Manipulation of Culture Conditions: Tool for Correlating/Improving Lipid and Carotenoid Production by Rhodotorula glutinis
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Yeast Strain and Media Compositions
2.2. Effect of Different Culture Conditions and Metals Stress on Growth, Lipid, and Carotenoid Production by R. glutinis
2.3. Fed-Batch Cultivation of R. glutinis under Optimized Condition
2.4. Detection of Dry Cell Weight (DCW) and Reducing Sugar in the Culture Media
2.5. Fluorescence Microscopic Examination of Nile-Red Treated Cells
2.6. Total Lipid Detection and GC Analysis of Fatty Acid Methyl Ester
2.7. Total Carotenoid Extraction, Quantifying, and Identification
2.8. Kinetics Analysis of Fermentation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different Culture Conditions on Growth, Lipid, and Carotenoid Production by R. glutinis
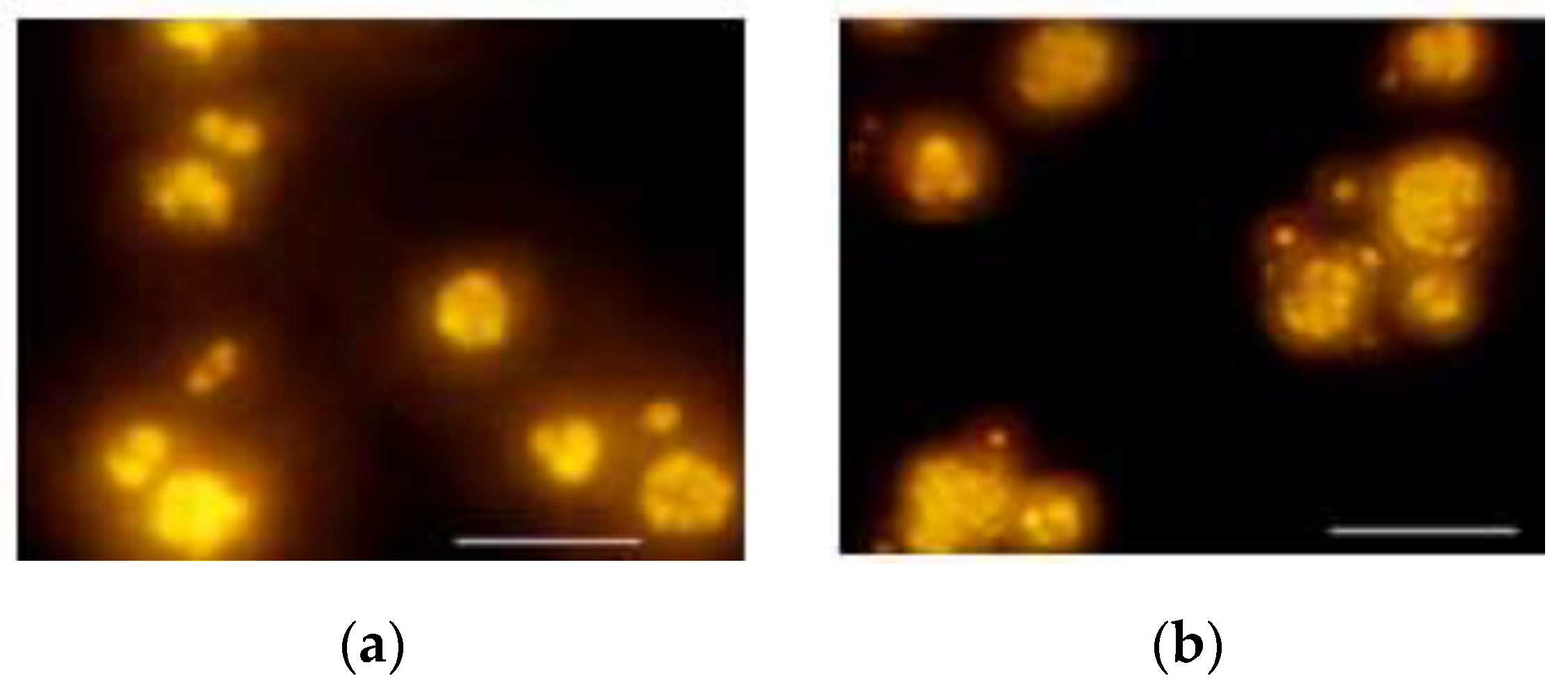
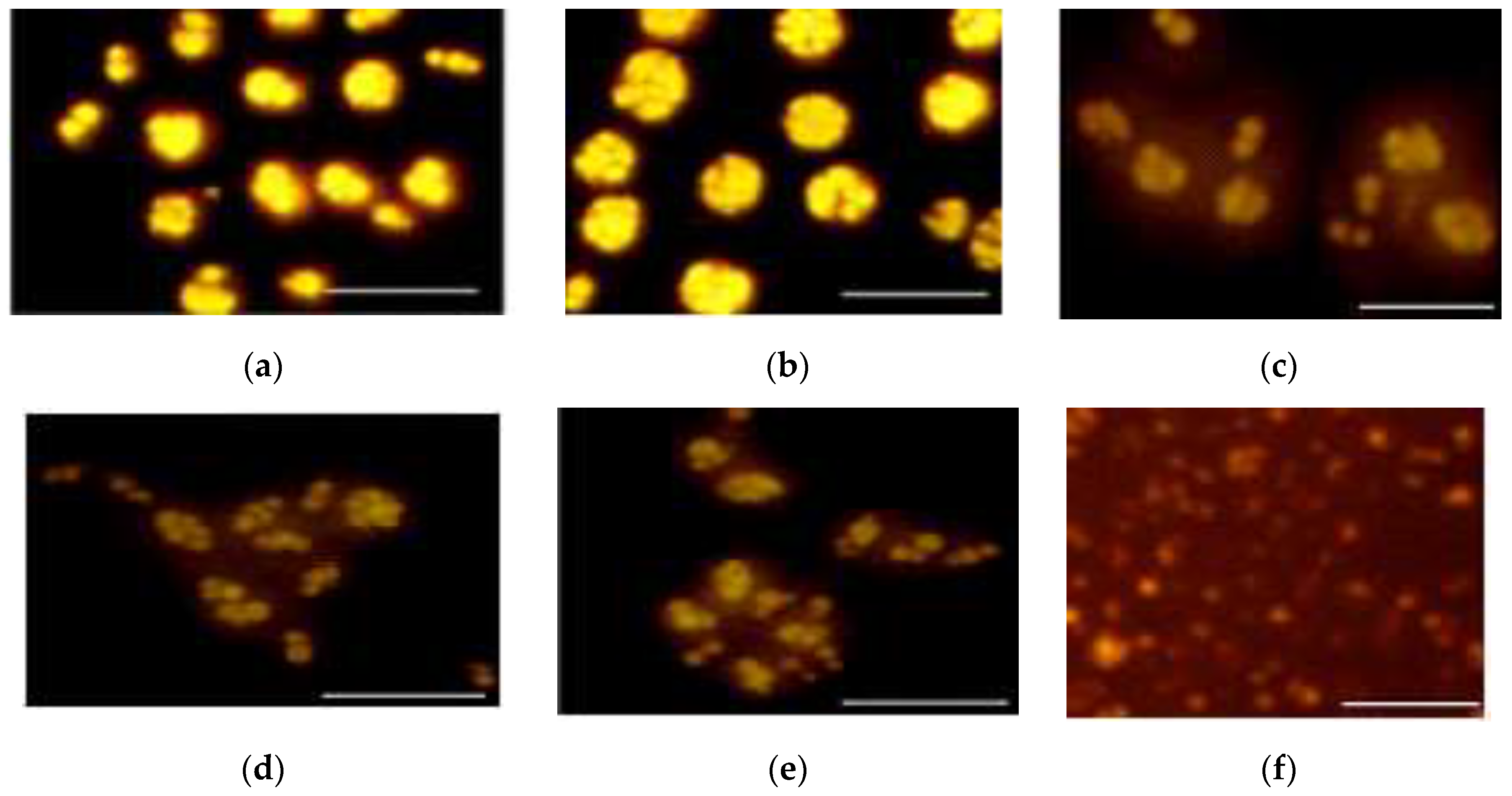
3.1.1. Microscopic Examination of R. glutinis Cellular Shapes and Lipid Bodies
3.1.2. The Growth, Lipid, and Carotenoid Production by R. glutinis under Different Culture Conditions
3.1.3. Lipid and Carotenoid Profile of R. glutinis under Different Culture Conditions
3.2. Effect of Different Metal Types and Concentrations on R. glutinis Growth, Lipid, and Carotenoid Production
3.2.1. Microscopic Examination of Cell and Lipid Bodies Accumulation of R. glutinis under Different Metal Treatments
3.2.2. Effect of Different Metal Stress on Lipid and Carotenoid Production by R. glutinis
3.3. Fed-Batch Cultivation of R. glutinis Using Metallo-Sulfo-Phospho-Glucose Feeding Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kot, A.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenge, C.; Cheirslip, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Modulation of immune function by dietary fatty acids. Proc. Nutr. Soc. 1998, 57, 277–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales-Campos, H.; Reis de Souza, P.; Peghini, C.B.; Da Silva, J.S.; Cardoso, C.R. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini. Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Phenolic compounds and squalene in olive oils: The concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignans and squalene. Food Chem. Toxicol. 2000, 38, 647–659. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Dimakopoulou, K.; Katsouyanni, K.; Bellander, T.; Grau, W.K.; Lanki, T.; Pistelli, R.; Schneider, A.; Peters, A. Mediterranean diet and inflammatory response in myocardial infarction survivors. Int. J. Epidemiol. 2009, 38, 856–866. [Google Scholar] [CrossRef] [Green Version]
- Tere’s, S.; Barcelo’-Coblijn, G.; Benet, M.; A’lvarez, R.; Bressani, R.; Halver, J.E.; Escriba, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA 2008, 37, 13811–13816. [Google Scholar] [CrossRef] [Green Version]
- Besler, H.T.; Grimble, R.F. Comparison of the modulatory influence of maize and olive oils and butter on metabolic responses to endotoxin in rats. Clin. Sci. 1995, 66, 59–66. [Google Scholar] [CrossRef]
