CFD Modeling and Experimental Validation of an Alkaline Water Electrolysis Cell for Hydrogen Production
Abstract
:1. Introduction
2. Methodology: Modeling and Experimental
2.1. Alkaline Water Electrolysis
- (1)
- Activation overpotentials: related to activation energies of hydrogen and oxygen formation reactions on the surface of electrodes;
- (2)
- Ohmic overpotentials: sum of the electrical resistance of several components such as electrodes, current collectors, etc., and the transport resistance related to gas bubbles, ionic transfer in the electrolyte, and resistivity of the diaphragm;
- (3)
- Concentration overpotentials: due to mass-transport limitations occurring on the surface of the electrodes at high currents.
2.2. CFD Modeling of an Alkaline Electrolysis Cell
2.2.1. Model Geometry and Mesh
2.2.2. Mathematical Procedure and Governing Equations
Electrical Current Conservation
- An empirical relationship for specific conductivity of electrolyte ( in S/m) with respect to temperature () and KOH concentration () was used [29]:
- The conductivity of the diaphragm ( in S/m) was defined as a function of the conductivity of electrolyte () and geometric parameters such as porosity () and tortuosity (), according to [28]:
- The Bruggeman equation ( in S/m) relates the variation of conductivity of electrolyte () with the volume fraction of gas () inside the cell [30]. The gas fraction for both electrolytic chambers at each current density value was calculated according to the equations described in the “liquid-gas flow distribution” section:
