Abstract
The term biolubricant applies to all lubricants that are easily biodegradable and non-toxic to humans and the environment. The uses of biolubricant are still very limited when compared to those of mineral oils, although this trend is increasing and depends on investment in research and development (R&D). The increase in demand for biodegradable lubricants is related to the evolution of environmental regulations, with more restrictive rules being implemented to minimize environmental impact caused by inappropriate disposal. This study provides an overview of the types, production routes, properties, and applications of biolubricants. Biolubricants are classified as either natural or synthetic oils according to chemical composition. Natural oils are of animal or vegetable origin and are rarely used because they are unstable at high temperatures and form compounds that are harmful to equipment and machines. Synthetic oils are obtained from chemical reactions and are the best lubricants for demanding applications. They are obtained by various routes, mainly by obtaining straight or branched-chain monoesters, diesters, triesters, and polyol esters from vegetable oils. The conversion of triglyceride to esters can be followed or preceded by one or more reactions to improve reactions such as epoxidation and hydrogenation.
1. Introduction
The increasing global population together with industrialization and modernization has led to an increase in energy consumption. Over the last century, societies have strongly depended on fossil fuels, causing a progressive depletion of fuel reserves in such a way that it has been predicted that this non-renewable energy source will be exhausted in the medium-term, thereby increasing search and development efforts for alternative chemicals and energy sources which could replace traditional fossil fuels. Many types of renewable energy sources, such as hydropower, geothermal, wind, solar, or biomass energy, have been proposed as potential energy sources. Among them, biomass is the only renewable source from which energy and chemical products can be obtained; therefore, this is the only current alternative able to replace petroleum in the synthesis of a wide range of valuable organic products.
It was reported that the annual production of lubricants is 30-40 million tons; these compounds are often used for many industrial applications in order to decrease friction and heat, protect against corrosion and wear, transmit energy, and eliminate contaminants or sealing processes, among others [1,2,3]. It was reported that about 50%–75% of total lubricant production is poured uncontrollably into the environment [2]. About 95% of total lubricant production is petroleum-based, generally denoted as mineral oil. These oils are composed of a complex mixture of paraffinic (linear/branch), olefinic, naphthenic, and aromatic hydrocarbons of 20 to 50 carbon atoms. These formulations are non-renewable and toxic in such a way that the lubricants are harmful to humans and the environment as a consequence of their low biodegradability and high toxicity; some are even considered to be carcinogenic. However, biolubricants display faster and easier biodegradability, thereby exerting less adverse effects on the environment [4]. In spite of the high toxicity of the biolubricants, lubricant discharge was also reported, causing strong contamination in air, soil, and drinking water. Considering the annual volume of lubricants and their environmental effects, governments have introduced strict regulations to mitigate the effects of the disposal of these lubricants [5].
In order to satisfy the new environmental regulations, the scientific community is developing new lubricants with greater biodegradability and less toxicity [6]. In this sense, the lubricants obtained from bio-based sources (biolubricants) have emerged as potential alternatives to replace traditional mineral oils synthesized from petroleum [7]. Biolubricants display excellent physicochemical properties, such as high viscosity indexes and flash points as well as good resistance to shear and high biodegradability, in such a way that these compounds can be considered renewable and readily biodegradable [8,9,10] (Table 1), allowing them to be used in various industrial applications, including as emulsifiers, lubricants, plasticizers, surfactants, plastics, solvents, and resins [9,10] (Table 2). Careful selection of the starting oil to synthesize a biolubricant is important, since, in most cases, vegetable oils are used in the food chain. The use of these oils for industrial applications could cause an increase in speculation, thereby increasing prices and social imbalances. Considering these data, the most sustainable alternative is the use of vegetable oils, which do not interfere with the food chain.

Table 1.
Advantages of biolubricant use.

Table 2.
Main applications of several vegetable oils.
Despite the great potential of vegetable oils as biolubricants, they are not widely commercialized due to their high heterogeneity and other undesirable physical properties related to poor oxidation stability, poor low temperature properties, and poor viscosity indexes, among others [11,12].
Considering these disadvantages, the main challenge of biolubricants is maintaining consistent chemical composition of the vegetable oils used as starting materials in order to meet final application and performance specifications.
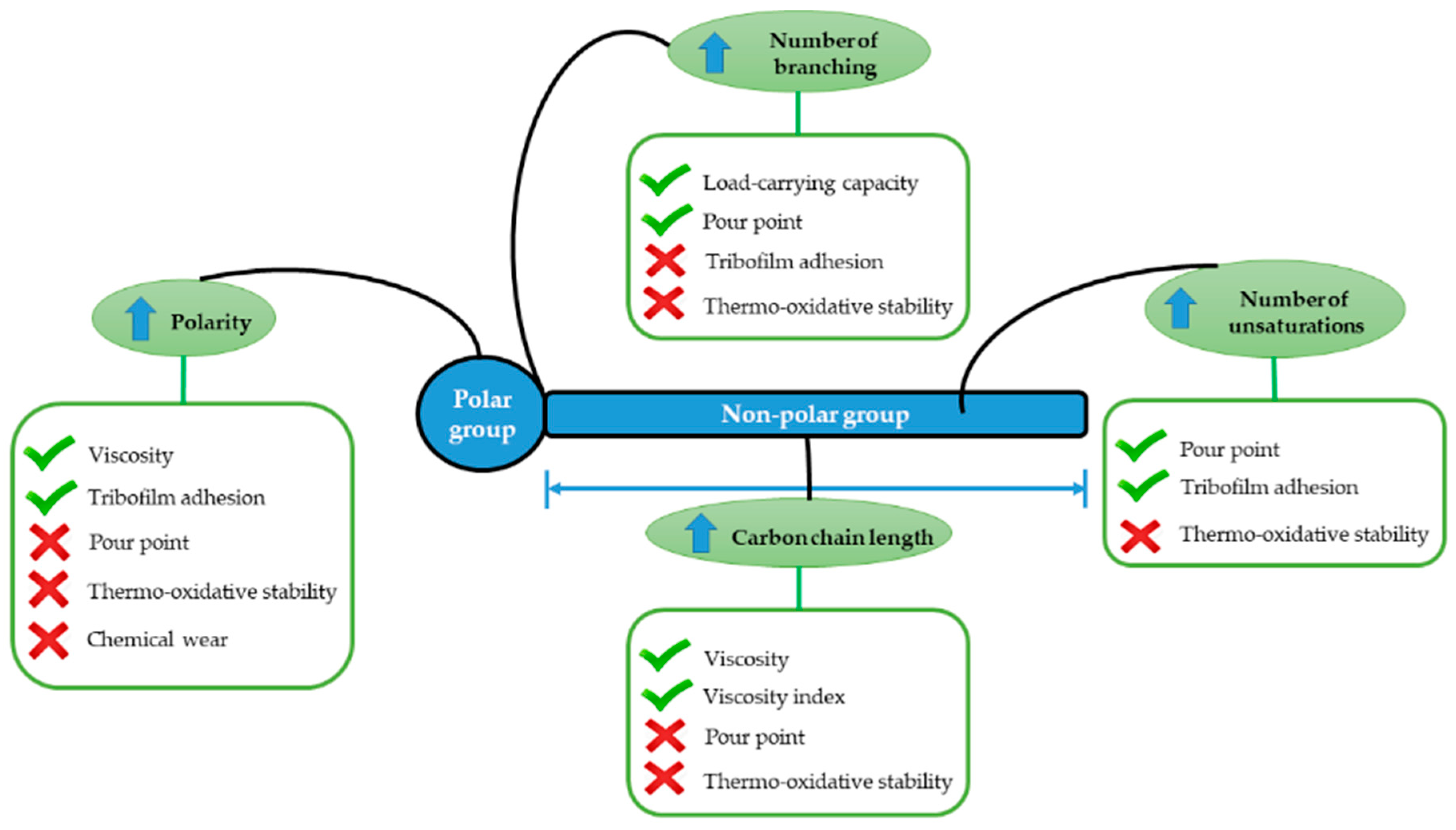
Viscosity is one of the key properties of a biolubricant, as it describes the internal friction within a liquid due to molecular interactions. This parameter is in the range 30–50 mm2/s for most vegetable oils, although castor oil displays a viscosity of 220 mm2/s as a consequence of the presence of a hydroxyl group on the C9-carbon. This implies that vegetable oils present high lubricity, good metal adherence, and a high viscosity index [13]. Generally, natural oils contain glycerol and polar fatty acids with long chains, which form a triacylglycerol molecule. The presence of large amounts of unsaturated fatty acids leads to poor thermal stability. However, other intrinsic properties, such as the ability to lubricate metal surfaces via the fatty acids forming a monolayer film using their closely packed polar carboxyl groups, drastically decrease friction and wear in such a way that these biolubricants can be used as boundary lubricants [14,15] (Figure 1).