- Ferrara, L.A.; Raimondi, A.S.; Episcopo, L.; Guida, L.; Dello Russo, A.; Marotta, T. Olive oil and reduced need for antihypertensive medications. Arch. Intern. Med. 2000, 160, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Kremer, M.; Lawrence, D.A.; Jubiz, W.; Digiacomo, R.; Rynes, R.; Bartholomew, L.E.; Sherman, M. Dietary Fish Oil And Olive Oil Supplementation In Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 1990, 33, 810–820. [Google Scholar] [CrossRef]
- Martin-Moreno, J.M.; Willett, W.C.; Gorgojo, L.; Banegas, J.R.; Rodriguez-Artalejo, F.; Fernandez-Rodriguez, J.C.; Maisonneuve, P.; Boyle, P. Dietary fat, olive oil intake and breast cancer risk. Int. J. Cancer 1994, 58, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, L.; Martinez, M.E.; Angell, J.; Hsieh, C.C.; Trichopoulos, D. Olive Oil and Human Cancer: An Assessment of the Evidence. Prev. Med. 1997, 26, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błazejak, S.; Kieliszek, M.; Gientka, I.; Bry’s, J. Simultaneous Production of Lipids and Carotenoids by the Red Yeast Rhodotorula from Waste Glycerol Fraction and Potato Wastewater. Appl. Biochem. Biotechnol. 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Kot, A.M.; Błazejak, S.; Gientka, I.; Kieliszek, M.; Bry’s, J. Torulene and torularhodin: “New” fungal carotenoids for industry? Microbl. Cell Fact. 2018, 100, 6103–6117. [Google Scholar] [CrossRef] [Green Version]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Brown, L.; Rimm, E.B.; Seddon, J.M.; Giovannucci, E.L.; Chasan-Taber, L.; Spiegelman, D.; Willett, W.C.; Hankinson, S.E. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am. J. Clin. Nutr. 1999, 70, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Beatty, S.; Nolan, J.; Kavanagh, H.; Donovan, O.O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. ABB 2004, 430, 70–76. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, A.H. Carotenoids and Flavonoids Contribute to Nutritional Protection against Skin Damage from Sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Mata-gómez, L.C.; Montañez, J.C.; Méndez-zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microbl. Cell Fact. 2014, 13, 1–11. Available online: http://www.microbialcellfactories.com/content/13/1/12 (accessed on 1 January 2014). [CrossRef] [PubMed] [Green Version]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef]
- Tkáčová, J.; Čaplová, J.; Klempová, T.; Čertík, M. Correlation between lipid and carotenoid synthesis in torularhodin-producing Rhodotorula Glutinis. Ann. Microbiol. 2017, 67, 541–551. [Google Scholar] [CrossRef]
- Elfeky, N.; Elmahmoudy, M.; Zhang, Y.; Guo, J.; Yongming, B. Lipid and Carotenoid Production by Rhodotorula glutinis with a Combined Cultivation Mode of Nitrogen, Sulfur and Aluminium Stress. Appl. Sci. 2019, 9, 2444. [Google Scholar] [CrossRef] [Green Version]
- Schulze, K.L. The Effect of Phosphate Supply on the Rate of Growth and Fat Formation in Yeasts. Appl. Microbiol. 1956, 4, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Baldrian, P.; Gabriel, I. Influence of Cadmium and Mercury on Activities of Ligninolytic Enzymes and Degradation of Polycyclic Aromatic Hydrocarbons by Pleurotus ostreatus in Soil. Appl. Environ. Microbiol. 2000, 66, 2471–2478. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cao, J.; Xing, G.; Yuan, H. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour. Technol. 2015, 175, 537–544. [Google Scholar] [CrossRef]
