Current Advances in Biofouling Mitigation in Membranes for Water Treatment: An Overview
Abstract
1. Introduction
2. The Main Factors Playing an Important Role in the Biofouling Phenomenon
2.1. Physico-Chemical Composition of the Feed Solution
2.2. Effect of Transmembrane Pressure
2.3. Effect of pH
2.4. Effect of the Feed Flow Rate
2.5. Effect of Feed Temperature
2.6. Effect of the Intrinsic Membrane Properties
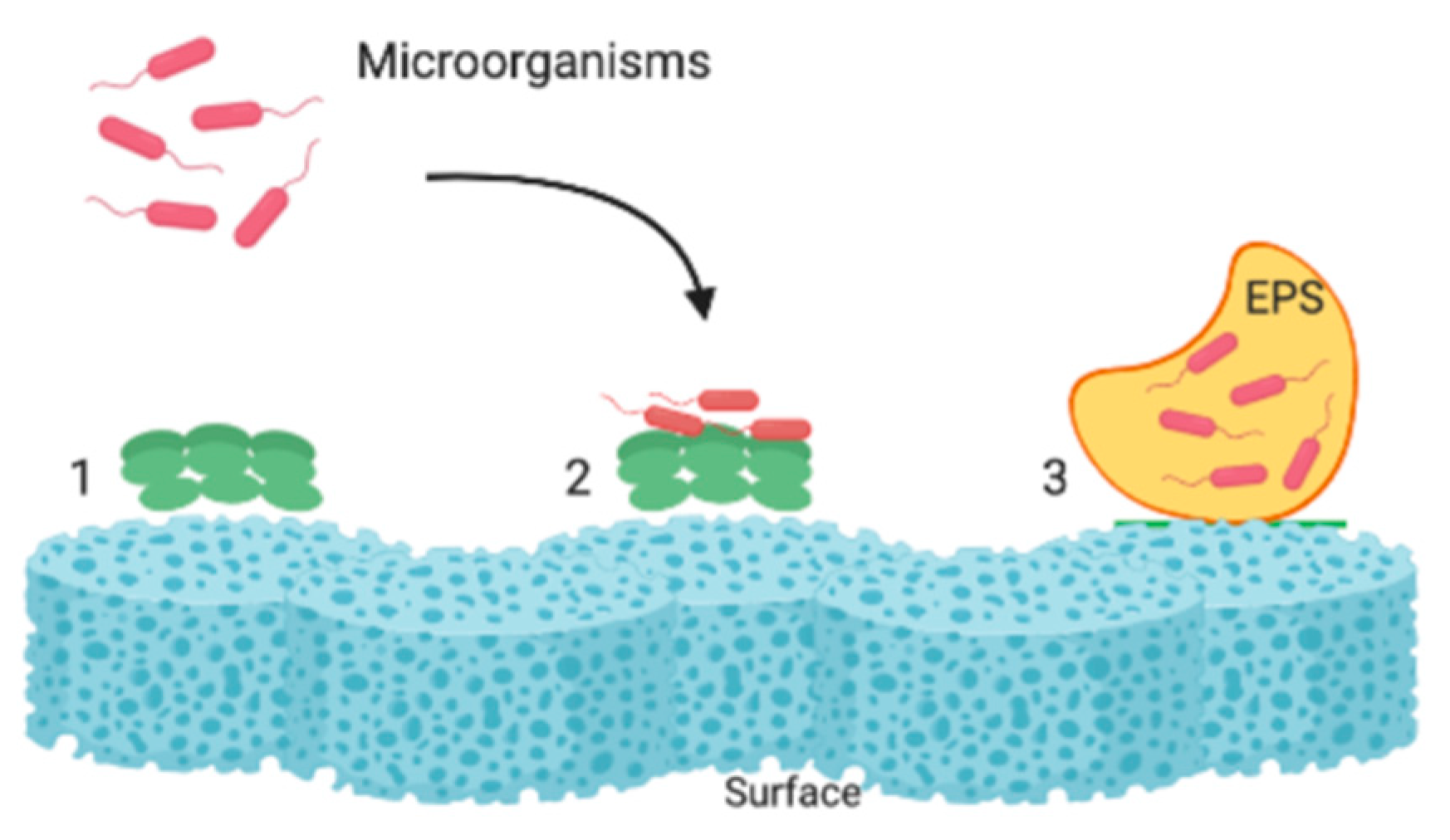
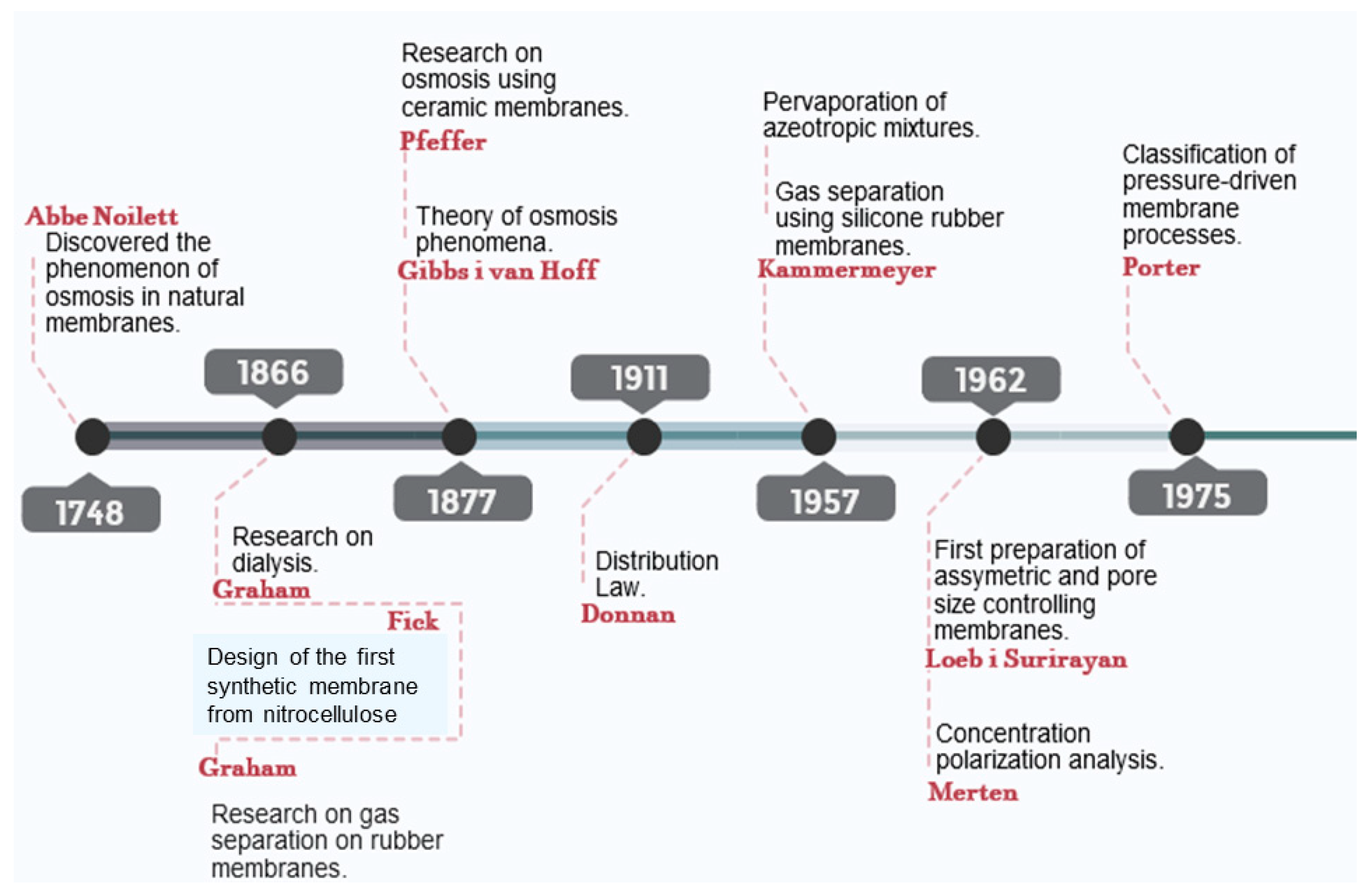
3. Beginnings of the Development Works Aimed at the Mitigation of Biofouling in Membranes
4. Current Advances in Biofouling Mitigation in Membranes
4.1. Polymer Blending
4.2. Nanocomposite Materials
4.3. Chemical Modification
4.4. Alternative Novel Strategies in Biofouling Mitigation
5. Concluding Remarks and Future Trends in the Field
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castro-Muñoz, R. Pressure-driven membrane processes involved in waste management in agro-food industries: A viewpoint. AIMS Energy 2018, 6, 1025–1031. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Cvejic, J.; Verardo, V.; Segura-Carretero, A. Food Use for Social Innovation by Optimizing Food Waste Recovery Strategies. In Innovation Strategies in the Food Industry: Tools for Implementation; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Yu, G.; Meng, X.; David Giraldo, J.; Thakur, V.K.; Gutiérrez, E. The History and State of Art in Membrane Technologies Tarragona, Erasmus 2005. J. Membr. Sci. 2013, 16, 1–28. [Google Scholar] [CrossRef]
- Kabsch-Korbutowicz, M.; Kutylowska, M. The Possibilities of Modelling the Membrane Separation Processes. Environ. Prot. Eng. 2008, 34, 15. [Google Scholar]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic membranes: Preparation and application for water treatment and desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Fíla, V.; Denis, P.C.; Ruby-Figueroa, R. Current role of membrane technology: From the treatment of agro-industrial by-products up to the valorization of valuable compounds. Waste Biomass Valorization 2018, 9, 513–529. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Membr. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.; Figoli, A. Progress of Nanocomposite Membranes for Water Treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed]
- Ozzello, E.; Mollea, C.; Bosco, F.; Bongiovanni, R. Factors Influencing Biofouling and Use of Polymeric Materials to Mitigate It. Adhes. Pharm. Biomed. Dent. Fields 2017, 185–206. [Google Scholar] [CrossRef]
- Kirschner, A.Y.; Cheng, Y.H.; Paul, D.R.; Field, R.W.; Freeman, B.D. Fouling mechanisms in constant flux crossflow ultrafiltration. J. Membr. Sci. 2019, 574, 65–75. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Perreault, F.; Jaramillo, H.; Xie, M.; Ude, M.; Nghiem, L.D.; Elimelech, M. Biofouling Mitigation in Forward Osmosis Using Graphene Oxide Functionalized Thin-Film Composite Membranes. Environ. Sci. Technol. 2016, 50, 5840–5848. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Tang, B.; Ding, J.; Zheng, Y.; Zhang, Z. Membrane fouling mechanism of biofilm-membrane bioreactor (BF-MBR): Pore blocking model and membrane cleaning. Bioresour. Technol. 2018, 250, 398–405. [Google Scholar] [CrossRef]
- Said, M.; Ahmad, A.; Mohammad, A.W.; Nor, M.T.M.; Sheikh Abdullah, S.R. Blocking mechanism of PES membrane during ultrafiltration of POME. J. Ind. Eng. Chem. 2015, 21, 182–188. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Conidi, C.; Cassano, A. Membrane-based technologies for meeting the recovery of biologically active compounds from foods and their by-products. Crit. Rev. Food Sci. Nutr. 2019, 59, 2927–2948. [Google Scholar] [CrossRef]