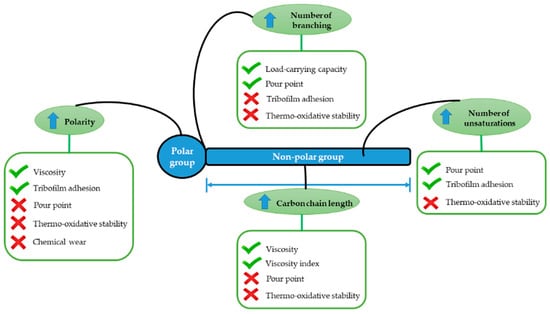
Figure 1.
Molecular structure of vegetable oils and the relationships between the structure and the physicochemical properties.
Over the last decades, the physicochemical properties of vegetable oils have been modified via several chemical routes to improve biodegradability, thereby maintaining and even improving specific properties various applications. In this sense, it was reported that chemical modification of vegetable oils causes improved thermo-oxidative stability, thereby allowing their use in a wider range of operating conditions.
2. Classification of Biolubricants
Biolubricants can be classified according to their chemical fluid composition in natural and synthetic oils. Natural oils are made using vegetable oils or animal fats, while synthetic oils use the natural oils as starting materials to form more advanced biolubricants. Among them, it was reported that ester synthesis, involving modification by microorganisms, alcohols, polyalcohols, polyglycols, perfluoroalkylethers, and other species, are able to graft to a natural oil [16] in such a way that the synthesized biolubricant exhibited thermo-oxidative stability, wear resistance, and lubricity properties even greater than those exhibited by mineral oils [17]. However, synthetic esters also display several limitations, since chemical modification raises the price of the lubricant, slightly increases the volatility and toxicity, diminishes the friction tolerance, and the esters do not work well with mineral oils in comparison to unmodified vegetable oils [15].
2.1. Natural Oils
As indicated above, oils obtained from vegetables, fruits, or seeds, as well as fats obtained from animals, have been used as starting materials to obtain their respective oils through several extraction and distillation methods [18,19]. These oils are widely available, are inexpensive, and exhibit higher biodegradability than those obtained from mineral oils [20]. Generally, the physicochemical properties of vegetable oils depend on the composition of free fatty acids (Table 3). Natural oils display variable compositions, since both environmental and biological parameters strongly influence chemical composition [21]. Nonetheless, some parameters of natural oils are generally better than those of traditional mineral oils, as indicated in Table 1, Table 2, and Table 4 [22,23,24,25,26]. However, the use of these natural oils to synthesize biolubricants is inconsistent, since most interfere with the food chain, thereby causing speculation regarding the prices of consumable vegetable oils and producing increased prices and social imbalances.

Table 3.
Chemical compositions of vegetable oils commonly used.

Table 4.
Physicochemical properties of some mineral and vegetable oils.
To date, most efforts concerning natural oils have focused on understanding the physicochemical properties of saturated and unsaturated fatty acids to be used as green solvents. These natural oils can be used directly or as additives in some formulations depending on the application [22]. Further, the scientific community is developing new additives to incorporate into natural oils in order to improve their physicochemical properties, including thermo-oxidative stability and sliding contact, among others [25].
2.2. Synthetic Oils
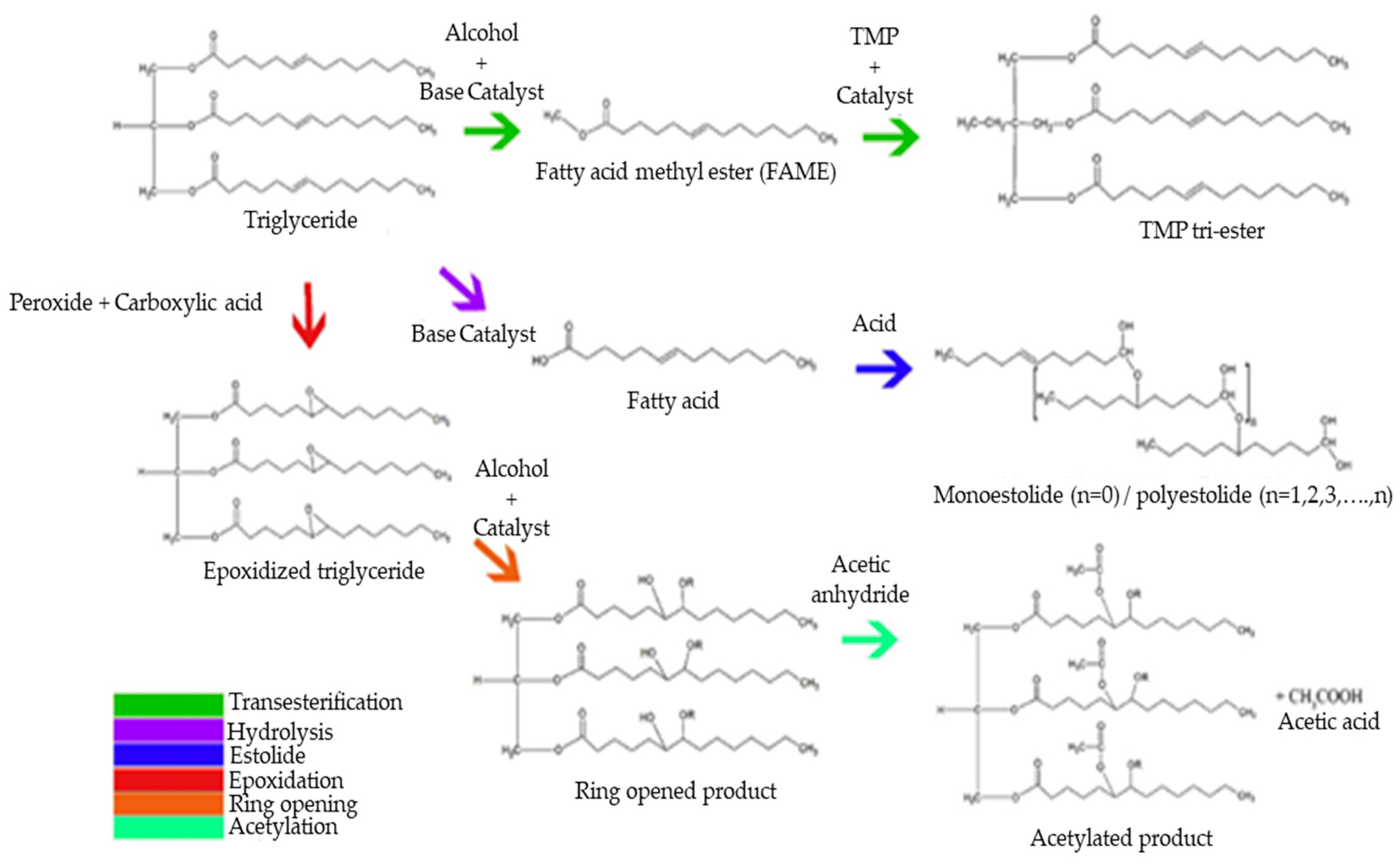
Synthetic esters are formed by chemical modification of plant-based oils or animal fats. To date, most biolubricant studies of natural and synthetic esters focused on improving their physicochemical properties via several chemical modification methods and different catalytic processes (Figure 2). However, development regarding these biolubricants must continue, since they still present less-than-ideal thermal degradation, hydrolysis sensibility, and oxidative stability as a consequence of unsaturated vegetable oils [27]. In this sense, the main challenge facing biolubricant quality improvement involves increasing oxidative stability and pour temperature [4]. The most promising processes focus on the modification of unsaturated oils through chemical processes, since double bonds are prone to reactions with atmospheric oxygen. The most common strategies to modify vegetable oils are transesterification/esterification reactions, epoxidation, hydrogenation, and estolide formation, among others. Generally, these methodologies improve some physicochemical properties of synthetic biolubricants, although these chemical modifications also present a series of disadvantages, as indicated in Table 5.
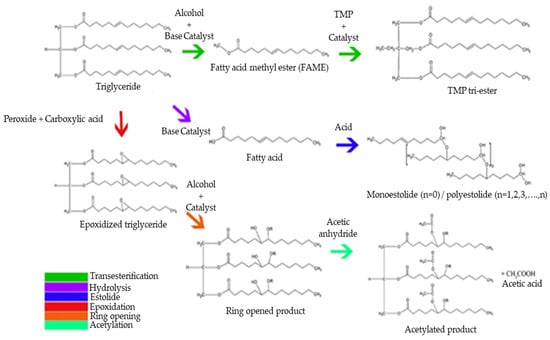
Figure 2.
Chemical modifications of vegetable oils to obtain biolubricants.

Table 5.
Chemical modifications of vegetable oils.