- Goswami, L.; Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Simultaneous heavy metal removal and anthracene biodegradation by the oleaginous bacteria Rhodococcus opacus. 3 Biotech 2017, 7, 1–9. [Google Scholar] [CrossRef]
- El-Banna, A.A.; Abd El-Razek, A.M.; El-Mahdy, A.R. Some Factors Affecting the Production of Carotenoids by Rhodotorula glutinis var. glutinis. Food Nutr. Sci. 2012, 3, 64–71. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Gadre, V. Production of β-carotene by a mutant of Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2001, 55, 423–427. [Google Scholar] [CrossRef]
- Sitepu, R.; Sestric, R.; Ignatia, L.; Levin, D.; German, J.; Gillies, L.A.; Almada, L.A.; Boundy-Mills, K.L. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour. Technol. 2013, 144, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Miloski, K.; Wallace, K.; Fenger, A.; Schneider, E.; Bendinskas, K. Comparison of Biochemical and Chemical Digestion and Detection Methods for Carbohydrates. Am. J. Undergrad. Res. 2019, 7, 7–18. [Google Scholar] [CrossRef]
- Kimura, K.; Yamaoka, M.; Kamisaka, Y. Rapid estimation of lipids in oleaginous fungi and yeasts using Nile red fluorescence. J. Microbiol. Methods 2004, 56, 331–338. [Google Scholar] [CrossRef]
- Available online: https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html (accessed on 1 January 2014).
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Van Wychen, S.; Ramirez, K.; Laurens, L.M. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by in situ Transesterification. NREL 2013, 303, 275–3000. [Google Scholar]
- Frengova, G.; Sirnova, E.; Pavlova, K.; Beshkova, D. Formation of Carotenoids by Rhodotorula glutinis in whey ultrafiltrate. Biotechnol. Bioeng. 1994, 44, 888–894. [Google Scholar] [CrossRef]
- Weber, R.W.; Anke, H.; Davoli, P. Simple method for the extraction and reversed-phase high-performance liquid chromatographic analysis of carotenoid pigments from red yeasts (Basidiomycota, Fungi). J. Chromatogr. A 2007, 1145, 118–122. [Google Scholar] [CrossRef]
- Certik, M.; Shimizu, S. Kinetic analysis of oil biosynthesis by an arachidonic acid-producing fungus, Mortierella alpina 1S-4. Appl. Microbiol. Biotechnol. 2000, 54, 224–230. [Google Scholar] [CrossRef]
- Patel, A.; Pruthi, V.; Singh, R.P.; Pruthi, P.A. Synergistic effect of fermentable and non-fermentable carbon sources enhances accumulation in oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour. Technol. 2015, 188, 136–144. [Google Scholar] [CrossRef]
- Karamerou, E. Bioprocessing Strategies for the Cultivation of Oleaginous Yeasts on Glycerol. PhD Thesis, School of Chemical Engineering and Analytical Science, University of Manchester, UK, 2016. [Google Scholar]
- Dias, C.; Sousa, S.; Caldeira, J.; Reis, A. New dual-stage pH control fed-batch cultivation strategy for the improvement of lipids and carotenoids production by the red yeast Rhodosporidium toruloides NCYC 921. Bioresour. Technol. 2015, 189, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, V.; Singh, M.; Saini, V.S.; Sista, V.R.; Yadav, N.K. Effect of pH on lipid accumulation by an oleaginous yeast: Rhodotorula glutinis IIP-30. World J. Microbiol. Biotechnol. 1992, 8, 382–384. [Google Scholar] [CrossRef]