- Zheng, X. Major Organic Foulants in Ultrafiltration of Treated Domestic Wastewater and their Removal by Bio-Filtration as Pre-Treatment. Doctoral Thesis, Technische Universität Berlin, Berlin, Germany, 2010. [Google Scholar]
- Abdul Ghani, D.; Radwan, A.-R.; Mohammad, J. Studies on organic foulants in the seawater feed of reverse osmosis plants of SWCC. Desalination 2000, 132, 217–232. [Google Scholar]
- Maartens, A.; Swart, P.; Jacobs, E.P. Feed-water pretreatment: Methods to reduce membrane fouling by natural organic matter. J. Membr. Sci. 1999, 163, 51–62. [Google Scholar] [CrossRef]
- Jepsen, K.L.; Bram, M.V.; Pedersen, S.; Yang, Z. Membrane fouling for produced water treatment: A review study from a process control perspective. Water (Switzerland) 2018, 10, 847. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, G.; Wang, H.; Zhao, J.; Su, H.; Huang, Q. Effect of operating conditions on separation performance of reactive dye solution with membrane process. J. Membr. Sci. 2008, 321, 183–189. [Google Scholar] [CrossRef]
- Nanda, D.; Tung, K.L.; Li, Y.L.; Lin, N.J.; Chuang, C.J. Effect of pH on membrane morphology, fouling potential, and filtration performance of nanofiltration membrane for water softening. J. Membr. Sci. 2010, 349, 411–420. [Google Scholar] [CrossRef]
- Franks, R.; Bartels, C.; Nagghappan, L.N.S.P. Performance of a reverse osmosis system when reclaiming high Ph—High temperature wastewater. In Proceedings of the 2009 AWWA Membrane Technology Conference and Exposition, Memphis, TN, USA, 15–18 March 2009; pp. 1–16. [Google Scholar]
- Kucera, J. Biofouling of polyamide membranes: Fouling mechanisms, current mitigation and cleaning strategies, and future prospects. Membranes 2019, 9, 111. [Google Scholar] [CrossRef]
- Koo, C.H.; Mohammad, A.W.; Suja’, F. Effect of cross-flow velocity on membrane filtration performance in relation to membrane properties. Desalin. Water Treat. 2015, 55, 678–692. [Google Scholar] [CrossRef]
- Choi, H.; Zhang, K.; Dionysiou, D.D.; Oerther, D.B.; Sorial, G.A. Effect of permeate flux and tangential flow on membrane fouling for wastewater treatment. Sep. Purif. Technol. 2005, 45, 68–78. [Google Scholar] [CrossRef]
- Rezaei, H.; Ashtiani, F.Z.; Fouladitajar, A. Fouling behavior and performance of microfiltration membranes for whey treatment in steady and unsteady-state conditions. Braz. J. Chem. Eng. 2014, 31, 503–518. [Google Scholar] [CrossRef]
- Jin, X.; Jawor, A.; Kim, S.; Hoek, E.M.V. Effects of feed water temperature on separation performance and organic fouling of brackish water RO membranes. Desalination 2009, 239, 346–359. [Google Scholar] [CrossRef]
- Wong, P.C.Y.; Kwon, Y.N.; Criddle, C.S. Use of atomic force microscopy and fractal geometry to characterize the roughness of nano-, micro-, and ultrafiltration membranes. J. Membr. Sci. 2009, 340, 117–132. [Google Scholar] [CrossRef]
- Costa, A.R.; de Pinho, M.N.; Elimelech, M. Mechanisms of colloidal natural organic matter fouling in ultrafiltration. J. Membr. Sci. 2006, 281, 716–725. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.; Pinnau, I. Fouling of reverse osmosis membranes by biopolymers in wastewater secondary effluent: Role of membrane surface properties and initial permeate flux. J. Membr. Sci. 2007, 290, 173–181. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y. Control and cleaning of membrane biofouling by energy uncoupling and cellular communication. Environ. Sci. Technol. 2011, 45, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.; Walton, A. Membrane pretreatment of reverse osmosis: Long-term experience on difficult waters. Desalination 1999, 122, 157–170. [Google Scholar] [CrossRef]