2.2.1. Esterification/Transesterification Reactions
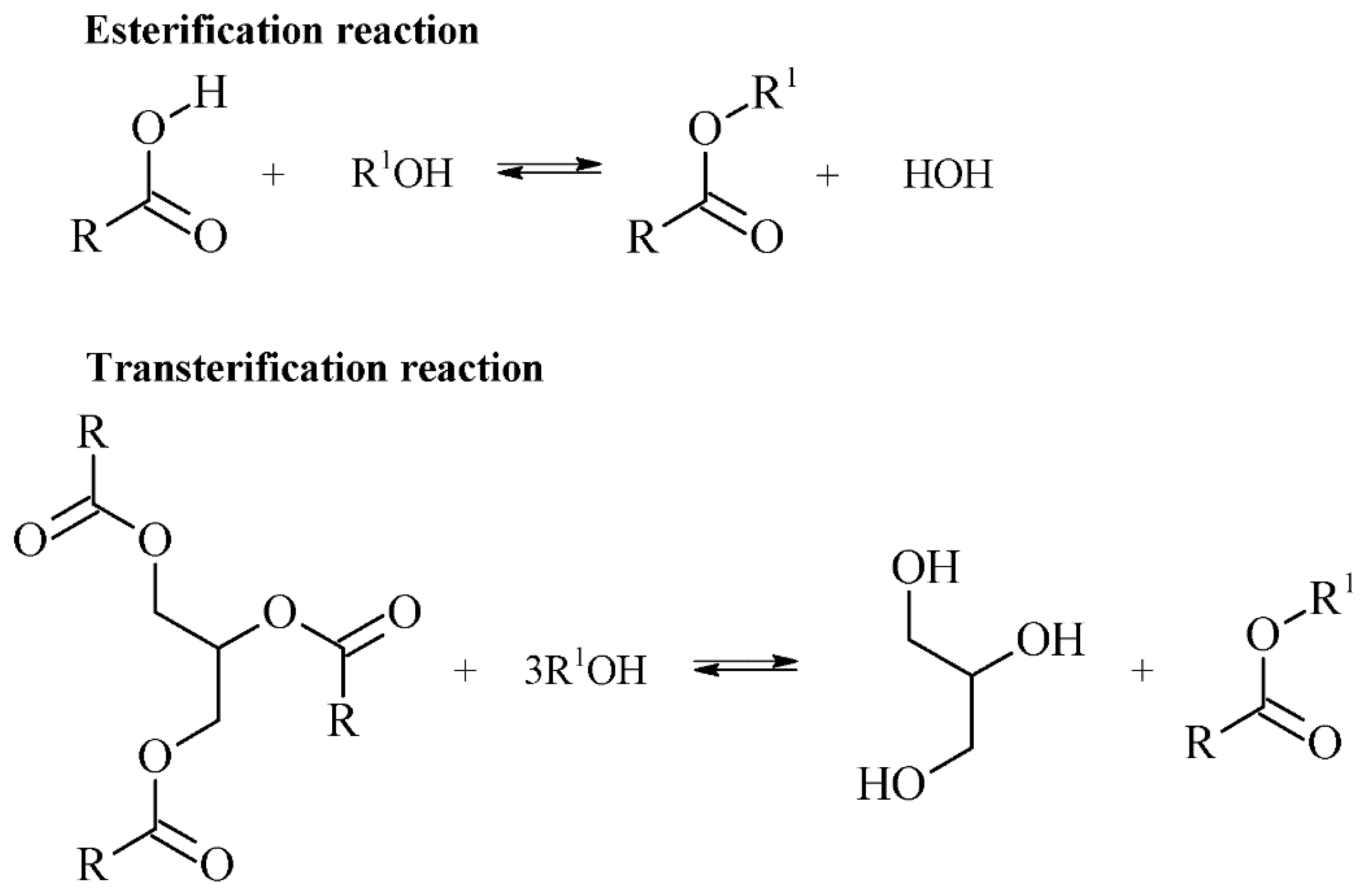
Biolubricants are prone to modification in the ester moieties of triacylglycerides. Transesterification reactions involve the glycerol moieties of the triacylglyceride being replaced by a long and/or branched-chain alcohol, while esterification reactions involve free fatty acids of natural oils reacting with long-chain alcohols to form their respective esters [28] (Figure 3).
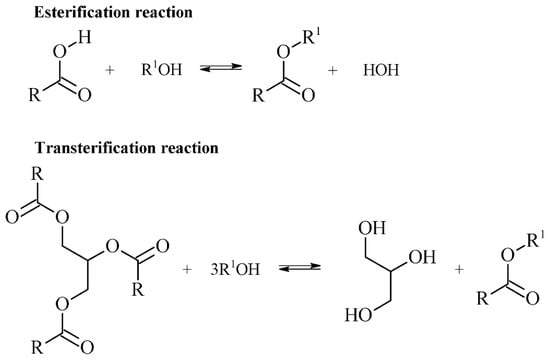
Figure 3.
Esterification of free fatty acids and transesterification of a triglyceride ester to fatty acids.
Generally, esterification/transesterification reactions are carried out using mineral oils (HCl, H2SO4, or p-toluensulfonic acid). In spite of the high yield of mineral oils, these processes also exhibit some drawbacks related to product separation and high acid corrosivity. Considering these disadvantages, solid catalysts were developed as sustainable alternative to carry out esterification and transesterification reactions. These reactions take place using basic catalysts, mainly CaO, obtaining full conversion during transesterification, although high temperatures are required to activate the catalyst and the regeneration step. The other alternative involves using solid acid catalysts, such as cationic exchange resins, zeolites, or oxides (Al2O3, ZrO2, TiO2, WO3, Nb2O5, Ta2O5), which can also be sulfated to improve their catalytic activity [5,6,28].
The physicochemical properties of the synthetic esters are directly related to the acids or alcohols used in the esterification/transesterification reactions. Generally, synthetic esters present better properties than their respective triglycerides. Thus, synthetic esters display lower temperature properties, higher flash temperatures, lower volatility, and higher oxidative stability in comparison with natural oils [26] (Table 6). As esters are susceptible to hydrolysis and thermal degradation, their thermal properties can be improved by alcohol substitution with longer alkyl chains or branched chains, such as 2-ethylhexanol, neopentylglycol (NPG), pentaerythritol (PE), trimethylolpropane (TMP), trimethylolhexane (TMH), and trimethylolethane (TME) [29,30,31,32,33,34]. As a representative example, the esterification of vegetable oil free fatty acids with NPG leads to a lubricant that is often used in aircraft engines, while the esterification of vegetable oil free fatty acids with PE forms a biolubricant that used in turbine gas [35]. Esterification with TMP generates a lubricant used in marine engine oils, compressor oil hydraulic fluids, gear oils, and grease formulations [35]. The use of sugars as an alcohol source also forms synthetic esters with higher lubricity, thermo-oxidative stability, and biodegradability [36], thereby allowing applications as food additives and in the pharmaceutics and cosmetics fields [36].

Table 6.
Physicochemical properties of vegetable oils and their respective lubricants.
Both esterification and transesterification reactions can proceed using acidic or basic catalysts. Transesterification has been extensively studied at the laboratory scale under acidic conditions, but this reaction is rarely used commercially; the base-catalyzed reaction is roughly 4000 times faster and requires lower temperatures and reaction times than the acid-catalyzed reaction. Generally, esterification/transesterification reactions are catalyzed by mineral acids, such as HCl, H2SO4, or H3PO4. In spite of these catalysts displaying good conversion, these processes also present serious disadvantages related to the corrosion of the reactor as well as the separation and purification of the obtained biolubricants. Considering these factors, heterogeneous solid acid catalysts were proposed as potential alternatives, and the use of ion-exchange resins or oxides, such as Al2O3, ZrO2, TiO2, Nb2O5, Ta2O5, WO3, zeolites, or heteropolyacids, were reported as potential catalysts to replace traditional mineral oils [32,33,34,37,38,39,40,41]. With regard to the basic catalysts used in esterification/transesterification, most studies used alkaline or alkaline earth oxides, hydroxides, alkoxides, or carbonates [42,43,44], obtaining yields higher than 98% in many cases with less catalyst and shorter reaction times than the solid acid catalysts.
2.2.2. Hydrogenation Reactions
As indicated above, the level of unsaturation has an adverse effect on the thermo-oxidative stability of the biolubricant. An alternative to improve biolubricant stability involves hydrogenation using Ni, Pd, Pt, or Ru-based catalysts [45,46,47,48], although greater pressures (25–35 MPa) and temperature conditions (250–300 °C) are required. This chemical modification causes isomerization in the respective cis- and trans-acids, as well as decreased fluidity of the lubricant; solid lubricants or waxes are often obtained in these situations, so the hydrogenation of the double C=C bond was not considered to be effective regarding physicochemical property improvement [49].
2.2.3. Epoxidation and Branching Reactions
As indicated previously, the number of double bonds has an important role in oxidation process resistance, since unsaturated molecules are prone to hydrolytic degradation [4,50,51]. Moser and Erhan proposed an auto-oxidation mechanism of vegetable oils, as indicated in Table 7 [52], pointing out that oxidation takes place via free radicals, which react with oxygen to generate peroxy-radicals. The obtained peroxy-radicals react with other lipid molecules, forming hydroperoxide and other free radicals, thereby propagating the oxidation process [53,54]. The oxidative degradation increased viscosity, which diminishes the half-life of the biolubricant and produces increased pour temperature of the lubricant.

Table 7.
Autoxidation mechanism of vegetable oils.