- Milkessa, T.; Marizeth, J.; Carolina, G.; Laurinda, P.; Pohl, C. Optimization of cultivation conditions for biotechnological production of lipid by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 for biodiesel preparation. 3 Biotech 2017, 7, 1–11. [Google Scholar]
- Dhaliwal, M.K.; Chandra, N. Optimization of carotenoids production by Rhodotorula mucilaginosa. IJPSR 2015, 3, 1161–1165. [Google Scholar]
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, Identification of Carotenoid-Producing Rhodotorula sp. from Marine Environment and Optimization for Carotenoid Production. Mar. Drugs 2019, 17, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, S. Effect of Temperature and pH on the Growth Kinetics and Carotenoid Production by Sporobolomyces ruberrimus H110 Using Technical Glycerol as Carbon Source. Iran. J. Chem. Chem. Eng. 2006, 25, 59–64. [Google Scholar]
- Saad, N.; Abdeshahian, P.; Kalil, M.S.; Wan Yusoff, W.M.; Abdul Hamid, A. Optimization of Aeration and Agitation Rate for Lipid and Gamma Linolenic Acid Production by Cunninghamella bainieri 2A1 in Submerged Fermentation Using Response Surface Methodology. Sci. World J. 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Han, L.; He, H.; Yu, D.; Feng, J.; Zhang, X. Effects of agitation, aeration and temperature on production of a novel glycoprotein gp-1 by streptomyces kanasenisi zx01 and scale-up based on volumetric oxygen transfer coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef] [Green Version]
- Vlaev, S.; Pavlova, K.; Rusinova-Videva, S.; Georgieva, K.; Georgiev, D. Agitation effects and kinetic constants of exoglucomannan production by Antarctic yeast strain in a stirred tank bioreactor. Chem. Biochem. Eng. 2016, 30, 393–400. [Google Scholar] [CrossRef]
- Dai, C.; Tao, J.; Xie, F.; Dai, Y.J.; Zhao, M. Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. Afr. J. Biotechnol. 2007, 6, 2130–2134. [Google Scholar]
- El-Fadaly, A.H.; El-Naggar, N.; El-Sayed, M. Single Cell Oil Production by an Oleaginous Yeast Strain in a Low cost cultivation medium. Res. J. Microbiol. 2009, 4, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, S.; Zhu, Z.; Shen, H.; Lin, X.; Jin, X.; Jiao, X.; Zhao, Z. Biotechnology for Biofuels Systems analysis of phosphate-limitation-induced lipid accumulation by the oleaginous yeast Rhodosporidium toruloides. Biotechnol. Biofuels 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Buzzini, P.; Martini, A.; Gaetani, M.; Turchetti, B.; Pagnonib, U.; Davoli, P. Optimization of carotenoid production by Rhodotorula graminis DBVPG 7021 as a function of trace element concentration by means of response surface analysis. Enzym. Microb. Technol. 2005, 36, 687–692. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Latha, B.V.; Jeevaratnam, K.; Murali, H.S.; Manja, K.S. Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DFR-PDY from natural source. Indian J. Biotechnol. 2004, 4, 353–357. [Google Scholar]
- Frengova, G.I.; Beshkova, D.M. Carotenoids from Rhodotorula and Phaffia: Yeasts of biotechnological importance. J. Ind. Microbiol. Biotechnol. 2009, 36, 163–180. [Google Scholar] [CrossRef]