- Amjad, Z. RO systems current fouling problems & solutions. Desalin. Water Reuse 1997, 6, 55–60. [Google Scholar]
- Isaias, N.P. Experience in reverse osmosis pretreatment. Desalination 2001, 139, 57–64. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.M. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Abu Tarboush, B.J.; Rana, D.; Matsuura, T.; Arafat, H.A.; Narbaitz, R.M. Preparation of thin-film-composite polyamide membranes for desalination using novel hydrophilic surface modifying macromolecules. J. Membr. Sci. 2008, 325, 166–175. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Valentine, R.L. Modeling the kinetics of ferrous iron oxidation by monochloramine. Environ. Sci. Technol. 2002, 36, 662–668. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: A review. Sep. Purif. Technol. 2010, 71, 137–143. [Google Scholar] [CrossRef]
- Surface, E.; Treatment, W. Committee Report: Recent advances and research needs in membrane fouling. J. Am. Water Work. Assoc. 2005, 97, 79–89. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Carretier, S.; Chen, L.A.; Venault, A.; Yang, Z.R.; Aimar, P.; Chang, Y. Design of PVDF/PEGMA-b-PS-b-PEGMA membranes by VIPS for improved biofouling mitigation. J. Membr. Sci. 2016, 510, 355–369. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, S.; Liang, H. Mechanisms for the enhancement of ultrafiltration and membrane cleaning by different ultrasonic frequencies. Desalination 2010, 263, 133–138. [Google Scholar] [CrossRef]
- Gule, N.P.; Begum, N.M.; Klumperman, B. Advances in biofouling mitigation: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 535–555. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef]
- Solís Carvajal, C.A.; Vélez Pasos, C.A.; Ramírez-Navas, J.S. Tecnología de membranas: Ultrafiltración. Entre Cienc. E Ing. 2017, 11, 26–36. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S. Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: Preparation, morphology, performance and antifouling properties. J. Membr. Sci. 2007, 305, 299–312. [Google Scholar] [CrossRef]
- Ma Wenzhong Rajabzadeh, S.; Shaikh, A.R.; Kakihana, Y.; Sun, Y.; Matsuyama, H. Effect of type of poly(ethylene glycol) (PEG) based amphiphilic copolymer on antifouling properties of copolymer/poly(vinylidene fluoride) (PVDF) blend membranes. J. Membr. Sci. 2016, 514, 429–439. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Zhao, X.; Li, Y.; Zhang, R.; Jiang, Z. Improved antifouling properties of polyethersulfone membrane by blending the amphiphilic surface modifier with crosslinked hydrophobic segments. J. Membr. Sci. 2015, 486, 195–206. [Google Scholar] [CrossRef]
- Ruan, H.; Li, B.; Ji, J.; Sotto, A.; Van Der Bruggen, B.; Shen, J.; Gao, C. Preparation and characterization of an amphiphilic polyamide nanofiltration membrane with improved antifouling properties by two-step surface modification method. RSC Adv. 2018, 8, 13353–13363. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, G.; Zhang, Q.; Zhan, X.; Chen, F. Improved Antifouling Properties of Poly(Ether Sulfone) Membrane by Incorporating the Amphiphilic Comb Copolymer with Mixed Poly(Ethylene Glycol) and Poly(Dimethylsiloxane) Brushes. Ind. Eng. Chem. Res. 2015, 54, 8789–8800. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V. Progress on incorporating zeolites in matrimid® 5218 mixed matrix membranes towards gas separation. Membranes 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; De La Iglesia, Ó.; Fila, V.; Téllez, C.; Coronas, J. Pervaporation-assisted esterification reactions by means of mixed matrix membranes. Ind. Eng. Chem. Res. 2018, 57. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Ong, C.S.; Goh, P.S.; Lau, W.J.; Misdan, N.; Ismail, A.F. Nanomaterials for biofouling and scaling mitigation of thin film composite membrane: A review. Desalination 2016, 393, 2–15. [Google Scholar] [CrossRef]