The thermo-oxidative stability and other physicochemical properties can be improved by modifying the alkene groups and incorporating alkyl side chains [55]. In this sense, epoxidation is another important ways of modifying -C=C- bonds to obtain biolubricants with better low-temperature properties, higher oxidative stability, better acidity, and higher adsorption to metal surfaces, thereby generating higher viscosity, better lubricity, a lower viscosity index, and increased pour point [4,56] (Table 8). Generally, epoxidation of unsaturated bonds takes place via alkyl hydroperoxides, using peroxy acids, dioxiranes, or peracids as sources [57]. In order to avoid the corrosive nature of the reaction medium, heterogeneous solid catalysts, such as acidic ion exchange resins or transition metal-based catalysts (TiO2/SiO2, NbVO/SiO2, polyoxomethaltes, sulfated/SnO2), are used as sustainable alternative in epoxidation reactions [6,58,59,60,61,62].

Table 8.
Physicochemical properties of modified epoxidized vegetable oils.
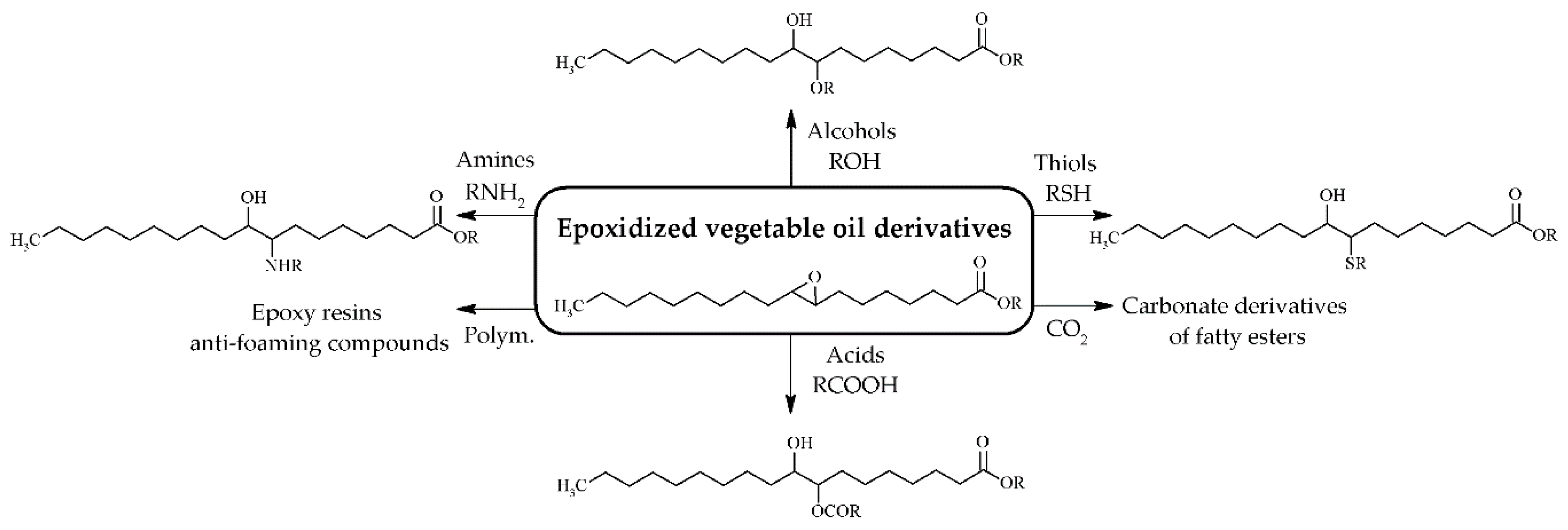
As epoxidized products still display poor temperature properties, it is necessary to modify these epoxidized compounds to improve physicochemical properties via esterification reactions or alkylation, acylation, acyloxylation, amino alkylation, cooligomerization, enereaction, hydroaminomethylation, or hydroformylation [24] (Figure 4). Several authors reported that these consecutive reactions improve low temperature properties, lubricity, viscosity index, and thermo-oxidative stability and reduce the friction coefficient [6,28,49,55,63]. Both esterification and acetylation reactions were carried out using homogeneous acids catalysts (H2SO4) mixed with alcohols [55,64,65]. However, the potential corrosion of this reaction medium led to the development of solid acid catalysts for use in these reactions, such as cationic exchange resins or sulfated-SnO2 [55,58,59].
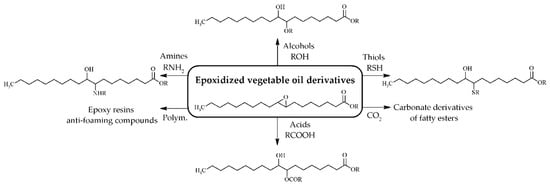
Figure 4.
Epoxidized vegetable oils used as starting reagents to synthesize biolubricants with different physicochemical properties.
2.2.4. Estolides of Fatty Acids
Estolides are branched esters which are formed when the carboxylic acid group of one fatty acid links to the site of unsaturation of another fatty acid (via carbocation) to form oligomeric esters using an acid catalyst (H2SO4) and an oxidant (HClO4). This carbocation can be subject to nucleophilic attacks by other fatty acids to form an ester, namely, an estolide. These branched esters are more resistant to hydrolytic degradation in comparison to triglycerides. On the other hand, it was reported that the length of the hydrocarbon chain and the estolide number are important for the physicochemical properties of the biolubricant, as indicated by the estolides obtained from 2-ethylhexanol, oleic acid, and lauric acid [66,67].
Generally, the obtained estolide has improved lubricity, thermo-oxidative stability, and viscosity index and a decreased pour point of the biolubricant [57,68] (Table 9). In addition, these estolide esters are more suitable as biodegradable lubricants than current commercial estolides, which are not used on their own but rather are mixed with other vegetable oils with different physicochemical properties; they can also be modified to highlight any specific property [69,70].

Table 9.
Physicochemical properties of estolides obtained from vegetable oils.
2.2.5. Use of Additives to Improve Lubricant Properties
The physicochemical properties of lubricants can be improved by incorporating additives in several fields, such as resistance to oxidation and hydrolysis, viscosity, corrosion or rust inhibition, decreased pour temperature, etc. [71,72]. In some cases, lubricant formulations present several heteroatoms, such as metals, sulfur, phosphorus, and chlorine; however, these additives have adverse effects on the environment due their high toxicity levels [73,74]. Layered and particulate materials, plant-derived compounds, polymers, and ionic liquids have emerged as potential additives of biolubricants to mediate these adverse environmental effects.
Plant-derived compounds, such as cystine schiff base ester, exhibit good properties regarding their use as additives in anticorrosion, antiwear or antifriction processes, since the disulfide groups (-S-S-) form a surface-complex film to protect the metal pieces in contact with each other [75]. On the other hand, ethyl cellulose and ethylene-vinyl acetate have emerged as potential additives to modify viscosity, since even the addition of a small amount of these compounds can double the viscosity of a biolubricant. Similarly, these additives diminish the wear and friction of metallic pieces [76,77].
The addition of non-toxic inorganic oxides nanoparticles that form smooth, thin films, such as ZnO, CuO, or TiO2, minimizes asperity contact and wear, due to the sheet alignment in the same direction, thereby causing sliding [78,79] and improvement of the friction and wear properties of the final biolubricant. Other layered materials used as additives for these applications include boron nitride, graphene, molybdenum, and tungsten sulfides [79]. These inorganic compounds are highly refractory, so the incorporation of “powder lubricants” extends the range of pressure and temperature for their applications [19,23,30,80,81]. These solid biolubricants also exhibit some disadvantages, since they can be forced out of the contact zone during dry sliding contact [82]. However, this problem can be minimized by the addition of natural oils, such as soybean or canola oil [81], allowing them to remain in contact with the pin–disk interface without degrading over time [70], thereby increasing friction and minimizing wear using a sustainable composite [23,30].
Ionic liquids have also shown great potential as additives in several types of sliding materials [83,84], since they display low flammability, negligible vapor pressure, low volatility, relatively high thermo-chemical stability [84], and interesting lubricating properties, such as high load-carrying capacity, antiwear, and friction reduction [84,85]. However, ionic liquids, mainly polar biolubricants, also have disadvantages related to their immiscibility, although these limitations can be solved by incorporating the ionic liquid as an emulsion [86].
3. Physicochemical Properties of the Biolubricants
3.1. Thermo-Oxidative Stability
As indicated above, unsaturated bonds have an adverse effect on the thermo-oxidative stability of biolubricants. However, it is possible to achieve an appropriate relationship between the behavior of the bonds with low temperature and thermo-oxidative stability; some biolubricants with excellent properties and a vegetable oil (sunflower, canola, or soybean) content above 80% were reported to show these properties [49]. Thermo-oxidative stability can also be improved by chemically modifying the vegetable oil via saturation of the –C=C– bond, thereby obtaining biolubricants that can be used hydraulic lubricants, transmission fluids, and engine or compressor lubricants [87]. Thermo-oxidative stability and the viscosity index can be further improved by saturating –C=C– bonds (via epoxidation) and then causing them to become branched. Therefore, it is possible to modulate the viscosity by modifying the length of the alkyl chain.
