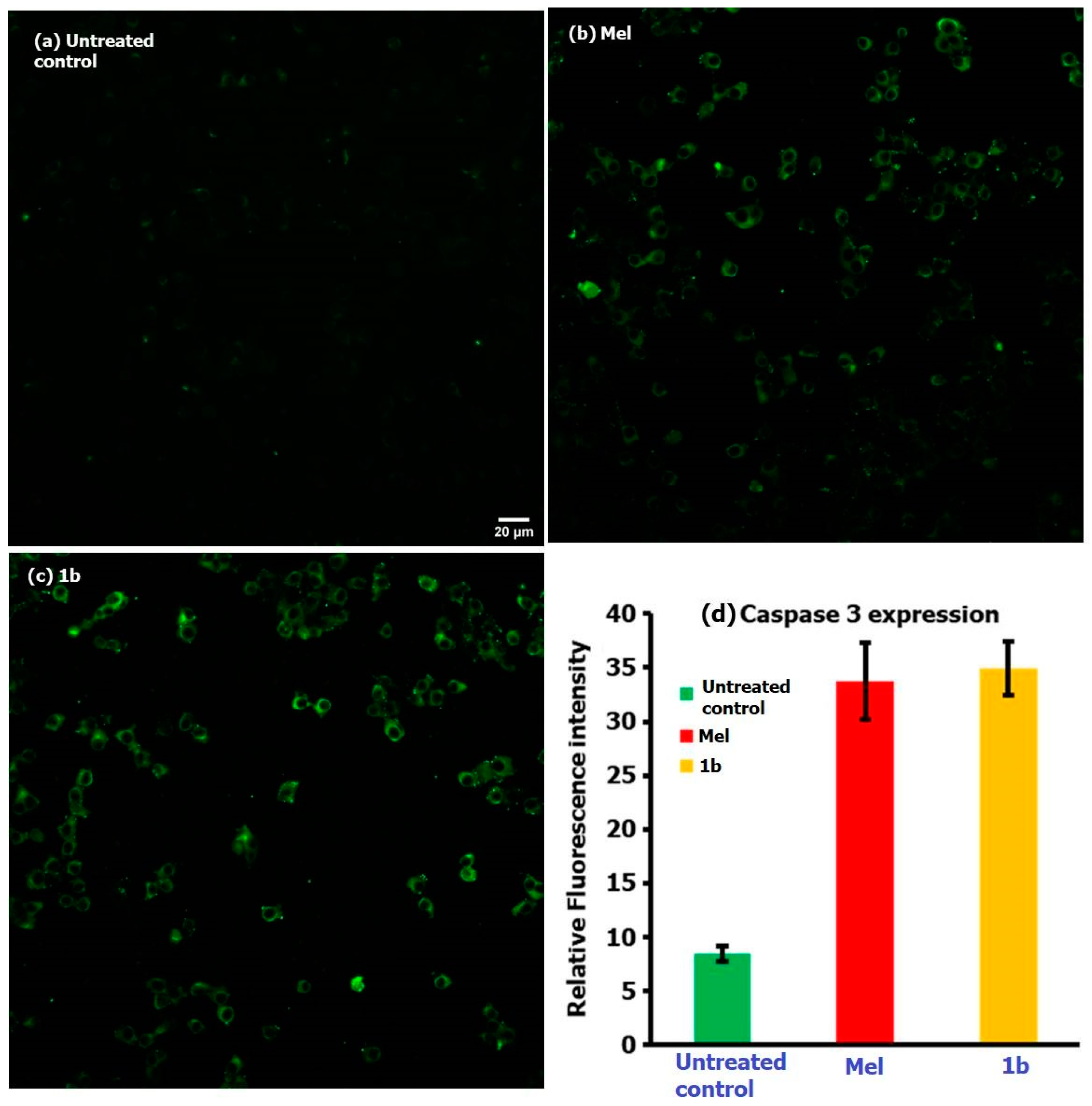
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef] [Green Version]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Roy, S.; Kumar, A.; Islam, M.S.; Rabbi, F.A.; Paul, P.; Mia, M.M.; Islam, A.K.M.K.; Ray, A.K. Drug resistance and its future perspectives in cancer treatment. Asian Oncol. Res. J. 2020, 3, 26–46. [Google Scholar]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer. 2009, 9, 501–507. [Google Scholar] [CrossRef]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Dysregulation of apoptotic signaling in cancer: Molecular mechanisms and therapeutic opportunities. J. Cell. Biochem. 2008, 104, 1124–1149. [Google Scholar] [CrossRef] [Green Version]
- Chande, M.S.; Verma, R.S.; Barve, P.A.; Khanwelkar, R.R.; Vaidya, R.B.; Ajaikumar, K.B. Facile synthesis of active antitubercular, cytotoxic and antibacterial agents: A Michael addition approach. Eur. J. Med. Chem. 2005, 40, 1143–1148. [Google Scholar] [CrossRef]
- Lundahl, K.; Schut, J.; Schlatmann, J.L.; Paerels, G.B.; Peters, A. Synthesis and antiviral activities of adamantane spiro compounds. 