- Zhu, B.; Myat, D.T.; Shin, J.W.; Na, Y.H.; Moon, I.S.; Connor, G.; Maeda, S.; Morris, G.; Gray, S.; Duke, M. Application of robust MFI-type zeolite membrane for desalination of saline wastewater. J. Membr. Sci. 2015, 475, 167–174. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, Q.; Lv, R.; Soyekwo, F.; Zhu, A.; Liu, Q. Highly efficient polymer–MOF nanocomposite membrane for pervaporation separation of water/methanol/MTBE ternary mixture. Chem. Eng. Res. Des. 2017, 117, 688–697. [Google Scholar] [CrossRef]
- Khalid, A.; Abdel-Karim, A.; Ali Atieh, M.; Javed, S.; McKay, G. PEG-CNTs nanocomposite PSU membranes for wastewater treatment by membrane bioreactor. Sep. Purif. Technol. 2018, 190, 165–176. [Google Scholar] [CrossRef]
- María Arsuaga, J.; Sotto, A.; del Rosario, G.; Martínez, A.; Molina, S.; Teli, S.B.; de Abajo, J. Influence of the type, size, and distribution of metal oxide particles on the properties of nanocomposite ultrafiltration membranes. J. Membr. Sci. 2013, 428, 131–141. [Google Scholar] [CrossRef]
- Wang, L.; Han, X.; Li, J.; Zheng, D.; Qin, L. Modified MCM-41 silica spheres filled polydimethylsiloxane membrane for dimethylcarbonate/methanol separation via pervaporation. J. Appl. Polym. Sci. 2013, 127, 4662–4671. [Google Scholar] [CrossRef]
- Sun, X.; Qin, J.; Xia, P.; Guo, B.; Yang, C.; Song, C.; Wang, S. Graphene oxide—Silver nanoparticle membrane for biofouling control and water purification. Chem. Eng. J. 2015, 281, 53–59. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, K.; Wang, K.; Xie, Z.; Ladewig, B.; Wang, H. Fabrication of polyethersulfone-mesoporous silica nanocomposite ultrafiltration membranes with antifouling properties. J. Membr. Sci. [CrossRef]
- Sotto, A.; Boromand, A.; Balta, S.; Darvishmanash, S.; Kim, J.; Van der Bruggen, B. Nanofiltration membranes enhanced with TiO 2 nanoparticles: A comprehensive study. Desalin. Water Treat. 2011, 34, 179–183. [Google Scholar] [CrossRef]
- Matteucci, S.; Kusuma, V.A.; Kelman, S.D.; Freeman, B.D. Gas transport properties of MgO filled poly(1-trimethylsilyl-1-propyne) nanocomposites. Polymer 2008, 49, 1659–1675. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.; Wang, J.; Cao, B.; Tang, C.Y. In situ reduction of silver by polydopamine: A novel antimicrobial modification of a thin-film composite polyamide membrane. Environ. Sci. Technol. 2016, 50, 9543–9550. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Mehrnia, M.R.; Rahmani, S.; Mohades Mojtahedi, Y. Fabrication of alumina/polysulfone nanocomposite membranes with biofouling mitigation approach in membrane bioreactors. J. Ind. Eng. Chem. 2015, 22, 357–367. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Faria, A.F.; Liu, C.; Xie, M.; Perreault, F.; Nghiem, L.D.; Ma, J.; Elimelech, M. Thin-film composite forward osmosis membranes functionalized with graphene oxide–silver nanocomposites for biofouling control. J. Membr. Sci. 2017, 525, 146–156. [Google Scholar] [CrossRef]
- Ma Wen Soroush, A.; Van Anh Luong, T.; Brennan, G.; Rahaman, M.S.; Asadishad, B.; Tufenkji, N. Spray- and spin-assisted layer-by-layer assembly of copper nanoparticles on thin-film composite reverse osmosis membrane for biofouling mitigation. Water Res. 2016, 99, 188–199. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Rahbari-Sisakht, M.; Daneshfar, A.; Ghanbari, M.; Mayahi, A.; Matsuura, T.; Ismail, A.F. A novel thin film nanocomposite reverse osmosis membrane with superior anti-organic fouling affinity for water desalination. Desalination 2015, 368, 106–113. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Zinadini, S.; Alizadeh, A.; Heydari, R.; Beygzadeh, M.; Ghouzivand, S. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A.; Arabi Shamsabadi, A.; Sharifian, M.G.; Soroush, M. Mitigation of Thin-Film Composite Membrane Biofouling via Immobilizing Nano-Sized Biocidal Reservoirs in the Membrane Active Layer. Environ. Sci. Technol. 2017, 51, 5511–5522. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Salehi, H.; Ghorbani, F.; Amini, M.; Naslhajian, H. Polyoxometalate based thin film nanocomposite forward osmosis membrane: Superhydrophilic, anti-fouling, and high water permeable. J. Colloid Interface Sci. 2019, 536, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Chen, M.; Ma, J.; Chen, S.; Wu, Z. Membrane biofouling control using polyvinylidene fluoride membrane blended with quaternary ammonium compound assembled on carbon material. J. Membr. Sci. 2017, 539, 229–237. [Google Scholar] [CrossRef]
- Mooss, V.A.; Hamza, F.; Zinjarde, S.S.; Athawale, A.A. Polyurethane films modified with polyaniline-zinc oxide nanocomposites for biofouling mitigation. Chem. Eng. J. 2019, 359, 1400–1410. [Google Scholar] [CrossRef]
- Carmalin Sophia, A.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater—A review. Desalin. Water Treat. 2016, 3994, 1–14. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly (vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-Gonzalez, J.; de la Iglesia, O.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly(vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water Through Helium-Leak–Tight Graphene-Based Membranes. Science 2012, 335, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Bian, X.; Lu, X.; Shi, L.; Liu, Z.; Chen, L.; Hou, Z.; Fan, K. Preparation and characterization of ZnO / polyethersulfone (PES) hybrid membranes. DES 2012, 293, 21–29. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, W.; Shi, M.; Wang, Z.; Wang, J. Improving permeability and antifouling performance of polyethersulfone ultra fi ltration membrane by incorporation of ZnO-DMF dispersion containing nano-ZnO and polyvinylpyrrolidone. J. Membr. Sci. 2015, 478, 105–116. [Google Scholar] [CrossRef]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van der Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Membr. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Ahn, S.; Cong, X.; Lebrilla, C.B.; Gronert, S. Zwitterion formation in gas-phase cyclodextrin complexes. J. Am. Soc. Mass Spectrom. 2005, 16, 166–175. [Google Scholar] [CrossRef][Green Version]
- Venault, A.; Huang, W.Y.; Hsiao, S.W.; Chinnathambi, A.; Alharbi, S.A.; Chen, H.; Zheng, J.; Chang, Y. Zwitterionic Modifications for Enhancing the Antifouling Properties of Poly(vinylidene fluoride) Membranes. Langmuir 2016, 32, 4113–4124. [Google Scholar] [CrossRef]
- Bott, T.R. Potential physical methods for the control of biofouling in water systems. Chem. Eng. Res. Des. 2001, 79, 484–490. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Jiang, B.B.; Sun, X.F.; Wang, L.; Wang, S.Y.; Liu, R.D.; Wang, S.G. Polyethersulfone membranes modified with D-tyrosine for biofouling mitigation: Synergistic effect of surface hydrophility and anti-microbial properties. Chem. Eng. J. 2017, 311, 135–142. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Barclay, T.G.; Michelmore, A.; Zou, L.; Saint, C.P.; Ginic-Markovic, M. Effective in-situ chemical surface modification of forward osmosis membranes with polydopamine-induced graphene oxide for biofouling mitigation. Desalination 2016, 385, 126–137. [Google Scholar] [CrossRef]