- Wongsnansilp, T.; Juntawong, N.; Wu, Z. Effects of phosphorus on the growth and chlorophyll fluorescence of a Dunaliella salina strain isolated from saline soil under nitrate limitation. J. Biol. Res. 2016, 89, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Forján, E.; Garbayo, I.; Bejarano, C.; Vílchez, C. Enhancement of carotenoid production in Nannochloropsis by phosphate and sulphur limitation. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Microbiology Book Series; Formatex: Badajoz, Spain, 2007; Volume 1, pp. 356–364. ISBN 978-84-611-9421-6. [Google Scholar]
- Kot, A.M.; Błazejak, S.; Kurcz, A.; Bry’s, J.; Gientka, I.; Bzducha-Wróbel, A.; Maliszewska, M.; Reczek, L. Effect of initial pH of medium with potato wastewater and glycerol on protein, lipid and carotenoid biosynthesis by Rhodotorula glutinis. Electron. J. Biotechnol. 2017, 27, 25–31. [Google Scholar] [CrossRef]
- Suutari, M.; Rintamliki, A.; Laakso, S. The Effect of Temperature on Lipid Classes and Their Fatty Acid Profiles in Lipomyces starkeyi. JAOCS 1996, 73, 1071–1073. [Google Scholar] [CrossRef]
- Miranti, A.; Arbianti, R.; Utami, T. Effect of pH, temperature and medium agitation rate in production of AA, DHA, EPA from Aspergillus oryzae with submerged fermentation. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012113. [Google Scholar] [CrossRef]
- Hu, H.; Wang, H.; Li, J.; Ma, L.; Shen, X.; Zeng, R. Evaluation of the effect of agitation speed on the growth and high-value LC-PUFA formation of Porphyridium cruentum based on basic rheological analysis. J. Chem. Technol. Biotechnol. 2019, 7, 2158–2166. [Google Scholar] [CrossRef]
- Davoli, P.; Mierau, V.; Weber, R.W.S. Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl. Biochem. Microbiol. 2004, 40, 392–397. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Tan, T. Lipid and carotenoid production by Rhodotorula glutinis under irradiation/high-temperature and dark/low-temperature cultivation. Bioresour. Technol. 2014, 157, 149–153. [Google Scholar] [CrossRef] [PubMed]












| Factor | T (Day) | DCW | Lipid | Carotenoid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DCW g/L | DCW-Y | TL g/L | TL-Y % | TL-RP | TP mg/L | Car-Y | Car-RP | |||
| g/g glu | g/gdcw | mg/100 g glu | µg/g dcw | |||||||
| Carbon sources | Glu | 3 rd | 6.71 ± 0.06 | 47.3 | 2.40 ± 0.20 | 16.8 | 35.6 | 0.56 ± 0.15 | 39.8 | 84.2 |
| C1 | 6 th | 10.5 ± 0.10 | 26.1 | 5.00 ± 0.14 | 18.0 | 47.7 | 0.81 ± 0.09 | 17.3 | 77.8 | |
| Suc | 3 rd | 5.60 ± 0.04 | 34.4 | 1.80 ± 0.05 | 11.0 | 31.9 | 0.45 ± 0.08 | 27.3 | 79.4 | |
| 6 th | 8.70 ± 0.08 | 24.0 | 4.00 ± 0.10 | 16.9 | 45.5 | 0.70 ± 0.03 | 19.9 | 80.6 | ||
| Glu + Suc | 3 rd | 7.20 ± 0.20 | 47.6 | 2.60 ± 0.05 | 17.3 | 36.4 | 0.67 ± 0.03 | 44.0 | 92.3 | |
| 6 th | 10.7 ± 0.50 | 21.1 | 5.30 ± 0.20 | 16.0 | 49.4 | 0.96 ± 0.07 | 17.9 | 89.9 | ||
| Mal | 3 rd | 6.00 ± 0.09 | 36.6 | 0.64 ± 0.20 | 3.90 | 10.6 | 0.33 ± 0.06 | 20.4 | 55.7 | |
| 6 th | 9.30 ± 0.10 | 35.0 | 2.70 ± 0.50 | 22.5 | 29.5 | 0.54 ± 0.11 | 21.8 | 58.0 | ||
| pH | 3 | 3 rd | 6.50 ± 1.70 | 33.0 | 2.80 ± 0.02 | 14.2 | 42.9 | 0.39 ± 0.06 | 19.7 | 59.5 |
| 6 th | 9.90 ± 2.20 | 35.7 | 4.90 ± 0.55 | 22.1 | 49.3 | 0.46 ± 0.15 | 7.80 | 46.8 | ||
| 4 | 3 rd | 6.50 ± 1.70 | 40.7 | 2.60 ± 0.38 | 16.4 | 40.4 | 0.44 ± 0.10 | 27.4 | 67.3 | |
| 6 th | 10.0 ± 1.00 | 30.2 | 5.20 ± 0.25 | 21.8 | 51.6 | 0.59 ± 0.16 | 12.8 | 58.6 | ||
| 5 | 3 rd | 6.71 ± 0.06 | 47.3 | 2.40 ± 0.20 | 16.8 | 35.6 | 0.56 ± 0.15 | 39.8 | 84.2 | |