1. Adamantane and analogous spiro-3’-pyrrolidines. J. Med. Chem. 1972, 15, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Wang, D.; Tian, M.; Han, S.; Feng, T.; Liu, X.; Mei, R.; Zhou, Y. Diversity-oriented one-pot multicomponent synthesis of spirooxindole derivatives and their biological evaluation for anticancer activities. Tetrahedron 2016, 72, 8523–8536. [Google Scholar] [CrossRef]
- Lotfy, G.; Said, M.M.; Ashry, E.E.; Tamany, E.S.; Al-Dhfyan, A.; Aziz, Y.M.; Barakat, A. Synthesis of new spirooxindole-pyrrolothiazole derivatives: Anti-cancer activity and molecular docking. Bioorg. Med. Chem. 2017, 254, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.; El-Senduny, F.F.; Badria, F.; Elshaier, Y.A.; Ghabbour, H. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335–14346. [Google Scholar] [CrossRef] [Green Version]
- Kornet, M.J.; Thio, A.P. Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem. 1976, 19, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Maclean, D.; Schullek, J.R.; Murphy, M.M.; Ni, Z.J.; Gordon, E.M.; Gallop, M.A. Encoded combinatorial chemistry: Synthesis and screening of a library of highly functionalized pyrrolidines. Proc. Natl. Acad. Sci. USA 1997, 94, 2805–2810. [Google Scholar] [CrossRef] [Green Version]
- Lo, M.M.-C.; Neumann, C.S.; Nagayama, S.; Perlstein, E.O.; Schreiber, S.L. A Library of Spirooxindoles Based on a Stereoselective Three-Component Coupling Reaction. J. Am. Chem. Soc. 2004, 126, 16077–16086. [Google Scholar] [CrossRef]