- Wibisono, Y.; Yandi, W.; Golabi, M.; Nugraha, R.; Cornelissen, E.R.; Kemperman, A.J.B.; Ederth, T.; Nijmeijer, K. Hydrogel-coated feed spacers in two-phase flow cleaning in spiral wound membrane elements: Anovel platform for eco-friendly biofouling mitigation. Water Res. 2015, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Alayande, A.B.; Chae, S.; Kim, I.S. Applications of nisin for biofouling mitigation of reverse osmosis membranes. Desalination 2018, 429, 52–59. [Google Scholar] [CrossRef]
- Katalo, R.; Okuda, T.; Nghiem, L.D.; Fujioka, T. Moringa oleifera coagulation as pretreatment prior to microfiltration for membrane fouling mitigation. Environ. Sci. Water Res. Technol. 2018, 4, 1604–1611. [Google Scholar] [CrossRef]
- Qasim, M.; Darwish, N.N.; Mhiyo, S.; Darwish, N.A.; Hilal, N. The use of ultrasound to mitigate membrane fouling in desalination and water treatment. Desalination 2018, 443, 143–164. [Google Scholar] [CrossRef]
- Ma Wen Panecka, M.; Tufenkji, N.; Rahaman, M.S. Bacteriophage-based strategies for biofouling control in ultrafiltration: In situ biofouling mitigation, biocidal additives and biofilm cleanser. J. Colloid Interface Sci. 2018, 523, 254–265. [Google Scholar] [CrossRef]
- Ali, J.; Wang, L.; Waseem, H.; Sharif, H.M.A.; Djellabi, R.; Zhang, C.; Pan, G. Bioelectrochemical recovery of silver from wastewater with sustainable power generation and its reuse for biofouling mitigation. J. Clean. Prod. 2019, 235, 1425–1437. [Google Scholar] [CrossRef]
- Ergön-Can, T.; Köse-Mutlu, B.; Koyuncu, İ.; Lee, C.H. Biofouling control based on bacterial quorum quenching with a new application: Rotary microbial carrier frame. J. Membr. Sci. 2017, 525, 116–124. [Google Scholar] [CrossRef]
- Al-Abri, M.; Al-Ghafri, B.; Bora, T.; Dobretsov, S.; Dutta, J.; Castelletto, S.; Rosa, L.; Boretti, A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. Npj Clean Water 2019, 2. [Google Scholar] [CrossRef]
- Klein, T.; Zihlmann, D.; Derlon, N.; Isaacson, C.; Szivak, I.; Weissbrodt, D.G.; Pronk, W. Biological control of biofilms on membranes by metazoans. Water Res. 2016, 88, 20–29. [Google Scholar] [CrossRef]
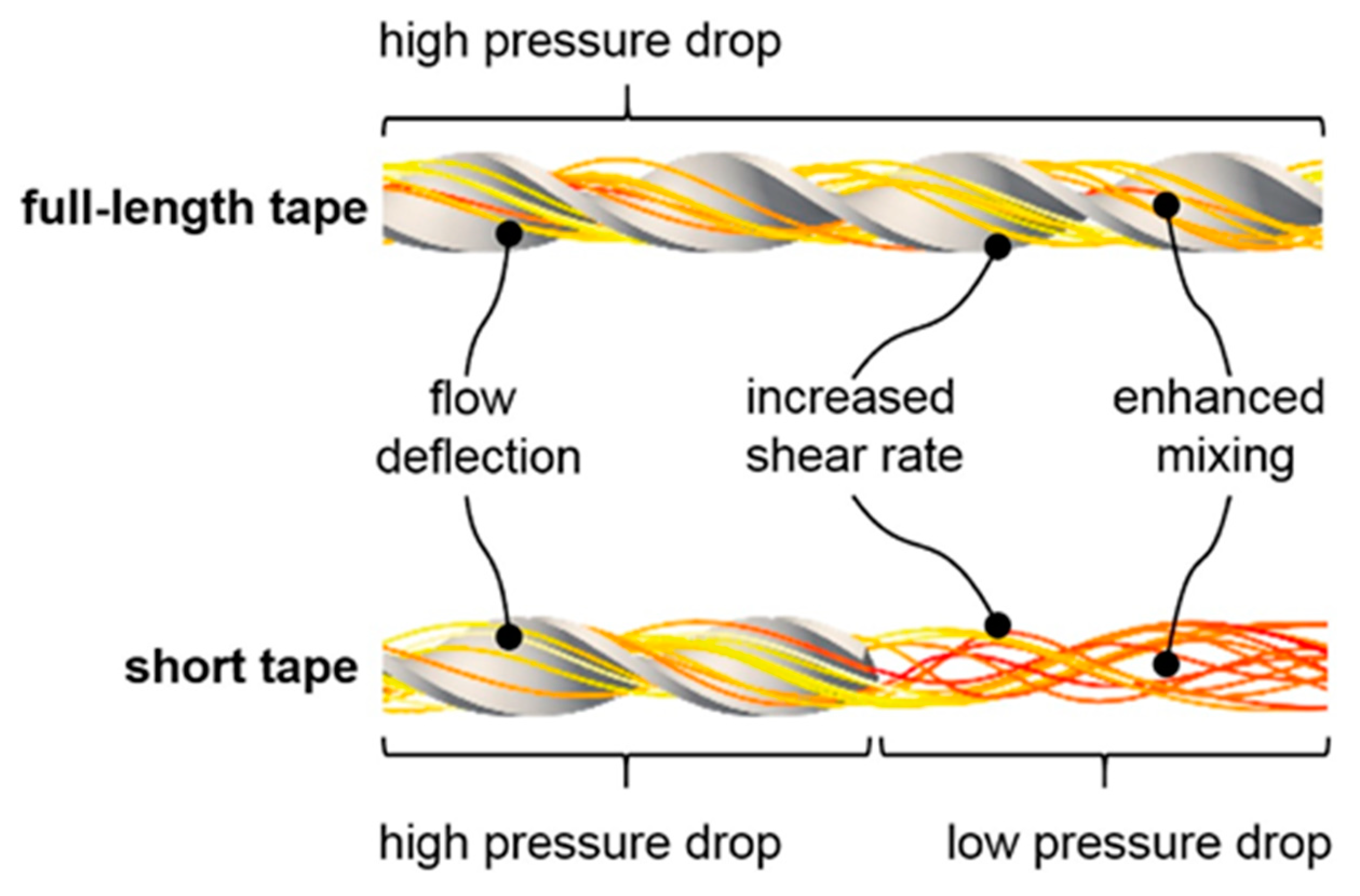
- Armbruster, S.; Stockmeier, F.; Junker, M.; Schiller-Becerra, M.; Yüce, S.; Wessling, M. Short and spaced twisted tapes to mitigate fouling in tubular membranes. J. Membr. Sci. 2020, 595, 117426. [Google Scholar] [CrossRef]


| Membrane Process | Required Pressure (bar) | Typical Separation Mechanism | |
|---|---|---|---|
| Min. | Max. | ||
| Microfiltration | 0.1 | 2 | Sieving |
| Ultrafiltration | 0.1 | 7 | Sieving |
| Nanofiltration | 3 | 25 | Sieving and charge effect |
| Classification | Compound | MW (Da) | Size (µm) |
|---|---|---|---|
| Dissolved Organic Matter (DOM) | Nutrients | 10–102 | <10−4 |
| Amino acids | >10–102.5 | <10−4–10−4 | |
| Recalcitrant Matter | >10–103 | <10−4–10–3.8 | |
| Carbohydrate | 102–103 | <104–10–3.6 | |
| Fatty acid | |||
| Chlorophyl | 103–103.5 | 10−4–10−3.3 | |
| Vitamin | 103–104 | 10−3.8–10−3.3 | |
| Humic acid | 103.3–106.5 | 10−3.5–10−1.8 | |
| Proteins | 103.6–107.5 | 10−3.5–10−1.2 | |
| RNA | 104–106.5 | 10−3.1–10−2.4 | |
| Extracellular enzyme | 104–105 | 10−4.8–10−3.5 | |
| Polysaccharide | 104–107 | 10−3–<10−1 | |
| Virus | 106–109 | 10−2–<10 | |
| Cell fragment | >107–<109 | >10–2–<10 | |
| DNA | >107–109 | 10−1–<10 | |
| Particular/Colloidal Organic Matter (POM/COM) | Organic debris | >108 | >10–1–103 |
| Bacteria | >109 | <10–<102 | |
| Algae and protozoa | >109 | 10–103 |
| Year | Authors | Remark of the Study | Reference |
|---|---|---|---|
| 1784 | J. Abbe Nollet | Discovery of the osmosis phenomenon in natural membranes | [4] |
| 1999 | Durham and Walton | Description of the early stages of pretreatment in desalination processes | [36] |
| 1997 | Amjad | Starting solutions for fouling | [37] |