- The reversible potential ( in V) was defined according to the LeRoy et al. [31] equation as a function of temperature ():
- Activation overpotentials ( in V) were defined for the cathode and the anode by the Butler–Volmer equation (Tafel equation form) for each current density (), according to:
Liquid-Gas Flow Distribution
2.2.3. Initial and Boundary Conditions
- (1)
- The gas density is negligible compared to the liquid density;
- (2)
- The movement of gas bubbles in relation to the liquid phase is determined by a balance between viscous drag and pressure forces;
- (3)
- The two phases share the same pressure field;
- (4)
- Hydrogen and oxygen crossover through the diaphragm is negligible;
- (5)
- The electrolysis cell works with a high enough flow rate of electrolyte to avoid the accumulation of gas bubbles in the cell;
- (6)
- The bubbles have a diameter of less than 1 mm, so the Hadamard–Rybczynski drag law for spherical gas bubbles in liquid is used for the gas velocity;
- (7)
- The electrical resistance of the electrodes (Ni) is negligible with respect to the rest of the elements of the electrolysis cell.
2.3. Experimental Test Facility and Methodology
2.3.1. Alkaline Water Electrolysis Test-Bench
2.3.2. Experimental Protocol
3. Results and Discussion
3.1. Polarization Curve
3.1.1. Influence of Temperature
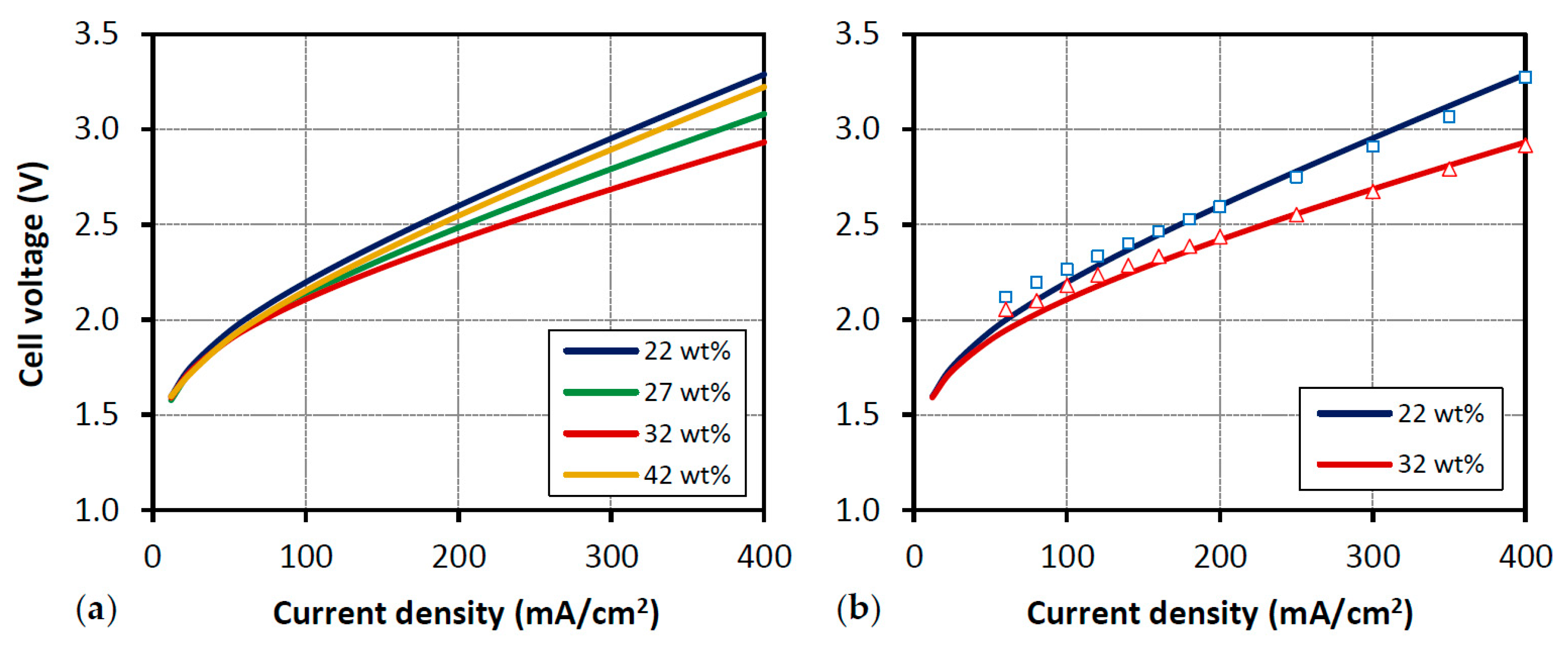
3.1.2. Influence of Electrolyte Conductivity