3.2. Hydrolytic Stability
The resistance of biolubricants to chemical attacks, where H2O is involved as a reactant or product, is another parameter to be considered regarding biolubricant stability. Generally, biolubricants are formed by esters, which are prone to hydrolysis by their respective carboxylic acid and alcohol. Previous authors reported that saturation improved hydrolytic stability. Similarly, saturated dicarboxylic esters were shown to be highly stable due to steric effects [82].
The hydrolytic stability of biolubricants is enhanced when short-chain alcohols are employed. This resistance can be further improved by using branched alcohols. The use of glycerol as the alcohol in a biolubricant results in hydrolytic stability, which is comparable to that shown in mono-esters formed by branched alcohols. Despite the hydrolytic stability causing the physicochemical biolubricant properties to deteriorate, this parameter is key to obtaining biolubricants with high biodegradability and lesser negative environmental effects.
3.3. Viscosity
The viscosity is a parameter that must be considered when replacing the traditional lubricants obtained from crude petroleum. Most biolubricants display high viscosity, and the viscosity directly increases with the length of the hydrocarbon chain of the carboxylic acid or alcohol in ester biolubricants. The presence of hydroxyl groups in the fatty acid or the addition of polyols via esterification modifies the viscosity index due to increased hydrogen bonds interactions [32,39].
Viscosity is also directly related to temperature. It was reported that biolubricants with high viscosity indexes displayed less viscosity modifications when the temperature increased. Biolubricant branching also affects the viscosity index, since increased branching in the alcohol or the carboxylic acid favors a lower viscosity index, whereas increased length has an adverse effect by increasing the viscosity index [88].
3.4. Pour Point
The pour point is defined as the temperature below which the liquid loses its flow characteristics. In biolubricants, the pour point is directly related to the viscosity index. It was reported that the presence of ternary alcohols, such as trimethylolpropane (TMP), diminishes the pour point of the biolubricant, although it also causes a decay in the thermo-oxidative stability of the alcohol. The use of bigger branched alcohols, such as neopentyl polyols, has emerged as a potential alternative to obtain biolubricants with low pour points and higher oxidative stability levels [28,29].
The presence of –C=C– bonds decreases the pour point, however, these lubricants are more vulnerable to oxidation processes. The optimum pour point is obtained for saturated fatty acids with short hydrocarbon chains, since an increase in the length of the carbon chain causes a higher pour point. In plants, chains generally contain between 16 and 18 carbon atoms, indicating that the saturation of these acids causes them to become solid at temperatures of 65–75 °C [82]. The position of the –C=C– unsaturated bond does not seem to affect the pour point temperature. However, its conformation slightly influences the pour point temperature, with cis-configuration chains, where the hydrogen atoms are located on the same side, being observed to have a lower pour point than hydrocarbon chains with trans-configuration [89].
3.5. Biodegradability
A lubricant is considered to be biodegradable when it can be broken down by enzymes in renewable raw materials derived through aerobic or anaerobic processes by organisms or their enzymes [90].
The biodegradability of a lubricant takes place via several steps. In the first step, the original biolubricant disappears to form another compound that may or may not be completely biodegraded. This primary degradation is measured by the analysis of the C-H bond through infra-red spectroscopy [91]. Then, in the second step, the organic compound determines the degradation of the organic compound into CO2 and H2O by biodegradation within 28 days [92].
3.6. Ecotoxicity
Ecotoxicity is an important parameter that must be controlled, since some components of lubricant formulations are environmentally toxic and can irreversibly affect living things. Aqueous ecosystems are more prone to strong damage, therefore it is necessary to determine lubricant water toxicity the potential to poison target organisms such as bacteria, algae, small fishes, or laboratory rats. A lubricant is considered to be degradable when it is biodegradated at least 80% within 28 days or at least 60% after 28 days. Ecotoxicity is measured using the LD50, i.e., the required dosage to kill 50% of a population. A biolubricant is not considered ecotoxic when the LD50 is above 1000 ppm [82].
4. Applications of the Biolubricants
Lubricants can be employed in both closed and open systems. In open systems, lubricants are continuously released into the environment, while in closed systems, lubricants should be kept insulated so uncontrolled releases cannot take place; however, mishandling or human error can cause undesired disposal of lubricants into the environment. Considering these factors, lubricants used as potential alternatives to traditional mineral oils must have high levels of biodegradability [93].
As indicated previously, the physicochemical properties of a biolubricant depend on its intended application. The applications of the biolubricants are indicated in Table 10.

Table 10.
Significant properties of biolubricants for several applications.
4.1. Engine Oils
Engine oil plays a key role in motors by diminishing friction and wear of moving pieces, inhibiting corrosion of the engine system, improving sealing, and cooling the motor [94]. In engine systems, lubricants must operate under severe pressure and/or temperature conditions and must be stable to prolonged exposure of contaminating acids, which can cause progressive deterioration of the biolubricant. Igartua et al. [95] reported that biolubricants with low sulfur and phosphorus contents exhibited less volatility with better properties than lubricants obtained from mineral oils.
Castor, palm, and coconut oil have proven to be potential lubricant alternatives to two-stroke engine oils [96], which may contain unburned hydrocarbons, particulate matter, CO, or CO2, which all have adverse effects on human health [97]. Singh pointed out that the use of castor oil as a biolubricant diminished volatile organic compounds emissions by 5%, probably because of improvement in the lubricity [96]. Similarly, Masjuki et al. established that palm oil was a more efficient lubricant in two-stroke engines than traditional mineral oils because it reduced CO and hydrocarbon emissions, although higher levels of CO2 and O2 were found [98].
As indicated above, one of the main challenges of lubricants is thermo-oxidative stability. Palm and coconut oils were found to display good performance in four-stroke engine oils, obtaining similar values to those obtained for conventional oils. However, after prolonged use, their behavior worsened as a consequence of the absence of additives, as well as their poorer thermo-oxidative stability [99]. Similarly, Reddy et al. [100] also reported that palm oil was a renewable and non-hazardous biolubricant, obtaining hopeful results in four-stroke engine oils. In another study, it was reported that a conventional diesel engine could operate with a rapeseed oil-based biolubricant–biodiesel combination, showing improvement in the brake’s thermal and mechanical efficiency as well as power [101].
Nowadays, several biolubricants used as engine oils are marketed by several companies, such as Green Earth Techologies (USA), Biosynthethic Technologies (USA), and Renewable Lubricants Inc. (USA). The physicochemical properties, mainly the viscosity, of these oils are adapted to diesel and gasoline engines following the International Lubricant Standards Association (ILSAC GF-5) and American Petroleum Institute (API SN) standards.
4.2. Hydraulic Fluids
Hydraulic fluids must be devised to transfer energy/power in a hydraulic system as well as to lubricate the system. A key parameter of hydraulic fluids is their compressibility, since the design of a hydraulic fluid with lower compressibility improves the pressure transmission velocity, which is directly related to a faster response and a better use of energy [102]. However, the compressibility must be modulated, since it can dampen the autogenous pressure in some cases [103].
Vegetable oils display low isothermal compressibility and appropriate viscosity for use as hydraulic fluids. It was reported that soybean oil displayed better power transmitting properties than mineral oils [1,102]. In addition, vegetable oils also remove air bubbles inside the oil seven times faster than the required standard (PN-C-96057-6) [103,104]. Several vegetable oils, such as rapeseed oil, palm oil, moringa oil, passionfruit oil, and rubber seed oil, have shown excellent properties when used as hydraulic oils [105,106,107] comparable with traditional mineral oils in all cases. Other authors reported that esterification of vegetable oils with alcohols and acids improved hydraulic fluid behavior due to the obtained formulation being more biodegradable and having a lower pour point, as previously indicated [108].
Nowadays, important companies, such as Shell, Mobil, or Chevron Texaco, are marketing hydraulic fluids and hydraulic systems using environmental friendly vegetable oils with approved several pump test standards.
4.3. Compressor Oils
Compressor oils must maintain stability under severe conditions of pressure and temperature. Among the parameters that compressor oils must satisfy, these lubricants should avoid corrosion of the component, seal the compression cylinder, and regulate temperature during the compression process [7]. The main challenge of compressor oils is related to their thermal stability at elevated temperatures, since some CO2 or propane systems can reach about 215 °C; therefore, compressor oils must tolerate a temperature of 250 °C, at least. It was reported that epoxidation of vegetable oils maintains thermal stability even above 260 °C [109], indicating that these oils can be used as highly efficient compressor oils by reaching similar temperatures as those obtained by commercial, mineral-based compressor oils, such as R-68 [110]. Despite the severe pressure and temperature conditions, the thermo-oxidative stability of the lubricant is not important for compressor oils, since these oils are not exposed to moisture and atmospheric oxygen. Under these conditions, compressor oils are expected to have a lifespan of between 10 and 20 years.
The market for bio-based compressor oils is very scarce; they are intended for use in compressors such as vacuum pumps, reciprocating compressors, centrifugal compressors, and stationary rotary compressors.
4.4. Metalworking Oils
Metalworking oils are lubricants that are highly employed in machining processes, since they cool and lubricate chips and edges, thereby preventing system heating. The main drawback of these lubricants is related to their disposal; their handling can cause environmental and health problems, particularly when inhaled [111]. It was reported that bio-based soybean oil generates a minor impact by forming a volatile species as a consequence of the high viscosity and low volatility [112]. Syahir et al. pointed out that use of bio-based vegetable oils as starting materials to obtain metalworking fluids diminished surface roughness, which resulted in decreased tool flank wear and cutting temperature [35].