- Jossang, A.; Jossang, P.; Hadi, H.A.; Sevenet, T.; Bodo, B. Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Org. Chem. 1991, 56, 6527–6530. [Google Scholar] [CrossRef]
- Pellegrini, C.; Weber, M.; Borschberg, H.-J. Total Synthesis of (+)-Elacomine and (−)-Isoelacomine, Two Hitherto Unnamed Oxindole Alkaloids from Elaeagnus commutate. Helvitica 1996, 79, 151–168. [Google Scholar]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H. Pyrrolizidine alkaloids in plants used in the traditional medicine of Madagascar and the Mascarene islands. Pharmazie 2011, 66, 637–647. [Google Scholar] [PubMed]
- Wang, S.; Zhao, Y.; Aguilar, A.; Bernard, D.; Yang, C.-Y. Targeting the MDM2–p53 Protein–Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harb. Perspect. Med. 2017, 7, a026245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almansour, A.I.; Kumar, R.S.; Beevi, F.; Shirazi, A.N.; Osman, H.; Ismail, R.; Choon, T.S.; Sullivan, B.; McCaffrey, K.; Nahhas, A.; et al. Facile, Regio- and Diastereoselective Synthesis of Spiro-Pyrrolidine and Pyrrolizine Derivatives and Evaluation of Their Antiproliferative Activities. Molecules 2014, 19, 10033–10055. [Google Scholar] [CrossRef] [Green Version]
- Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Subbarayan, P.V.; Alshatwi, A.A.; Ghabbour, H.A. Anticancer Agents. U.S. Patent 9,486,444 B1, 8 November 2016. [Google Scholar]
- Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Subbarayan, P.V.; Alshatwi, A.A.; Athinarayanan, J. Anticancer Agents. U.S. Patent 9,873,699B1, 23 January 2018. [Google Scholar]
- Kia, Y.; Osman, H.; Kumar, R.S.; Basiri, A.; Murugaiyah, V. Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Filosa, R.; Peduto, A.; de Caprariis, P.; Saturnino, C.; Festa, M.; Petrella, A.; Pau, A.; Pinna, G.A.; Colla, P.L.; Busonera, B.; et al. Synthesis and antiproliferative properties ofN3/8-disubstituted3,8-diazabicyclo [3.2.1]octane analogues of 3,8-bis[2-(3,4,5-trimethoxyphenyl)pyridin-4-yl]methyl-piperazine. Eur. J. Med. Chem. 2007, 42, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Carreira, E.M.; Fessard, T.C. Four-membered ring-containing spirocycles: Synthetic strategies and opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef]
- Zheng, Y.-J.; Tice, C.M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 831–834. [Google Scholar] [CrossRef] [Green Version]
- Al-thamili, D.M.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Kumar, R.S. Functionalized N-pyridinylmethyl engrafted bisarylmethylidenepyridinones as anticancer agents. Processes 2020, 8, 1154. [Google Scholar] [CrossRef]
- Kumar, R.S.; Al-thamili, D.M.; Almansour, A.I.; Arumugam, N.; Dege, N. [Bmim]Br accelerated one-pot three-component cascade protocol for the construction of spirooxindole–pyrrolidine heterocyclic hybrids. Molecules 2020, 25, 4779. [Google Scholar] [CrossRef]

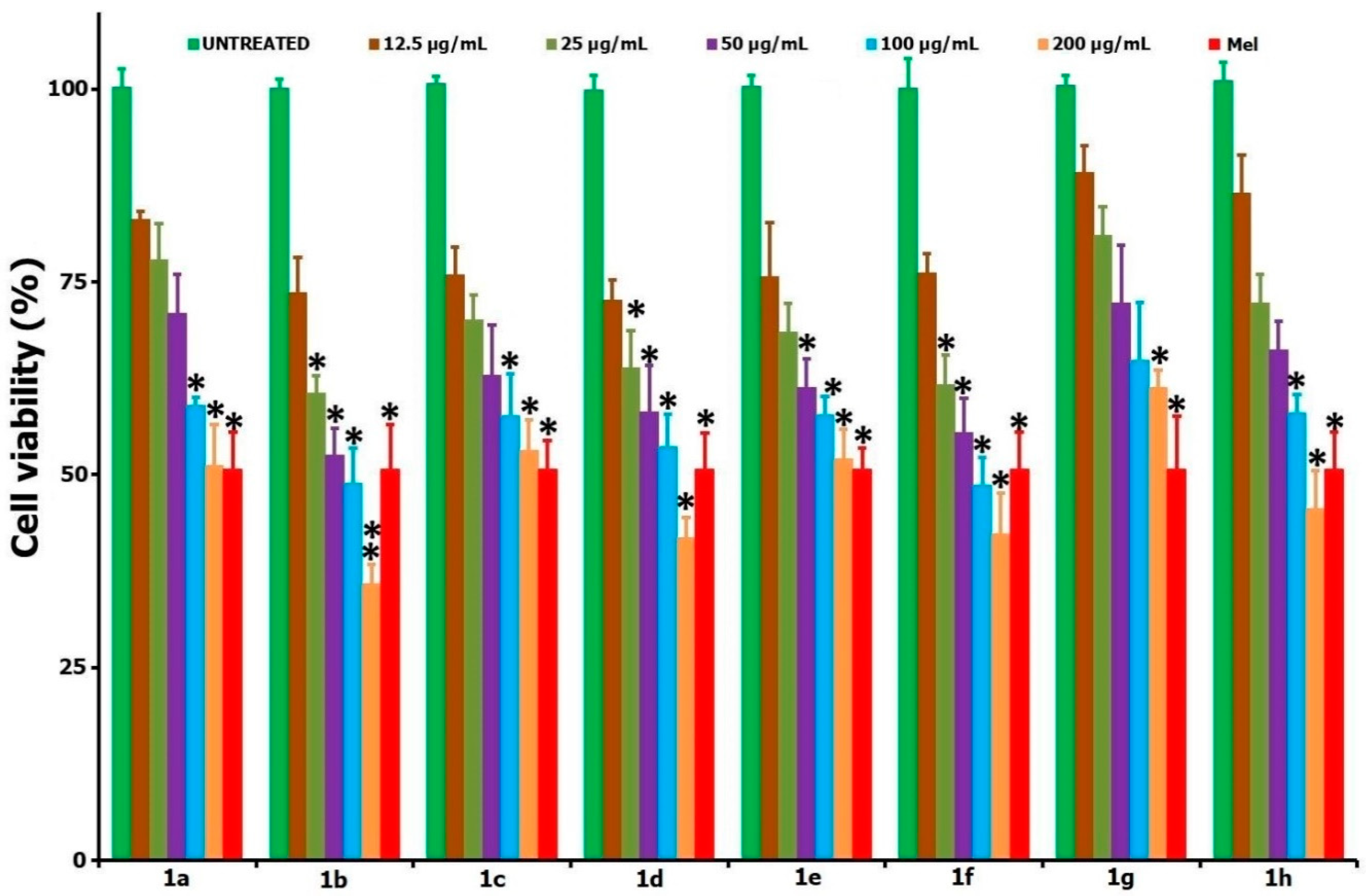
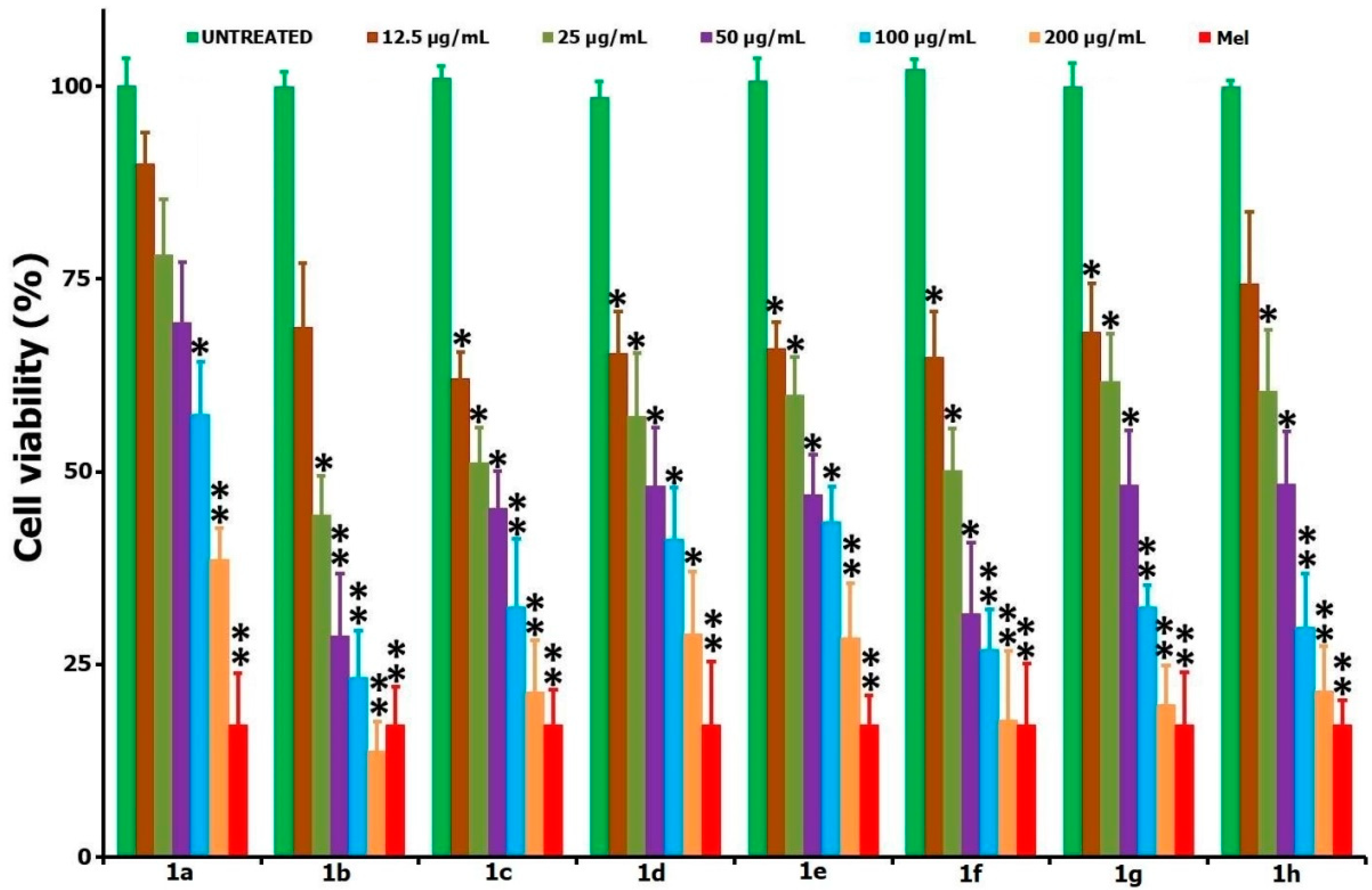

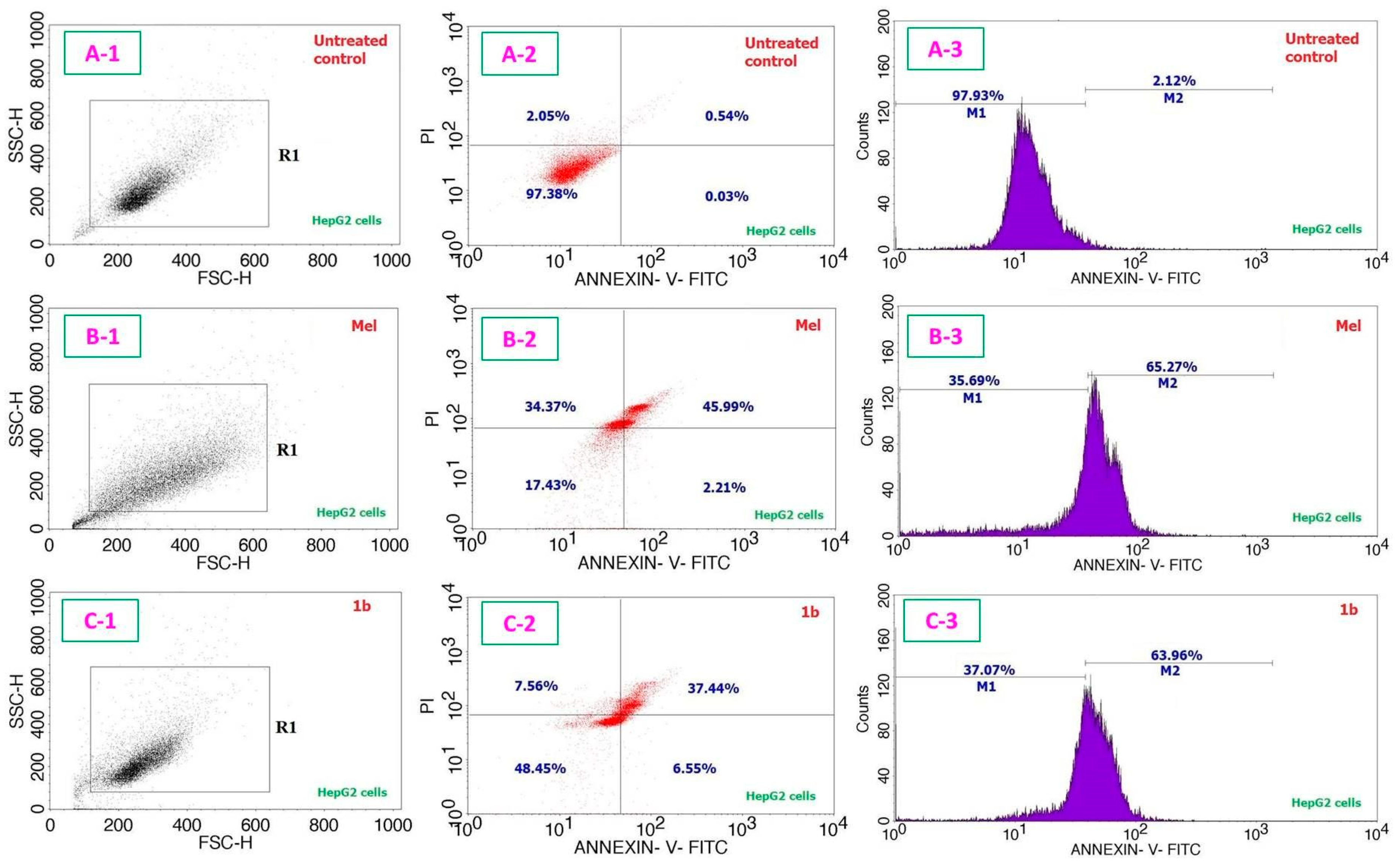

| Entry | Compound | IC50 Values in µg/mL (µM); 24 h | IC50 Values in µg/mL (µM); 48 h | |
|---|---|---|---|---|
| HepG2 Cells | HepG2 Cells | L929 Cells | ||
| 1 |  | 237.59 ± 12.5 (385.23 ± 20.27) | 126.81 ± 10.4 (205.61 ± 16.86) | - |
| 2 |  | 72.24 ± 8.7 (112.03 ± 13.49) | 23.81 ± 2.3 (36.92 ± 3.57) | 301.72 ± 12.51 (467.93 ± 19.40) |
| 3 | 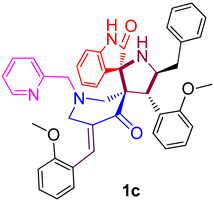 | 265.24 ± 9.4 (391.90 ± 13.89) | 29.83 ± 2.1 (44.07 ± 3.10) | - |
| 4 | 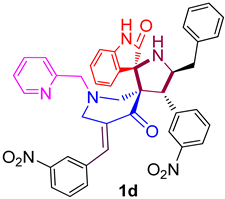 | 107.74 ± 7.5 (152.44 ± 10.62) | 44.1 ± 2.6 (62.40 ± 3.68) | - |
| 5 | 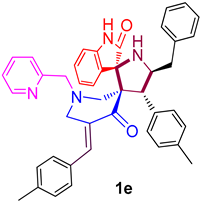 | 237.64 ± 8.9 (368.55 ± 13.80) | 45.86 ± 2.8 (71.12 ± 4.34) | - |
| 6 | 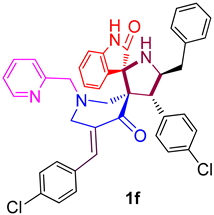 | 90.01 ± 5.6 (131.28 ± 8.17) | 25.01 ± 3.2 (36.48 ± 4.67) | 257.16 ± 9.6 (375.06 ± 14.00) |