- (1)
- As previously reported [10], increasing the electrolyte conductivity implies a better electrolysis performance due to a reduction of ohmic losses, which turns out in a lower required energy.
- (2)
- (3)
- The model shows an accurate agreement with the experimental polarization curves for the different electrolyte conductivity values considered (Figure 8b).
3.1.3. Influence of Electrode-Diaphragm Distance
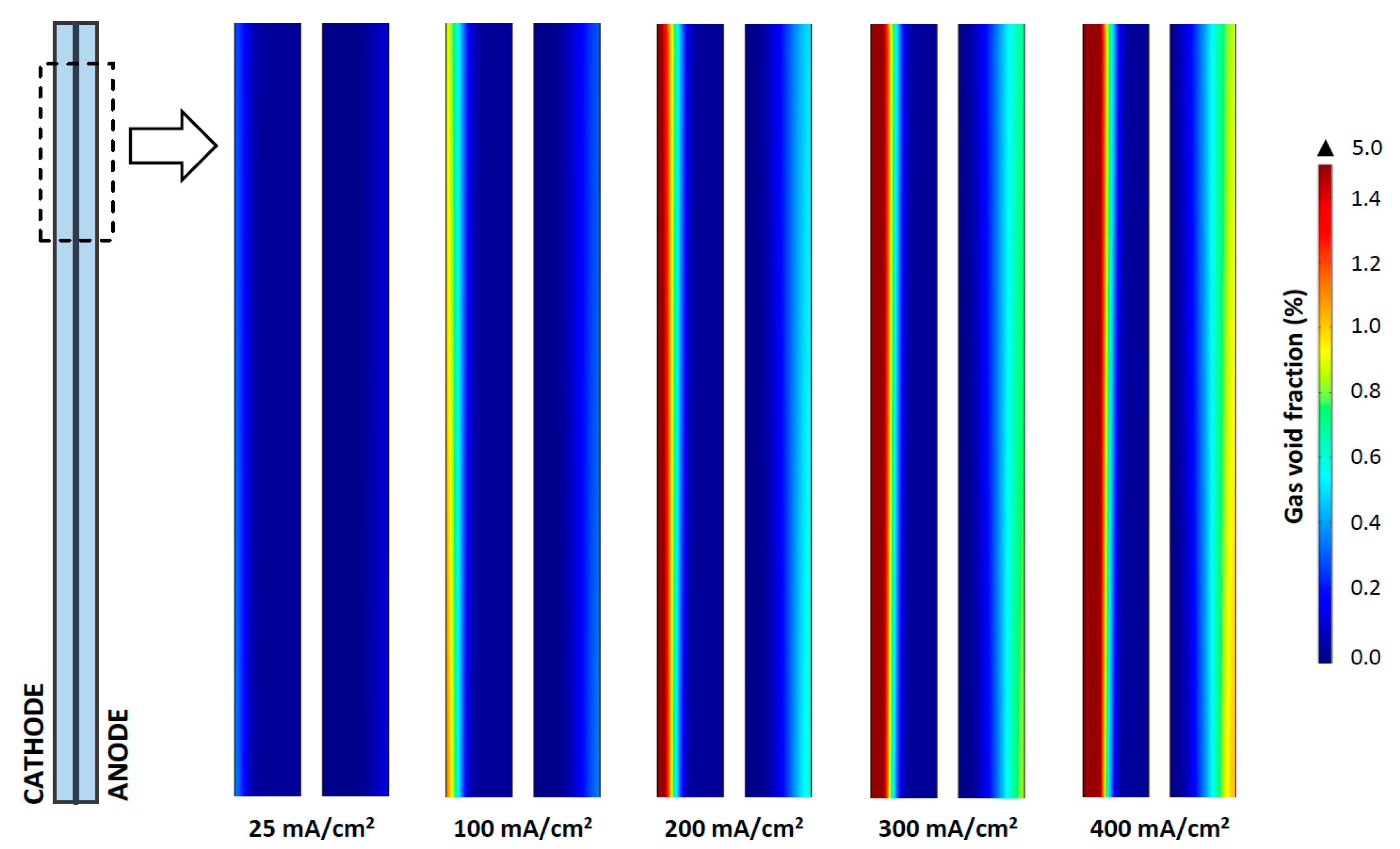
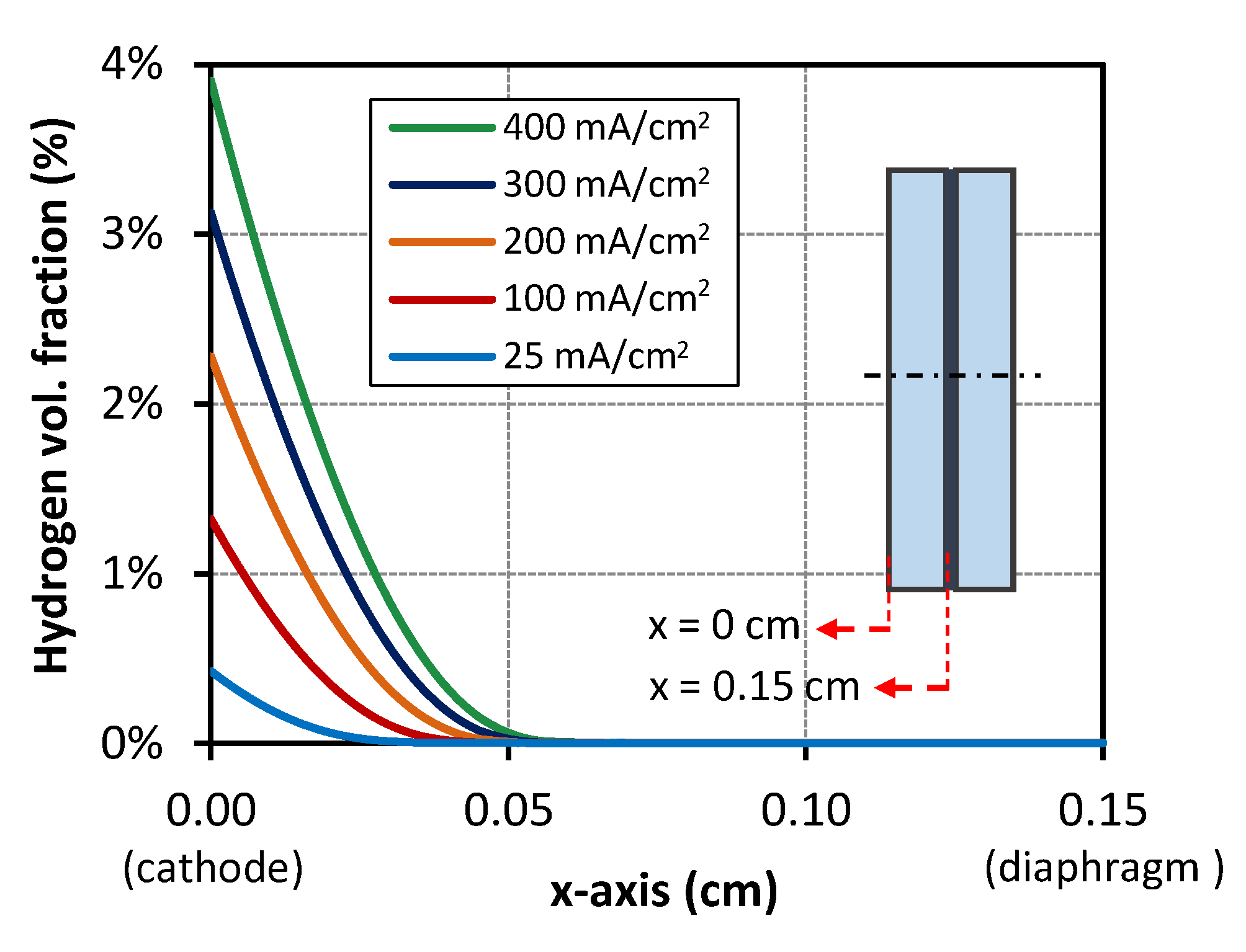
3.2. Gas Generation Profile
4. Conclusions and Future Work
- (1)
- Charge transfer coefficient (): the influence of this term over the performance of real electrolysis systems has hardly been studied. Even though it is possible to find different values in the literature, some authors have claimed significant differences when working with a laboratory electrochemical cell and with an electrolysis stack. Experimental values up to 1.035 [35] have been reported, which contrast with that which is considered to be the common range (0.3–0.7). In fact, it greatly depends on operating conditions: electrode materials, electrolyte, etc. For these reasons, it would be very interesting to experimentally determine this parameter in the test bench shown in Figure 5 in order to improve the accuracy of the model, especially in the activation overpotentials zone where the model fits worse with real data. This is extensible to exchange current density, which is another critical kinetic parameter.