As metalworking fluid frequently works by mixing with water to form emulsions, antirust and emulsifiability are two other parameters that should be considered. It was reported that esterification of soybean oils improved the thermal stability of metalworking fluids, while the presence of –OH or –SH groups improved its resistance to oxidation processes [113]. These data agreed with those obtained by Singh and Gupta, who showed improved thermal stability of metalworking fluid by modifying karanja oil [114]. Similarly, Lawal et al. [114] established that a lubricant obtained from coconut oil showed excellent behavior as a metalworking fluid in milling and ferrous metal turning operations, demonstrating an even better performance than that used for turning process of stainless steel and better surface roughness. These authors also reported that the addition of bio-derived palm oil to the previous metalworking fluid improved the metal removal rate during the turning process of steel [115].
The tool wear, cutting forces, tool life, and heat transfer efficiency levels were analyzed using several bio-based metalworking fluids, with a decrease in thrust force and an improvement in tool line being observed in most cases [116,117]. Tazehkandi et al. pointed out that spray diffusion of bio-based vegetable oil diminished the cutting fluid consumption [117].
Nowadays, biodegradable vegetable oils used as metalworking fluids are marketed together with several additives, such as emulsifiers (sodium sulfonate, sodium oleate, or sodium carboxylate), fungicides, antirust compounds, and antioxidants. These formulations are applied in drilling, machining, sawing, grinding, cutting, or tapping processes. Several studies reported that the use of these bio-based lubricants was able to increase tool life up to about 40%,while simultaneously improving the surface finish and accuracy [35].
4.5. Transmission Oils
Transmission oils must display high viscosity, high thermal stability, and high friction resistance and must be able to dissipate heat efficiently since these processes involve great amounts of friction. The temperature at which a transmission engine is regulated is another very important parameter to consider, since decreases stabilization temperatures imply decreased friction coefficients, improved thermo-oxidative stability, allowing oils to have longer service lives, and improved process efficiency [118].
Nowadays, the use of bio-based lubricants as starting materials for transmission oils is limited. It was reported that the addition of tetraoleate ester was possible, although this bio-based lubricant exhibited a higher weld load and lower wear scar diameter than commercial transmission oils [108,119]. Currently, the few commercialized bio-based gear and transmission oils that are employed mainly in gear in roller machinery are based on rapeseed oil.
4.6. Chainsaw Oils
Chainsaw lubricants must be renewable and biodegradable since they are often thrown to the ground or into water during their handling [120]. Moreover, these bio-based lubricants must display high flash point temperatures and low vapor pressures to minimize the inhalation of volatile compounds [121]. In order to improve chainsaw oil formulations, Stanovský et al. [122] established that less bio-based lubricants and fuels were consumed than mineral chainsaw lubricants.
The need for chainsaw lubricants with high biodegradability has led to high commercialization of these products from several companies, mainly soybean oil and rapeseed oil [35]. The main challenge in this field is related to the incorporation of additives, which elongate tool life and improve performance, in order to replace traditional mineral oils.
4.7. Grease
In general, grease can be classified based on the thickener, which is between 10 wt.% and 15 wt.% of the total grease. The thickener used most often on the industrial scale is a lithium compound [123], but this compound presents low biodegradability; the challenge in this field, therefore, involves the synthesis of bio-based thickeners as environmentally benign alternatives. It was reported that 12-hydroxystearic acid and/or its respective alkyl-ester derivatives could become a natural alternative due to the presence of intermolecular and intramolecular interactions, which favor its use as a thickener [124].
Nowadays, most efforts are focused on the development of degradable greases. Several studies reported that several compounds obtained from the biomass, such as quitin, quitosan, glycerol stearates, sorbitan stearates, cellulose, and alkyl cellulose, reached similar rheological, mechanical, or thermal properties to those exhibited by traditional lithium-salt greases [125,126,127,128]. Similarly, castor oil was mixed with glyceryl sterate, glyceryl sorbitan, amd acylated chitosan, demonstrating a decrease in the friction coefficient in comparison to the lithium–salt greases [35]. In another study, a raw clay mineral was mixed with waste cooking oil to obtain a good thickener, since the high adsorption capacity of the clay mineral allowed it to hold and release oil gradually, however, the oxidative stability was low [129]. In a similar study, castor oil was mixed with several cellulose sources (ethylated cellulose, methylated cellulose, and Kraft cellulose) as a thickener, resulting in greases with higher decomposition temperatures than the conventional lithium–salt greases, but the mechanical stability did not improve in all cases [130]. On the other hand, Boiko et al. [131] employed a glycerin-based boron mixture as a grease on railway curves, obtaining a product with excellent antiscoring and antiwear properties in comparison to traditional grease. Most bio-based greases that are currently marketed are based on rapeseed or soybean oils using non-harmful thickeners, such as silica or clay mineral.
4.8. Insulating Oils
Insulating fluids are usually used to prevent electrical discharge, dissipate heat, and lubricate and insulate surfaces. These fluids must display high electrical resistivity to avoid electrical arcing. Nowdays, traditional mineral oils are employed as insulating fluids in capacitors, bushings, or transformers, although strict environmental regulations have led to continuous modifications of their formulations [132].
In a study carried out with vegetable oils, Beroual et al. [133] reported that all bio-based formulations displayed greater breakdown voltage than traditional mineral oils, since the vegetable oils adsorbed moisture up to 30 times more than the mineral oils. On the other hand, the vegetable oils usually displayed higher dielectric constant in comparison to the mineral oils, thereby allowing them to be considered as sustainable insulating fluid alternatives in electrical transformers [134]. The presence of a hydroxyl group in the C-9 position in castor oil further increased the dielectric constant [135]. In spite of the good behavior observed in the bio-based insulating fluids, they still demonstrated poorer oxidative degradation due to the presence of copper wires [135]. Nonetheless, the degradation of these bio-based insulating fluids can be minimized by chemical modification of vegetable oils via esterification/transesterification reactions or by the addition of stabilizers.
Industrially, several formulations based on canola or sunflower oils have been marketed, which were proven to improve transformer loading by up to 20% [35].
5. Challenges and Future Perspective of Biolubricants
Biolubricants have not been strongly implanted in the market in many applications, which could be attributed to their poor oxidative stability and their poor properties at lower temperatures. With regard to the pour point, it is well known that the most vegetable oils solidify at about −10 °C, which prevents their use [29]. In the case of the low thermo-oxidative stability, unsaturated vegetable oils are prone to chemical attack, which causes modification of the physicochemical properties of the biolubricant. The main challenge is related to the high variability and seasonality of the feedstock, implying different chemical compositions of the starting vegetable oil. Another challenge is the selection of the non-edible oil. Most previous studies were carried out with vegetable oils that could interfere with the food chain, such as soybean, sunflower, rapeseed, or palm oil, since the use of these oils could cause price speculation and social imbalances. Due to this, the cultivation of non-edible oils, such as jojoba oil, karanja oil, jatropha oil, and castor oil, has grown over the last few years as sustainable alternative [35]. In recent years, microalgae has also emerged due to its high oil content, even over 70% in some cases [136].
The future perspectives of biolubricants should focus on better lubrication properties and nontoxicity in comparison with traditional mineral oils. Alongside strict environmental regulations, more sustainable formulations are expected to be developed in many fields, such as engine oils, hydraulic oils, compressor oils, and insulating fluids, among others. In order to market competitive biolubricants, it is necessary to search for reliable and cheaper starting vegetable oils. Efforts must also be focused on chemical modification of the starting vegetable oils to improve the final formulation of the biolubricants.
Despite biolubricants showing physicochemical properties very similar to mineral oils in most cases, the synthesis and use of biolubricants is relatively low compared to mineral oils. The conversion of lubricant-synthesizing companies requires investments and time, therefore, these companies tend to be reluctant to make changes in their production chains. Because of this, governments must enact laws that favor the planting of crops from which non-edible oils can be obtained. In addition, governments must act to obtain lubricants with greater proportions of bio-components with lower degradation and toxicity levels than mineral oils.
The scientific community is currently optimizing processes to increase efficiency in the synthesis of biolubricants. Several authors reported that the use of mechanochemical treatments [7], microwaves [137], or ultrasound [137,138,139] improved process yields under mild conditions. Over the last few years, the use of enzymatic processes has emerged as an alternative to obtain precursors or even biolubricants at low reaction temperatures. The use of nanoparticles was shown to improve biolubricant physiochemistry by minimizing asperity contact and wear [78,79]. However, the use of these nanoparticles has caused controversy, as other authors reported that nanoparticles carry potential risks of nano-object releases and highlighted the interest in the management of nano-risk at workplaces [140]. Furthermore, several studies highlighted nano-object emissions in the field of occupational hygiene at workplaces [141,142,143,144]. Considering the potential adverse effects regarding the addition of nanoparticles, the use of nanoparticle-free biolubricants is a challenge when synthesizing harmless lubricants [145].