| 2001 | Isaias | Pretreatment for fouling in desalination processes | [38] |
| 2002 | Vikesland and Valentine | Studies in monochloramine as an oxidant for Fe (II) removal in drinking water treatment. | [41] |
| 2006 | Le-Clench, Chen and Fane | Early stages of studies in membrane fouling for bioreactors used in wastewater treatment | [44] |
| 2008 | Khawaji, Kutubkhanah and Wie | Basic aspects and advances in seawater desalination, and fouling. | [39] |
| 2008 | Abu, Tarboush, Rana, Matsuura, Arafat and Narbaitz. | Research in polyamide membranes via surface modification for desalination | [40] |
| 2010 | Porcelli and Judd | Cleaning of drinking water using membranes | [42] |
| 2011 | Xu and Liu | Membrane fouling and cleaning. | [35] |
| Nanomaterial | Polymer | Remark of the Study | Reference |
|---|---|---|---|
| TiO2 | Polyamide (PA) | Good flux recovery by incorporating TiO2. Enhanced foulant removal than pristine membrane. | [60] |
| Al2O3 | Polysulfone (PS) | Water flux increase. Membrane fouling was reduced by 83%. | [71] |
| GO-Ag | Thin-film composite (TFC) | Static antimicrobial assays showed a significant inhibition to the attachment of Pseudomonas aeruginosa cells. | [74] |
| Cu | Thin-film composite (TFC) | The nanomaterial was deposited via spray- and spin-assisted layer-by-layer. The method was efficient and improved the distribution compared to conventional dip coating techniques. Cu nanoparticles improved the anti-biofouling properties. Cu nanoparticles effectively inhibited the permeate flux reduction caused by bacterial deposition. | [75] |
| NH2-TNTs | Polyamide (PA) | The water flux of the membrane was significantly increased. The nanomaterial significantly mitigated the BSA fouling and achieved a promising water flux recovery rate after rinsing. | [76] |
| Fe3O4 | Polyethersulfone (PES) | Iron oxide nanoparticles resulted in an increase in hydrophilicity and growth in the membrane sub-layer porosity. The pore radius was affected. | [77] |
| Silver-based MOF | Thin-film composite (TFC) | The MOF improved both the biocidal activity and the hydrophilicity of the membrane active layer. No effect was observed on the membrane selectivity. | [78] |
| Silica/QA/POM | Thin-film composite (TFC) | Membrane with 0.2 wt. % nanoparticle incorporation showed superior water flux in forward osmosis processes and minimal increase in reverse salt flux. Moreover, enhanced antifouling propensity toward BSA and sodium alginate foulant was noted. | [79] |
| QAC/Carbon | Polyvinylidene Fluoride (PVDF) | The introduction of Quatery Ammonium Compound assembled on Carbon into polymeric membranes was an effective way to prepare anti-biofouling membranes for water and wastewater treatment. | [80] |
| ZnO | Polyaniline (PANI) | The resulting membranes showed a good mechanical strength with moderate elasticity. The membranes showed good antifouling properties toward marine bacteria V. harveyi and B. licheniformis. | [81] |
| Material | Remarks | References |
|---|---|---|
| Divalent Cations | Calcium enhanced the fouling properties due to its bridging effects between carboxylic active groups contained in NOM and the negatively charged functional groups in the membrane surface. | [19] |
| Metal ions (Al3+ and Fe3+) | They are being used to form large precipitating complexes with the Humic acid and fulvic acid, and thus to facilitate their elimination. | [20] |
| Sulfonic groups | The attaching of sulfonic groups to the aromatic backbone of polysulfone and polyethersulfone membranes generated an electrophilic aromatic substitution reaction, in which hydrogen is replaced by sulfonic acid. | [92] |