| C1 | 6 th | 10.5 ± 0.10 | 26.1 | 5.00 ± 0.14 | 18.0 | 47.7 | 0.81 ± 0.09 | 17.3 | 77.8 | |
| 6 | 3 rd | 6.60 ± 0.53 | 47.6 | 1.97 ± 0.06 | 14.3 | 30.2 | 0.59 ± 0.02 | 42.9 | 90.1 | |
| 6 th | 10.5 ± 1.00 | 27.6 | 4.74 ± 0.46 | 19.2 | 45.1 | 0.89 ± 0.10 | 21.1 | 85.0 | ||
| 7 | 3 rd | 6.40 ± 1.40 | 46.7 | 1.86 ± 0.37 | 13.5 | 28.9 | 0.56 ± 0.04 | 40.8 | 87.5 | |
| 6 th | 9.50 ± 0.80 | 26.8 | 4.04 ± 0.36 | 18.9 | 42.3 | 0.80 ± 0.06 | 20.7 | 84.2 | ||
| 8 | 3 rd | 6.10 ± 1.10 | 46.3 | 0.55 ± 0.31 | 4.20 | 9.00 | 0.48 ± 0.02 | 36.4 | 78.5 | |
| 6 th | 9.00 ± 1.10 | 21.4 | 2.30 ± 0.30 | 12.6 | 25.3 | 0.73 ± 0.01 | 18.3 | 80.9 | ||
| Temp (°C) | 25 | 3 rd | 5.70 ± 0.34 | 47.6 | 1.36 ± 0.11 | 11.4 | 24.0 | 0.44 ± 0.10 | 36.7 | 77.1 |
| 6 th | 8.20 ± 0.30 | 24.7 | 3.63 ± 0.33 | 22.1 | 44.2 | 0.63 ± 0.12 | 19.0 | 77.0 | ||
| 28 | 3 rd | 6.71 ± 0.06 | 47.3 | 2.40 ± 0.20 | 16.8 | 35.6 | 0.56 ± 0.15 | 39.8 | 84.2 | |
| C1 | 6 th | 10.5 ± 0.1 | 26.1 | 5.00 ± 0.14 | 18.0 | 47.7 | 0.81 ± 0.09 | 17.3 | 77.8 | |
| 30 | 3 rd | 6.90 ± 0.11 | 43.8 | 2.39 ± 0.18 | 15.1 | 34.5 | 0.58 ± 0.15 | 36.8 | 83.9 | |
| 6 th | 10.9 ± 0.3 | 24.4 | 5.33 ± 0.37 | 18.2 | 49.1 | 0.83 ± 0.10 | 15.5 | 76.6 | ||
| 32 | 3 rd | 5.00 ± 0.11 | 45.4 | 1.29 ± 0.11 | 11.7 | 25.7 | 0.37 ± 0.10 | 33.8 | 74.5 | |
| 6 th | 7.41 ± 0.18 | 20.2 | 3.29 ± 0.35 | 16.9 | 44.4 | 0.58 ± 0.10 | 17.2 | 77.9 | ||
| Agitation (rpm) | 150 | 3 rd | 6.30 ± 0.27 | 48.3 | 1.77 ± 0.35 | 13.6 | 28.1 | 0.47 ± 0.10 | 36.4 | 75.3 |
| 6 th | 9.50 ± 0.48 | 26.4 | 3.50 ± 0.33 | 14.6 | 37.2 | 0.68 ± 0.10 | 17.1 | 71.7 | ||
| 180 | 3 rd | 6.70 ± 0.05 | 47.5 | 2.40 ± 0.20 | 16.6 | 35.0 | 0.57 ± 0.20 | 39.8 | 83.8 | |
| C1 | 6 th | 10.4. ± 0.1 | 25.6 | 5.00 ± 0.16 | 18.2 | 47.8 | 0.81 ± 0.10 | 16.7 | 77.2 | |
| 200 | 3 rd | 7.50 ± 0.50 | 47.2 | 2.60 ± 0.12 | 16.6 | 35.3 | 0.61 ± 0.20 | 38.5 | 81.6 | |
| 6 th | 11.0 ± 0.70 | 24.9 | 5.40 ± 0.30 | 19.3 | 48.8 | 0.89 ± 0.20 | 19.9 | 81.0 | ||
| 220 | 3 rd | 7.60 ± 0.50 | 43.9 | 2.60 ± 0.32 | 15.0 | 34.2 | 0.61 ± 0.20 | 35.4 | 80.6 | |
| 6 th | 11.0 ± 0.80 | 23.3 | 5.20 ± 0.22 | 17.7 | 47.1 | 0.89 ± 0.03 | 19.2 | 81.2 | ||
| C/P | 527 | 3 rd | 6.56 ± 1.60 | 41.5 | 2.61 ± 0.04 | 16.5 | 39.8 | 0.50 ± 0.17 | 31.6 | 76.0 |
| 6 th | 10.2 ± 1.90 | 21.6 | 5.30 ± 0.08 | 16.1 | 52.1 | 0.62 ± 0.11 | 7.33 | 60.9 | ||
| 176 | 3 rd | 6.80 ± 2.20 | 47.5 | 2.36 ± 0.20 | 16.6 | 35.0 | 0.57 ± 0.16 | 39.8 | 83.8 | |
| C1 | 6 th | 10.4 ± 2.60 | 25.3 | 5.10 ± 0.16 | 19.0 | 49.1 | 0.80 ± 0.10 | 16.6 | 77.4 | |
| 88 | 3 rd | 7.60 ± 2.60 | 51.2 | 1.60 ± 0.04 | 10.8 | 21.2 | 0.65 ± 0.02 | 43.5 | 85.0 | |
| 6 th | 11.0 ± 0.60 | 25.9 | 3.50 ± 0.04 | 14.5 | 31.8 | 0.94 ± 0.06 | 22.3 | 85.4 | ||
| 59 | 3 rd | 8.09 ± 1.70 | 53.7 | 0.64 ± 0.05 | 4.20 | 7.90 | 0.69 ± 0.04 | 45.8 | 85.3 | |
| 6 th | 11.5 ± 1.70 | 26.7 | 2.30 ± 0.06 | 12.6 | 19.5 | 0.99 ± 0.04 | 23.3 | 85.9 | ||
| Factor | Time (Day) | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | |
|---|---|---|---|---|---|---|---|---|
| Carbon sources | Glu C1 | 3 rd | 12.2 ± 1.4 | nd | 8.80 ± 9.9 | 68.8 ± 5.7 | 5.90 ± 8.0 | 4.30 ± 8.5 |
| 6 th | 20.4 ± 3.0 | nd | 11.7 ± 2.8 | 48.8 ± 7.1 | 10.4 ± 4.9 | 8.70 ± 3.1 | ||
| Suc | 3 rd | 12.7 ± 6.4 | nd | 5.50 ± 2.9 | 61.3 ± 10 | 15.2 ± 2.8 | 5.20 ± 6.2 | |