6. Conclusions
The worldwide trend in the lubricant and base-oil market is shows increased demand for products from renewable sources and higher quality biolubricants, which is related to the evolution of environmental regulations, with more restrictive rules to minimize impacts caused by inadequate disposal in the environment. The use of biolubricants is expected to reduce costs related to mineral lubricating oils. The trend toward higher quality lubricants is mainly the result of technological development. New equipment requires higher quality lubricants to guarantee their protection against overheating and excessive wear of parts, for example, in new operating conditions. Synthetic esters make up a large group of products, which can be of both petrochemical and oleochemical origin. Due to the great interest in environmentally friendly products, synthetic esters obtained from vegetable oils have received special attention. However, this raw material has the potential to compete with the food industry, which has generated great efforts regarding the development of products from sources that do not interfere in the food sector. The use of biolubricants is still very small, but the trend is growing and depends on investment in research & development (R&D). Biolubricants have higher quality and longer lifespans than minerals, in addition to several environmental advantages, but the high cost of synthetic oils with renewable bases compared to minerals prevents further accelerated growth. However, there is currently a lack of technology capable of producing renewable lubricants at competitive costs and in the volume necessary to show greater representation in the market. More competitive prices could be achieved when there is an increase in scale, which depends directly on the security of the supply of vegetable oils as the main raw material.
Author Contributions
Conceptualization, J.A.C. and F.M.T.d.L.; methodology, J.A.C., D.B.P., and R.M.A.S.; validation, C.L.C.J.; formal analysis, F.M.T.d.L.; investigation, J.A.C., D.B.P., and R.M.A.S.; writing—original draft preparation, J.A.C., D.B.P., and R.M.A.S.; review and editing, E.R.-C., F.M.T.d.L., and C.L.C.J.; funding acquisition E.R.-C.; visualization, F.M.T.d.L.; supervision, C.L.C.J.; project administration, J.A.C. and E.R.-C.; funding acquisition, E.R.-C., F.M.T.d.L., and C.L.C.J. All authors have read and agreed to the published version of the manuscript.
Funding
Project RTI2018-099668-BC22 of Ministerio de Ciencia, Innovación y Universidades, and project UMA18-FEDERJA-126 of Junta de Andalucía and FEDER funds.
Acknowledgments
J.A.C., D.B.P. and E.R.C. thank project RTI2018-099668-BC22 of Ministerio de Ciencia, Innovación y Universidades, project UMA18-FEDERJA-126 of Junta de Andalucía, and FEDER funds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mang, T.; Dresel, W. Lubricants and Lubrication; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Menezes, P.L.; Ingole, S.P.; Nosonovsky, M.; Kailas, S.V.; Lovell, M.R. Tribology for Scientists and Engineers; Springer: New York, NY, USA, 2013. [Google Scholar]
- Schneider, M.P. Plant-oil-based lubricants and hydraulic fluids. J. Sci. Food Agric. 2006, 86, 1769–1780. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Asadauskas, S. Lubricant base stocks from vegetable oils. Ind. Crops. Prod. 2000, 11, 277–282. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Liu, Z.; Adhvaryu, A. Lubricant base stock potential of chemically modified vegetable oils. J. Agric. Food Chem. 2008, 56, 8919–8925. [Google Scholar] [CrossRef] [PubMed]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. Biolubricants—Science and Technology; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Soni, S.; Agarwal, M. Lubricants from renewable energy sources—A review. Green Chem. Lett. Rev. 2014, 7, 359–382. [Google Scholar] [CrossRef]
- Shashidhara, Y.M.; Jayaram, S.R. Vegetable oils as a potential cutting fluid—An evolution. Tribol. Int. 2010, 43, 1073–1081. [Google Scholar] [CrossRef]
- Hsien, W.L.Y. Utilization of vegetable oil as Bio-lubricant and additive. In Towards Green Lubrication in Machining; Hsien, W.L.Y., Ed.; Springer: Berlin, Germany, 2015; Volume 1, pp. 7–17. [Google Scholar]
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar] [CrossRef]
- Panchal, T.; Chauhan, D.; Thomas, M.; Patel, J. Synthesis and characterization of bio lubricants from tobacco seed oil. Res. J. Agric. Environ. Manag. 2013, 3, 97–105. [Google Scholar]
- Jahanmir, S.; Beltzer, M. Effect of additive molecular structure on friction coefficient and adsorption. J. Tribol. 1986, 108, 109–116. [Google Scholar] [CrossRef]
- Jain, A.; Suhane, A. Research approach & prospects of non edible vegetable oil as a potential resource for biolubricant—A review. Adv. Eng. Appl. Sci. Int. J. 2012, 1, 23–32. [Google Scholar]
- Chang, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Backe, W. The present and future of fluid power. Proc. Inst. Mech. Eng. Part I 1993, 207, 193–212. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas): A review. Ind. Crops Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Lovell, M.; Higgs, C.F.; Deshmukh, P.; Mobley, A. Increasing formability in sheet metal stamping operations using environmentally friendly lubricants. J. Mater. Process Technol. 2006, 177, 87–90. [Google Scholar] [CrossRef]
- Duzcukoglu, H.; Sahin, O. Investigation of wear performance of canola oil containing boric acid under boundary friction condition. Tribol. Trans. 2011, 54, 57–61. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Obahiagbon, K.O.; Ori-jesu, M. Biodegradation of vegetable oils: A review. Sci. Res. Essays 2009, 4, 543–548. [Google Scholar]
- Gunstone, F.D. Vegetable Oils in Food Technology: Composition, Properties and Uses; Blackwell Publishing: Oxford, UK, 2000. [Google Scholar]
- Fox, N.J.; Tyrer, B.; Stachowiak, G.W. Boundary lubrication performance of free fatty acids in sunflower oil. Tribol. Lett. 2004, 16, 275–281. [Google Scholar] [CrossRef]
- Lovell, M.R.; Menezes, P.L.; Kabir, M.A.; Higgs, C.F. Influence of boric acid additive size on green lubricant performance. Philos. Trans. Math. Phys. Eng. Sci. 2010, 368, 4851–4868. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Choi, U.S.; Ahn, B.G.; Kwon, O.K.; Chun, Y.J. Tribological behavior of some antiwear additives in vegetable oils. Tribol. Int. 1997, 30, 677–683. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Birova, A.; Pavlovicov, A.; Cvenros, J. Lubricating oils based on chemically modified vegetable oils. J. Synth. Lubr. 2002, 18, 291–299. [Google Scholar] [CrossRef]
- Salih, N.; Salimon, J.; Yousif, E. The physicochemical and tribological properties of oleic acid based triester biolubricants. Ind. Crops Prod. 2011, 34, 1089–1096. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Perez, J.M. Oxidation and low temperature stability of vegetable oil-based lubricants. Ind. Crops Prod. 2006, 24, 292–299. [Google Scholar] [CrossRef]
- Deshmukh, P.; Lovell, M.; Sawyer, W.G.; Mobley, A. On the friction and wear performance of boric acid lubricant combinations in extended duration operations. Wear 2006, 260, 1295–1304. [Google Scholar] [CrossRef]
- Menezes, P.L.; Lovell, M.R.; Kabir, M.A.; Higgs, C.F.; Rohatgi, P.K. Green lubricants: Role of additive size. In Green Tribology; Nosonovsky, M., Bhushan, B., Eds.; Springer: Berlin, Germany, 2012; pp. 265–286. [Google Scholar]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Sales, A.V.; Tavares de Luna, F.M.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. Assessment of commercial resins in the biolubricants production fromfree fatty acids of castor oil. Catal. Today 2017, 279, 274–285. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Sales, A.V.; Tavares de Luna, F.M.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. Synthesis of biolubricants by the esterification of free fatty acids from castor oil with branched alcohols using cationic exchange resins as catalysts. Ind. Crops Prod. 2017, 104, 52–61. [Google Scholar] [CrossRef]
- Åkerman, C.O.; Gabera, Y.; Ghani, N.A.; Lämsä, M.; Hatti-Kaul, R. Clean synthesis of biolubricants for low temperature applications using heterogeneous catalysts. J. Mol. Catal. B Enzym. 2011, 72, 263–269. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Havet, L.; Blouet, J.; Valloire, F.R.; Brasseur, E.; Slomka, D. Tribological characteristics of some environmentally friendly lubricants. Wear 2001, 248, 140–146. [Google Scholar] [CrossRef]
- Marx, S. Glycerol-free biodiesel production through transesterification: A review. Fuel Process. Technol. 2016, 151, 139–147. [Google Scholar] [CrossRef]