| Carboxylation | The presence of carboxylic groups increased the membrane hydrophilicity. | [92] |
| Plasma treatment | The bombarded surface of the membrane with ionized plasma components generated radical sites. Active components generated by such plasma contributed to increasing the hydrophilicity without affecting the bulk of the polymer. | [92] |
| CO2-plasma | The addition of oxygen into the membrane’ surface, in the form of carbonyl, acid and ester groups, increased in hydrophilicity. | [92] |
| D-Tyrosine | D-amino acids inhibited the microbial attachment. D-tyrosine enhanced the membrane hydrophilicity and provided a smoother surface to the membrane without modifying its transport properties, and also reduced the propensity for biofouling. | [93] |
| GO-pDA | The attaching of graphene oxide nanosheets to the membrane surface, by chemical modification with polydopamine through an oxidative polymerization, reduced the loss of the draw solution and increased both membrane water flux and biofouling resistance. | [94] |
| Charged hydrogel | Anti-biofouling properties of neutral (polyHEMA-co-PEG10MA), cationic (polyDMAEMA) and anionic (polySPMA) hydrogels in feed spacers were tested with E. coli. The membranes showed reduced attachment and biofouling in the spacer-filled channels, resulting in delayed biofilm growth. | [95] |
| Antimicrobial peptides | A polycyclic antimicrobial peptide, like nisin, decreased the viability of Bacillus sp., and the dislodging of P. aeruginosa P60. Nisin served as a biological agent for the mitigation of membrane biofouling. | [96] |
| Approach | Description | References |
|---|---|---|
| Addition of bacteriophages as biocidal agents | T4 bacteriophage-facilitated biofouling control in the membrane ultrafiltration to inhibit the propagation of E. coli in situ. | [99] |
| Bio-electrochemistry | Silver was bioelectrochemically recovered from wastewater. It is an eco-friendly method showing the potential in anti-biofouling applications with recovered nano-flakes, particularly in membrane bioreactors. | [100] |
| Quorum quenching | The quorum quenching caused to prevent biofouling since quorum sensing interrupts the biological communication mechanism between microorganisms. This was achieved with rotational membrane filtration modules | [101] |
| UV light | Ultraviolet (UV) light penetrates the cell wall and damages the DNA and RNA, thus stopping the microorganism from reproducing. Furthermore, the main advantage is that it does not produce chemical by-products that can affect health. | [102] |
| Metazoans | The presence of an oligochaete (Aelosoma hemprichi), and a nematode (Plectus aquatilis) strongly affected the formation of biofilm. | [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pichardo-Romero, D.; Garcia-Arce, Z.P.; Zavala-Ramírez, A.; Castro-Muñoz, R. Current Advances in Biofouling Mitigation in Membranes for Water Treatment: An Overview. Processes 2020, 8, 182. https://doi.org/10.3390/pr8020182
Pichardo-Romero D, Garcia-Arce ZP, Zavala-Ramírez A, Castro-Muñoz R. Current Advances in Biofouling Mitigation in Membranes for Water Treatment: An Overview. Processes. 2020; 8(2):182. https://doi.org/10.3390/pr8020182
Chicago/Turabian StylePichardo-Romero, Daniela, Zahirid Patricia Garcia-Arce, Alejandra Zavala-Ramírez, and Roberto Castro-Muñoz. 2020. "Current Advances in Biofouling Mitigation in Membranes for Water Treatment: An Overview" Processes 8, no. 2: 182. https://doi.org/10.3390/pr8020182
APA StylePichardo-Romero, D., Garcia-Arce, Z. P., Zavala-Ramírez, A., & Castro-Muñoz, R. (2020). Current Advances in Biofouling Mitigation in Membranes for Water Treatment: An Overview. Processes, 8(2), 182. https://doi.org/10.3390/pr8020182