| 7 | 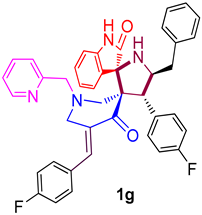 | 509.28 ± 14.3 (780.23 ± 21.91) | 40.51 ± 3.6 (62.06 ± 5.52) | - |
| 8 | 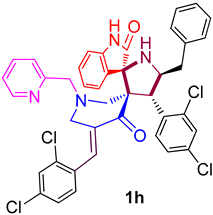 | 157.27 ± 11.3 (208.43 ± 14.98) | 43.17 ± 4.5 (57.21 ± 5.96) | - |
| 9 | 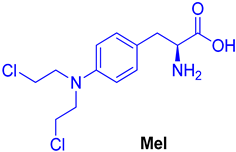 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Al-thamili, D.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Suresh Kumar, R. In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids. Processes 2020, 8, 1473. https://doi.org/10.3390/pr8111473
M. Al-thamili D, Almansour AI, Arumugam N, Mohammad F, Suresh Kumar R. In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids. Processes. 2020; 8(11):1473. https://doi.org/10.3390/pr8111473
Chicago/Turabian StyleM. Al-thamili, Dhaifallah, Abdulrahman I. Almansour, Natarajan Arumugam, Faruq Mohammad, and Raju Suresh Kumar. 2020. "In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids" Processes 8, no. 11: 1473. https://doi.org/10.3390/pr8111473
APA StyleM. Al-thamili, D., Almansour, A. I., Arumugam, N., Mohammad, F., & Suresh Kumar, R. (2020). In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids. Processes, 8(11), 1473. https://doi.org/10.3390/pr8111473