| 6 th | 17.1 ± 2.0 | nd | 2.40 ± 1.4 | 46.6 ± 5.7 | 9.10 ± 5.0 | 15.7 ± 2.0 | ||
| Glu+Suc | 3 rd | 7.20 ± 4.0 | nd | 5.10 ± 5.0 | 69.6 ± 4.2 | 13.5 ± 7.1 | 4.60 ± 5.0 | |
| 6 th | 11.4 ± 2.6 | nd | 8.00 ± 6.4 | 55.5 ± 5.6 | 14.6 ± 12 | 10.4 ± 4.9 | ||
| Mal | 3 rd | 8.40 ± 3.50 | nd | 15.3 ± 4.2 | 48.1 ± 1.0 | 12.7 ± 13 | 15.6 ± 4.9 | |
| 6 th | 10.1 ± 8.0 | nd | 6.10 ± 6.2 | 52.2 ± 6.4 | 21.1 ± 9.2 | 10.5 ± 11 | ||
| pH | 3 | 3 rd | 10.1 ± 4.2 | nd | 4.80 ± 9.9 | 60.7 ± 3.5 | 16.1 ± 9.2 | 8.40 ± 11 |
| 6 th | 13.6 ± 2.1 | nd | 10.3 ± 1.4 | 49.3 ± 9.2 | 19.9 ± 7.0 | 6.90 ± 2.8 | ||
| 4 | 3 rd | 10.9 ± 4.1 | nd | 5.80 ± 4.5 | 67.8 ± 5.4 | 10.5 ± 5.4 | 5.00 ± 5.6 | |
| 6 th | 14.5 ± 2.5 | 1.9 ± 2.3 | 8.30 ± 4.2 | 48.5 ± 2.9 | 20.1 ± 6.2 | 6.60 ± 6.4 | ||
| 5 C1 | 3 rd | 12.2 ± 1.4 | nd | 8.80 ± 9.9 | 68.8 ± 5.7 | 5.90 ± 8.0 | 4.30 ± 8.5 | |
| 6 th | 20.4 ± 3.0 | nd | 11.7 ± 2.8 | 48.8 ± 7.1 | 10.4 ± 4.9 | 8.70 ± 3.1 | ||
| 6 | 3 rd | 14.9 ± 4.5 | 2.5 ± 3.5 | 3.50 ± 3.7 | 64.1 ± 4.5 | 12.0 ± 3.0 | 3.00 ± 4.7 | |
| 6 th | 20.3 ± 4.4 | 2.3 ± 4.8 | 6.70 ± 3.6 | 48.4 ± 3.0 | 11.4 ± 4.3 | 11.0 ± 2.5 | ||
| 7 | 3 rd | 18.4 ± 2.3 | 1.0 ± 2.6 | 1.60 ± 2.2 | 57.6 ± 3.0 | 11.2 ± 5.7 | 10.2 ± 6.7 | |
| 6 th | 20.5 ± 5.8 | 3.1 ± 3.3 | 7.80 ± 6.0 | 46.3 ± 3.9 | 15.2 ± 4.6 | 7.20 ± 4.6 | ||
| 8 | 3 rd | 13.7 ± 4.0 | 1.3 ± 1.4 | 7.70 ± 4.7 | 58.0 ± 5.2 | 17.8 ± 4.2 | 1.40 ± 3.3 | |
| 6 th | 24.5 ± 4.9 | nd | 12.0 ± 2.4 | 44.0 ± 3.1 | 14.3 ± 4.0 | 5.30 ± 5.1 | ||
| Temp (°C) | 25 | 3 rd | 7.8 ± 0.40 | nd | 4.60 ± 1.0 | 66.4 ± 4.3 | 15.8 ± 6.2 | 5.30 ± 8.5 |
| 6 th | 10.4 ± 4.4 | nd | 3.80 ± 2.8 | 56.3 ± 7.1 | 20.6 ± 4.9 | 8.90 ± 3.1 | ||
| 28 C1 | 3 rd | 12.2 ± 1.4 | nd | 8.80 ± 9.9 | 68.8 ± 5.7 | 5.90 ± 8.0 | 4.30 ± 8.5 | |
| 6 th | 20.4 ± 3.0 | nd | 11.7 ± 2.8 | 48.8 ± 7.1 | 10.4 ± 4.9 | 8.70 ± 3.1 | ||
| 30 | 3 rd | 16.8 ± 0.3 | nd | 3.10 ± 4.3 | 62.6 ± 4.9 | 12.2 ± 4.2 | 5.20 ± 5.7 | |
| 6 th | 23.8 ± 3.5 | nd | 10.3 ± 4.9 | 46.5 ± 4.9 | 14.9 ± 6.4 | 4.50 ± 7.1 | ||
| 32 | 3 rd | 17.4 ± 3.2 | nd | 9.40 ± 0.7 | 59.8 ± 2.1 | 11.1 ± 7.1 | 2.20 ± 10 | |
| 6 th | 25.0 ± 5.7 | nd | 12.5 ± 2.1 | 45.7 ± 9.2 | 10.2 ± 7.8 | 6.50 ± 2.8 | ||
| Agitation (rpm) | 150 | 3 rd | 7.10 ± 2.10 | nd | 6.40 ± 5.0 | 69.5 ± 11.0 | 10.9 ± 2.8 | 6.10 ± 8.5 |
| 6 th | 21.2 ± 10.0 | nd | 8.40 ± 1.0 | 54.3 ± 1.0 | 10.1 ± 8.5 | 5.80 ± 5.7 | ||
| 180 C1 | 3 rd | 12.2 ± 1.4 | nd | 8.80 ± 10 | 68.8 ± 5.7 | 5.90 ± 8.0 | 4.30 ± 8.5 | |
| 6 th | 20.4 ± 3.0 | nd | 11.7 ± 3.0 | 48.8 ± 7.1 | 10.4 ± 4.9 | 8.70 ± 3.1 | ||
| 200 | 3 rd | 10.2 ± 1.4 | nd | 5.70 ± 4.0 | 60.8 ± 12 | 12.1 ± 6.4 | 11.2 ± 5.7 | |
| 6 th | 14.2 ± 3.5 | nd | 5.80 ± 2.0 | 45.9 ± 2.1 | 20.3 ± 6.3 | 13.7 ± 7.0 | ||
| 220 | 3 rd | 10.7 ± 1.0 | nd | 5.20 ± 8.0 | 56.8 ± 5.0 | 16.4 ± 7.1 | 10.9 ± 9.9 | |
| 6 th | 15.3 ± 5.0 | nd | 9.10 ± 9.0 | 46.6 ± 9.2 | 21.7 ± 7.5 | 7.30 ± 2.9 | ||
| C/P | 527 | 3 rd | 15.4 ± 5.0 | nd | 3.40 ± 3.2 | 69.8 ± 13 | 7.20 ± 14 | 4.20 ± 7.9 |
| 6 th | 19.7 ± 4.9 | 0.90 ± 0.71 | 3.10 ± 1.3 | 59.6 ± 4.2 | 12.1 ± 2.3 | 4.60 ± 2.1 | ||
| 176 C1 | 3 rd | 12.2 ± 1.4 | nd | 8.80 ± 10 | 68.8 ± 5.7 | 5.90 ± 8.0 | 4.30 ± 8.5 | |
| 6 th | 20.4 ± 3.0 | nd | 11.7 ± 3.0 | 48.8 ± 7.1 | 10.4 ± 4.9 | 8.70 ± 3.1 | ||
| 88 | 3 rd | 16.7 ± 6.4 | 1.0 ± 4.5 | 8.90 ± 1.6 | 60.1 ± 3.8 | 11.3 ± 7.1 | 2.00 ± 4.9 | |