- (2)
- Zero-gap cell design: this is one of the most extended alkaline water electrolysis cell designs in commercial systems. This configuration allows us to “ideally” eliminate bubbles between the electrodes because cathode and anode are placed directly over the diaphragm. For this to happen, the electrodes have to be porous. Therefore, in order to improve the model applicability to real systems, this geometry should be implemented in the model, making it a more useful design tool.
Author Contributions
Funding
Conflicts of Interest
References
- Armaroli, N.; Balzani, V. Solar electricity and solar fuels: Status and perspectives in the context of the energy transition. Chem. Eur. J. 2016, 22, 32–57. [Google Scholar] [CrossRef]
- Sánchez, M.; Amores, E.; Rodríguez, L.; Clemente-Jul, C. Semi-empirical model and experimental validation for the performance evaluation of a 15 kW alkaline water electrolyzer. Int. J. Hydrog. Energy 2018, 43, 20332–20345. [Google Scholar] [CrossRef]
- Mais, L.; Palmas, S.; Mascia, M.; Sechi, E.; Casula, M.F.; Rodriguez, J.; Vacca, A. Porous Ni photocathodes obtained by selective corrosion of Ni-Cu films: Synthesis and photoelectrochemical characterization. Catalysts 2019, 9, 453. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.; Rojas, N.; Sánchez-Molina, M.; Rodriguez, L.G.; Campana, R.; Rodríguez, L. Hybrid membranes based in Nafion-metallic oxides: Performance evaluations. Chem. Eng. Trans. 2016, 47, 415–420. [Google Scholar] [CrossRef]
- Amores, E.; Rodríguez, J.; Oviedo, J.; de Lucas-Consuegra, A. Development of an operation strategy for hydrogen production using solar PV energy based on fluid dynamic aspects. Open Eng. 2017, 7, 141–152. [Google Scholar] [CrossRef]
- Amores, E.; Sánchez, M.; Rojas, N.; Sánchez-Molina, M. Renewable hydrogen production by water electrolysis. In Sustainable Fuel Technologies Handbook, 1st ed.; Dutta, S., Hussain, C.M., Eds.; Academic Press: London, UK, 2021; pp. 271–313. [Google Scholar] [CrossRef]
- Lehner, M.; Tichler, R.; Steinmüller, H.; Koppe, M. Power-To-Gas: Technology and Business Models, 1st ed.; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Development of Water Electrolysis in the European Union. FCH-JU Funded Studies 2014: Element Energy, E4tech Sarl. Available online: https://www.fch.europa.eu/sites/default/files/FCHJUElectrolysisStudy_FullReport%20(ID%20199214).pdf (accessed on 10 November 2020).