| 6 th | 15.1 ± 7.0 | 3.0 ± 1.1 | 10.2 ± 3.0 | 44.6 ± 2.5 | 17.0 ± 7.1 | 10.0 ± 6.2 | ||
| 59 | 3 rd | 16.0 ± 11 | 0.8 ± 1.4 | 5.30 ± 3.3 | 60.9 ± 4.1 | 12.3 ± 21 | 4.60 ± 7.6 | |
| 6 th | 17.3 ± 0.8 | 1.2 ± 0.7 | 10.5 ± 4.0 | 43.6 ± 1.8 | 17.1 ± 1.6 | 10.3 ± 7.1 | ||
| Factor | C12:0 | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 ± 0.2 | 0.1 ± 0.03 | 13.2 ± 6.2 | 0.9 ± 6.7 | 5.8 ± 5.6 | 44.1 ± 4.7 | 29.3 ± 4.0 | 6.40 ± 6.0 | |
| FeSO4 0.1 mM | 1.2 ± 1.2 ** | 2.4 ± 3.5 ** | 23.9 ± 6.3 ** | 1.2 ± 6.5 * | 6.0 ± 1.9 | 55.3 ± 5.7 ** | 9.60 ± 2.1 ** | 0.40 ± 6.9 ** | |
| FeCl3 0.1 mM | 1.1 ± 0.3 ** | 2.3 ± 3.5 ** | 19.1 ± 5.5 ** | 1.1 ± 5.0 * | 7.5 ± 5.1 ** | 56.2 ± 6.7 ** | 11.0 ± 6.7 ** | 1.70 ± 3.3 ** | |
| CuSO4 0.1 mM | 2.3 ± 5.6 ** | 2.1 ± 1.8 ** | 31.3 ± 6.6 ** | 2.6 ± 4.5 ** | 6.1 ± 4.5 | 38.5 ± 6.6** | 14.5 ± 5.9 ** | 2.60 ± 7.5 ** | |
| CuCl2 0.1 mM | 2.1 ± 4.0 ** | 1.3 ± 1.2 ** | 26.4 ± 3.8 ** | 1.7 ± 5.1 ** | 7.5 ± 2.6 ** | 43.0 ± 7.9 ** | 14.8 ± 8.9 ** | 3.10 ± 4.0 ** | |
| MnCl2 | 0.1 mM | 0.1 ± 0.8 | 0.04 ± 0.7 ** | 15.2 ± 7.0 ** | 1.1 ± 3.0 * | 3.4 ± 6.6 ** | 29.5 ± 3.6 ** | 36.3 ± 5.8 ** | 14.3 ± 6.7 ** |
| 1 mM | 0.9 ± 0.5 ** | 1.7 ± 0.4 ** | 14.1 ± 2.0 * | 1.1 ± 2.0 * | 3.3 ± 4.8 ** | 34.3 ± 6.0 ** | 34.7 ± 5.0 ** | 9.90 ± 3.9 ** | |
| BaCl2 | 0.1 mM | 0.5 ± 0.5 ** | 0.1 ± 0.2 | 14.2 ± 9.0 * | 1.1 ± 1.8 * | 2.7 ± 10 ** | 33.1 ± 3.0 ** | 35.5 ± 3.8 ** | 12.7 ± 8.4 ** |
| 1 mM | 0.02 ± 0.5 ** | 0.2 ± 0.4 * | 15.2 ± 1.5 ** | 1.2 ± 4.0 * | 0.3 ± 5.0 ** | 35.0 ± 7.0 ** | 35.7 ± 2.8 ** | 12.3 ± 4.0 ** | |
| LiCl | 0.1 mM | 0.04 ± 0.3 ** | 0.1 ± 1.5 | 14.6 ± 5.4 * | 1.2 ± 4.5 * | 2.8 ± 4.0 ** | 36.1 ± 4.0 ** | 35.1 ± 6.8 ** | 10.0 ± 5.5 ** |
| 1 mM | nd | 0.1 ± 0.7 | 14.6 ± 6.0 * | 1.2 ± 5.0 * | 3.0 ± 6.0 ** | 33.0 ± 8.0 ** | 36.3 ± 3.9 ** | 11.9 ± 6.8 ** | |
| NiSO4 | 0.1 mM | nd | 0.1 ± 0.9 | 12.3 ± 5.0 * | 1.5 ± 6.0 ** | 6.0 ± 5.0 | 60.7 ± 3.8 ** | 16.2 ± 2.0 ** | 3.20 ± 5.5 ** |
| 1 mM | 0.2 ± 0.7 ** | 0.1 ± 0.8 | 13.6 ± 8.0 | 1.6 ± 5.0** | 6.0 ± 8.8 | 60.0 ± 7.0 ** | 15.6 ± 2.8 ** | 3.00 ± 1.2 ** | |
| ZnCl2 | 0.1 mM | 0.1 ± 1.3** | 0.04 ± 0.1 ** | 13.2 ± 5.7 | 0.6 ± 4.2 * | 11.2 ± 4.2 ** | 40.5 ± 6.6 ** | 27.8 ± 2.1 * | 6.60 ± 2.6 |
| 1 mM | 0.1 ± 0.6 ** | 0.05 ± .3.0 ** | 13.2 ± 4.2 | 0.7 ± 2.0 * | 12.2 ± 2.1 ** | 40.4 ± 3.3 ** | 27.3 ± 1.0 ** | 6.10 ± 3.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfeky, N.; Elmahmoudy, M.; Bao, Y. Manipulation of Culture Conditions: Tool for Correlating/Improving Lipid and Carotenoid Production by Rhodotorula glutinis. Processes 2020, 8, 140. https://doi.org/10.3390/pr8020140
Elfeky N, Elmahmoudy M, Bao Y. Manipulation of Culture Conditions: Tool for Correlating/Improving Lipid and Carotenoid Production by Rhodotorula glutinis. Processes. 2020; 8(2):140. https://doi.org/10.3390/pr8020140
Chicago/Turabian StyleElfeky, Nora, Mostafa Elmahmoudy, and Yongming Bao. 2020. "Manipulation of Culture Conditions: Tool for Correlating/Improving Lipid and Carotenoid Production by Rhodotorula glutinis" Processes 8, no. 2: 140. https://doi.org/10.3390/pr8020140
APA StyleElfeky, N., Elmahmoudy, M., & Bao, Y. (2020). Manipulation of Culture Conditions: Tool for Correlating/Improving Lipid and Carotenoid Production by Rhodotorula glutinis. Processes, 8(2), 140. https://doi.org/10.3390/pr8020140