- Amores, E.; Rodríguez, J.; Merino, C.; García, P. Study of an alkaline electrolyzer powered by renewable energy. In Proceedings of the COMSOL Conference, Stuttgart, Germany, 26–28 October 2011; COMSOL AB: Burlington, VT, USA, 2011. [Google Scholar]
- Amores, E.; Rodríguez, J.; Carreras, C. Influence of operation parameters in the modeling of alkaline water electrolyzers for hydrogen production. Int. J. Hydrog. Energy 2014, 39, 13063–13078. [Google Scholar] [CrossRef]
- Nagai, N.; Takeuchi, M.; Nakao, M. Influences of bubbles between electrodes onto efficiency of alkaline water electrolysis. In Proceedings of the PSFVIP-4, Chamonix, France, 3–5 June 2003. [Google Scholar]
- Olivier, P.; Bourasseau, C.; Bouamama, B. Low-temperature electrolysis system modelling: A review. Renew. Sustain. Energy Rev. 2017, 78, 280–300. [Google Scholar] [CrossRef]
- Ulleberg, Ø. Modeling of advanced alkaline electrolyzers: A system simulation approach. Int. J. Hydrog. Energy 2003, 28, 21–33. [Google Scholar] [CrossRef]
- Aldas, K. Application of a two-phase flow model for hydrogen evolution in an electrochemical cell. Appl. Math. Comput. 2004, 154, 507–519. [Google Scholar] [CrossRef]
- Mat, M.D.; Aldas, K.; Ilegbusi, O.J. A two-phase flow model for hydrogen evolution in an electrochemical cell. Int. J. Hydrog. Energy 2004, 29, 1015–1023. [Google Scholar] [CrossRef]
- Hawkes, G.; O’Brien, J.; Stoots, C.; Hawkes, B. 3D CFD model of a multi-cell high-temperature electrolysis stack. Int. J. Hydrog. Energy 2009, 34, 4189–4197. [Google Scholar] [CrossRef] [Green Version]
- Hreiz, R.; Abdelouahed, L.; Fünfschilling, D.; Lapicque, F. Electrogenerated bubbles induced convection in narrow vertical cells: PIV measurements and Euler–Lagrange CFD simulation. Chem. Eng. Sci. 2015, 134, 138–152. [Google Scholar] [CrossRef]
- El-Askary, W.A.; Sakr, I.M.; Ibrahim, K.A.; Balabel, A. Hydrodynamics characteristics of hydrogen evolution process through electrolysis: Numerical and experimental studies. Energy 2015, 90, 722–737. [Google Scholar] [CrossRef]
- Zarghami, A.; Deen, N.G.; Vreman, A.W. CFD modeling of multiphase flow in an alkaline water electrolyzer. Chem. Eng. Sci. 2020, 227, 115926–115935. [Google Scholar] [CrossRef]
- Le Bideau, D.; Mandin, P.; Benbouzid, M.; Kim, M.; Sellier, M.; Ganci, F.; Inguanta, R. Eulerian two-fluid model of alkaline water electrolysis for hydrogen production. Energies 2020, 13, 3394. [Google Scholar] [CrossRef]
- Hydrogen Roadmap Europe, FCH-JU 2019. Available online: https://www.fch.europa.eu/sites/default/files/Hydrogen%20Roadmap%20Europe_Report.pdf (accessed on 10 November 2020).
- Divisek, J. Water electrolysis in a low- and medium-temperature regime. In Electrochemical Hydrogen Technologies: Electrochemical Production and Combustion of Hydrogen; Wendt, H., Ed.; Elsevier: Oxford, UK, 1990; pp. 137–212. [Google Scholar]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- AC/DC Module User’s Guide v4.3. Part nº CM020101; COMSOL AB: Stockholm, Sweden, 2012.
- Kibria, M.F.; Mridha, M.S.; Khan, A.H. Electrochemical studies of a nickel electrode for the hydrogen evolution reaction. Int. J. Hydrog. Energy 1995, 20, 435–440. [Google Scholar] [CrossRef]
- Kibria, M.F.; Mridha, M.S. Electrochemical studies of the nickel electrode for the oxygen evolution reaction. Int. J. Hydrog. Energy 1996, 21, 179–182. [Google Scholar] [CrossRef]
- AGFA: Zirfon H2 Advanced. Available online: https://www.agfa.com/specialty-products/solutions/membranes/zirfon/ (accessed on 30 October 2020).
- Rodríguez, J.; Palmas, S.; Sánchez-Molina, M.; Amores, E.; Mais, L.; Campana, R. Simple and precise approach for determination of ohmic contribution of diaphragms in alkaline water electrolysis. Membranes 2019, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, R.J.; Graydon, J.W.; Kirk, D.W.; Thorpe, S.J. A review of specific conductivities of potassium hydroxide solutions for various concentrations and temperatures. Int. J. Hydrog. Energy 2007, 32, 359–364. [Google Scholar] [CrossRef]
- Weijs, M.P.M.G.; Janssen, L.J.J.; Visser, G.J. Ohmic resistance of solution in a vertical gas-evolving cell. J. Appl. Electrochem. 1997, 27, 371–378. [Google Scholar] [CrossRef]
- LeRoy, R.L.; Bowen, C.T.; LeRoy, D.J. The thermodynamics of aqueous water electrolysis. J. Electrochem. Soc. 1980, 127, 1954–1962. [Google Scholar] [CrossRef]
- Chemical Engineering Module User’s Guide v3.5a. Part nº CM020501; COMSOLAB: Stockholm, Sweden, 2008.
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Santos, D.M.F.; Sequeira, C.A.C.; Macciò, D.; Saccone, A.; Figuereido, J.L. Platinum–rare earth electrodes for hydrogen evolution in alkaline water electrolysis. Int. J. Hydrog. Energy 2013, 38, 3137–3145. [Google Scholar] [CrossRef]
- Tijani, A.S.; Kamarudin, N.A.B.; Mazlan, F.A.B. Investigation of the effect of charge transfer coefficient (CTC) on the operating voltage of polymer electrolyte membrane (PEM) electrolyzer. Int. J. Hydrog. Energy 2018, 43, 9119–9132. [Google Scholar] [CrossRef]
- Nagai, N.; Takeuchi, M.; Oka, T. Existence of optimum space between electrodes on hydrogen production by water electrolysis. Int. J. Hydrog. Energy 2003, 28, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Nagai, N.; Murai, Y.; Yamamoto, F. Particle image velocimetry measurement of bubbly flow induced by alkaline water electrolysis. In Proceedings of the PSFVIP-4, Chamonix, France, 3–5 June 2003. [Google Scholar]
- Aldas, K.; Pehlivanoglu, N.; Mat, M.D. Numerical and experimental investigation of two-phase flow in an electrochemical cell. Int. J. Hydrog. Energy 2008, 33, 3668–3675. [Google Scholar] [CrossRef]


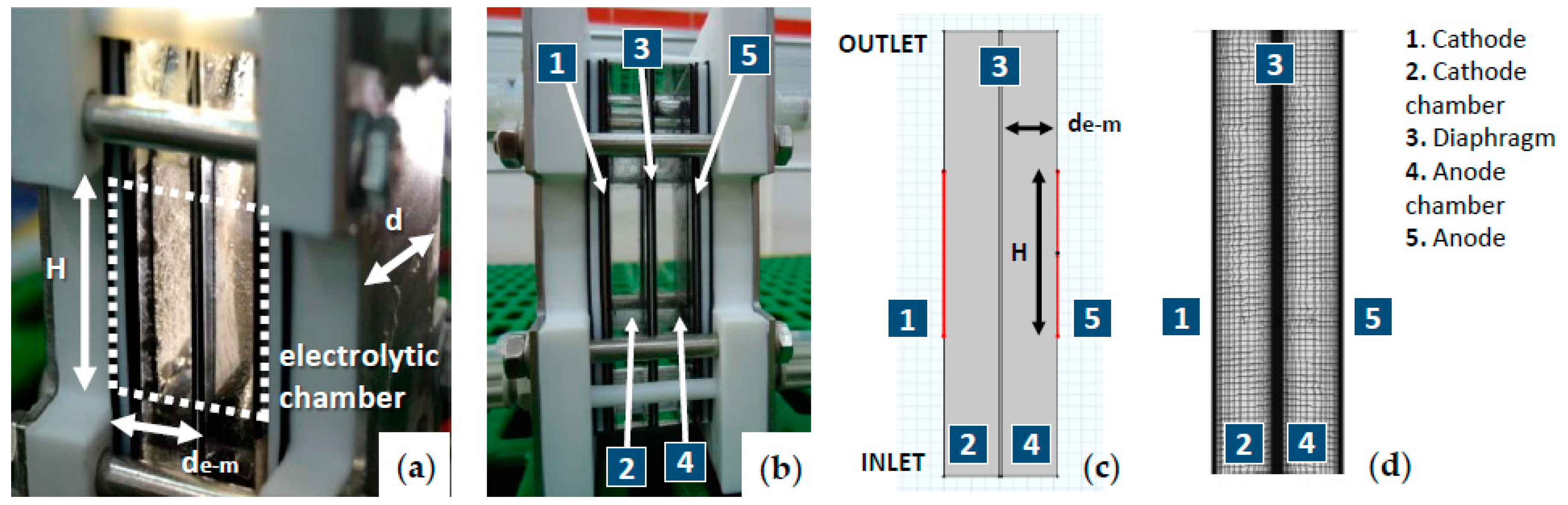
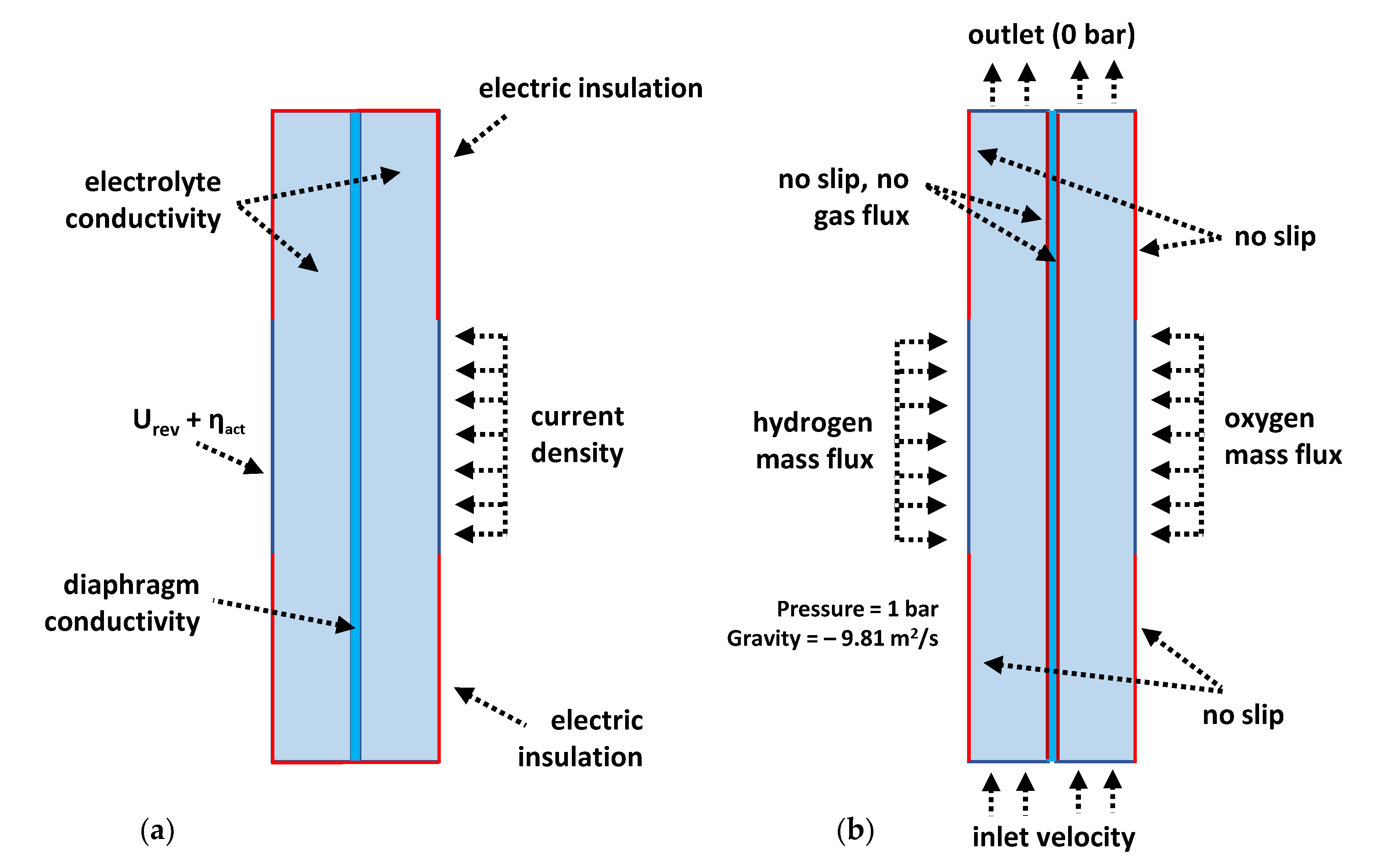
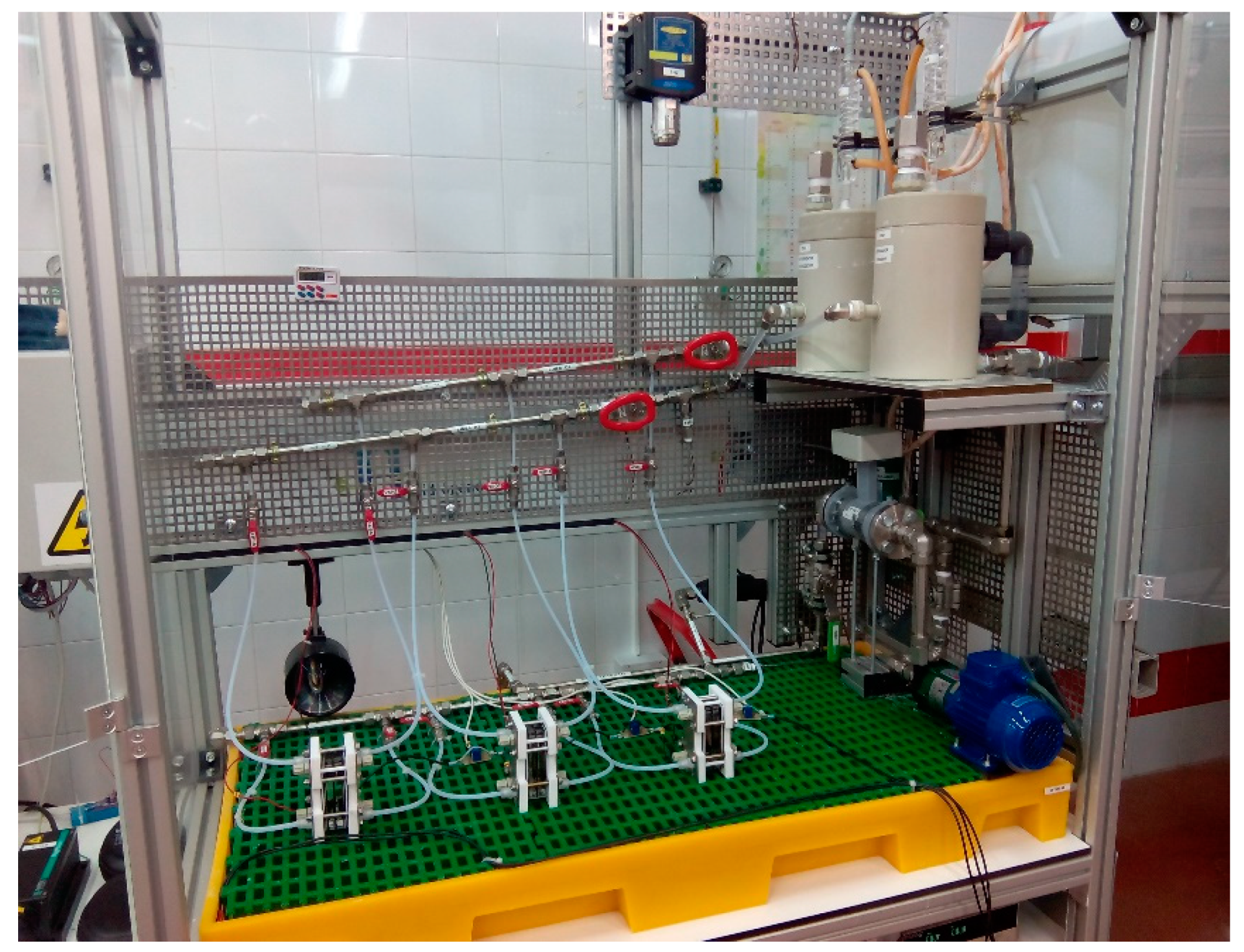



| Symbol | Value | Unit | Description |
|---|---|---|---|
| 1.5 to 10 | mm | Electrode-diaphragm distance | |
| 96485 | C | Faraday constant | |
| 250 to 4000 | A·m−2 | Current density (polarization curve) | |
| 21.1 to 93.5 | A·m−2 | Exchange current density (cathode) [25] | |
| 1.1 to 9.3 | A·m−2 | Exchange current density (anode) [26] | |
| 2 | g·moL−1 | Hydrogen molecular weight | |
| 32 | g·moL−1 | Oxygen molecular weight | |
| 1 | bar | Pressure operation | |
| 8.314 | J·K−1·moL−1 | Ideal gas constant | |
| 303 to 343 | K | Temperature operation | |
| 700 | ml·min−1 | Electrolyte flow rate (inlet velocity) | |
| 5 to 10 | M | Electrolyte concentration (22–42 wt% KOH) | |
| 0.60 | - | Charge transfer coefficient (anode) | |
| 0.77 | Charge transfer coefficient (cathode) | ||
| 0.55 | - | Diaphragm porosity [27] | |
| 1.89 | - | Diaphragm tortuosity [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, J.; Amores, E. CFD Modeling and Experimental Validation of an Alkaline Water Electrolysis Cell for Hydrogen Production. Processes 2020, 8, 1634. https://doi.org/10.3390/pr8121634
Rodríguez J, Amores E. CFD Modeling and Experimental Validation of an Alkaline Water Electrolysis Cell for Hydrogen Production. Processes. 2020; 8(12):1634. https://doi.org/10.3390/pr8121634
Chicago/Turabian StyleRodríguez, Jesús, and Ernesto Amores. 2020. "CFD Modeling and Experimental Validation of an Alkaline Water Electrolysis Cell for Hydrogen Production" Processes 8, no. 12: 1634. https://doi.org/10.3390/pr8121634
APA StyleRodríguez, J., & Amores, E. (2020). CFD Modeling and Experimental Validation of an Alkaline Water Electrolysis Cell for Hydrogen Production. Processes, 8(12), 1634. https://doi.org/10.3390/pr8121634