- Go, A.W.; Sutanto, S.; Ong, L.K.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. Developments in in-situ (trans) esterification for biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2016, 60, 284–305. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. WO3-based catalysts supported on porous clay heterostructures (PCH)with Si–Zr pillars for synthetic esters production. Appl. Clay Sci. 2016, 124, 69–78. [Google Scholar] [CrossRef]
- García-Sancho, C.; Saboya, R.M.A.; Cecilia, J.A.; Sales, A.V.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. Influence of pore size and loading for Nb2O5/SBA-15 catalysts on synthetic ester production from free fatty acids of castor oil. Mol. Catal. 2017, 436, 267–275. [Google Scholar] [CrossRef]
- Kuzminska, M.; Backov, R.; Gaigneaux, E.M. Behavior of cation-exchange resins employed as heterogeneous catalysts for esterification of oleic acid with trimethylolpropane. Appl. Catal. A Gen. 2015, 504, 11–16. [Google Scholar] [CrossRef]
- Freedman, B.; Pryde, E.H.; Mounts, T.L. Variables affecting the yields of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Schwab, A.W.; Bagby, M.O.; Freedman, B. Preparation and properties of diesel fuels from vegetable oils. Fuel 1987, 66, 1372–1378. [Google Scholar] [CrossRef]
- Kim, H.K.; Kang, B.S.; Kim, M.J.; Lee, K.Y. Development of heterogeneous catalyst system for esterification of free fatty acid contained in used vegetable oil. Stud. Surf. Sci. Catal. 2004, 153, 201–204. [Google Scholar]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crops Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Fang, K.; Ren, J.; Sun, Y. Effect of nickel precursors on the performance of Ni/AlMCM-41 catalysts for n-dodecane hydroconversion. J. Mol. Catal. A Chem. 2005, 229, 51–58. [Google Scholar] [CrossRef]
- Sánchez, M.A.; Mazzieri, V.A.; Vicerich, M.A.; Vera, C.R.; Pieck, C.L. Influence of the support material on the activity and selectivity of Ru–Sn–B catalysts for the selective hydrogenation of methyl oleate. Ind. Eng. Chem. Res. 2015, 54, 6845–6854. [Google Scholar] [CrossRef]
- Giraldo, L.; Camargo, G.; Tirano, J.; Moreno-Pirajan, J.C. Synthesis of fatty alcohol from oil palm using a catalyst of Ni-Cu supported onto zeolite. J. Chem. 2010, 7, 1138–1147. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Improvement of pour point and oxidative stability of synthetic ester base stocks for biolubricant applications. Arab. J. Chem. 2012, 5, 193–200. [Google Scholar] [CrossRef]
- King, J.W.; Holliday, R.L.; List, G.R.; Snyder, J.M. Hydrogenation of vegetable oils using mixtures of supercritical carbon dioxide and hydrogen. J. Am. Oil Chem. Soc. 2001, 78, 107–113. [Google Scholar] [CrossRef]
- Moser, B.R.; Erhan, S.Z. Preparation and evaluation of a series of α-hydroxy ethers from 9,10-epoxystreates. Eur. Lipid Sci. Technol. 2007, 109, 206–213. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Abdullah, B.M. Diesters biolubricant base oil: Synthesis, optimization, characterization, and physicochemical characteristics. Int. J. Chem. Eng. 2012, 896598. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Triester Derivatives of oleic acid: The effect of chemical structure on low temperature, thermo-oxidation and tribological properties. Ind. Crops Prod. 2012, 38, 107–114. [Google Scholar] [CrossRef]
- Hwang, H.S.; Erhan, S.Z. Synthetic Lubricant basestocks from epoxidized soybean oil and Guerbet alcohols. Ind. Crops Prod. 2006, 23, 311–317. [Google Scholar] [CrossRef]
- Sharma, B.K.; Stipanovic, A.J. Development of a new oxidation stability test method for lubricating oils using high pressure differential scanning calorimetry. Thermochim. Acta 2003, 402, 1–18. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N. Preparation and characteristic of 9,10-epoxyoleic acid a-hydroxy ester derivatives as biolubricant base oil. Eur. J. Sci. Res. 2009, 31, 265–272. [Google Scholar]
- Somidi, A.K.R.; Sharma, R.V.; Dalai, A.K. Synthesis of epoxidized canola oil using a sulfated-SnO2 catalyst. Ind. Eng. Chem. Res. 2014, 53, 18668–18677. [Google Scholar] [CrossRef]
- Mungroo, R.; Pradhan, N.C.; Goud, V.V.; Dalai, A.K. Epoxidation of canola oil with hydrogen peroxide catalyzed by acidic ion exchange resin. J. Am. Oil Chem. Soc. 2008, 85, 887–896. [Google Scholar] [CrossRef]
- Dinda, S.; Goud, V.V.; Patwardhan, A.V.; Pradhan, N.C. Selective epoxidation of natural triglycerides using acidic ion exchange resin as catalyst. Asia-Pac. J. Chem. Eng. 2011, 6, 870–878. [Google Scholar] [CrossRef]
- Guidotti, M.; Gavrilova, E.; Galarneau, A.; Coq, B.; Psaro, R.; Ravasio, N. Epoxidation of methyl oleate with hydrogen peroxide. The use of Ti-containing silica solids as efficient heterogeneous catalysts. Green Chem. 2011, 13, 1806–1811. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bisio, C.; Carniato, F.; Marchese, L.; Gallo, A.; Ravasio, N.; Psaro, R.; Guidotti, M. Epoxidation with hydrogen peroxide of unsaturated fatty acid methyl esters over Nb(V)-silica catalysts. Eur. J. Lipid Sci. Technol. 2013, 115, 86–93. [Google Scholar] [CrossRef]
- Ahn, B.J.K.; Kraft, S.; Sun, X.S. Solvent-free acid-catalyzed ring-ppening of epoxidized oleochemicals using stearates/stearic acid, and its applications. J. Agric. Food Chem. 2012, 60, 2179–2189. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Deshpande, P.S.; Mahajan, S.U.; Mahulikar, P.P. Epoxidation of mustard oil and ring opening with 2-ethylhexanol for biolubricants with enhanced thermo-oxidative and cold flow characteristics. Ind. Crops Prod. 2013, 49, 586–592. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Synthesis, characterization and physicochemical properties of oleic acid ether derivatives as biolubricants base stocks. J. Oleo Sci. 2011, 60, 613–618. [Google Scholar] [CrossRef]
- Brimberg, U.I.; Kamal-Eldin, A. On the kinetics of the autoxidation of fats: Influence of pro-oxidants, antioxidants and synergists. Eur. J. Lipid Sci. Technol. 2003, 105, 83–91. [Google Scholar] [CrossRef]
- García-Zapateiro, L.A.; Franco, J.M.; Valencia, C.; Delgado, M.A.; Gallegos, C.; Ruiz-Méndez, M.V. Viscosity modification of high-oleic sunflower and castor oils with acid oils-derived estolides for lubricant applications. Eur. J. Lipid Sci. Technol. 2013, 115, 1173–1182. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A. Biodegradable Oleic Estolide Ester Having Saturated Fatty Acid End Group Useful as Lubricant Base Stock. U.S. Patent Application No. 09/490,360, 13 November 2000. [Google Scholar]
- Cermak, S.C.; Brandon, K.B.; Isbell, T.A. Synthesis and physical properties of estolides from lesquerella and castor fatty acid esters. Ind. Crops Prod. 2006, 23, 54–64. [Google Scholar] [CrossRef]
- Cermak, S.C.; Bredsguard, J.W.; John, B.L.; McCalvin, J.S.; Thompson, T.; Isbell, K.N.; Feken, K.A.; Isbell, T.A.; Murraya, R.E. Synthesis and physical properties of new estolide esters. Ind. Crops Prod. 2016, 46, 386–391. [Google Scholar] [CrossRef]
- Schober, S.; Mittellbach, M. The impact of antioxidants on biodiesel oxidation stability. Eur. J. Lipid Sci. Technol. 2004, 106, 382–389. [Google Scholar] [CrossRef]
- Ruger, C.W.; Klinker, E.J.; Hammond, E.G. Abilities of some antioxidants to stabilize soybean oil in industrial use conditions. J. Am. Oil Chem. Soc. 2002, 79, 733–736. [Google Scholar] [CrossRef]
- Kim, B.; Mourhatch, R.; Aswath, P.B. Properties of tribofilms formed with ashless dithiophosphateand zinc dialkyl dithiophosphate under extreme pressure conditions. Wear 2010, 268, 579–591. [Google Scholar] [CrossRef]
- Hewstone, R.K. Environmental health aspects of lubricant additives. Sci. Total Environ. 1994, 156, 243–254. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, S.; Saxena, R.C.; Thakre, G.D.; Atray, N.; Ray, S.S. Study of cysteine schiff base esters as new environmentally benign multifunctional biolubricant additives. J. Ind. Eng. Chem. 2015, 26, 149–156. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Reddyhoff, T.; Gallegos, C.; Spikes, H.A. Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol. Int. 2014, 69, 110–117. [Google Scholar] [CrossRef]
- Delgado, M.A.; Quinchia, L.A.; Spikes, H.A.; Gallegos, C. Suitability of ethyl cellulose as multifunctional additive for blends of vegetable oil-based lubricants. J. Clean. Prod. 2017, 151, 1–9. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Lovell, M.R.; Jen, T.C. The influence of surface roughness and particulate size on the tribological performance of bio-based multi-functional hybrid lubricants. Tribol. Int. 2015, 88, 40–55. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Mufti, R.A.; Zulkifli, N.W.M.; Yunus, R.; Zahid, R. Improving the AW/EP ability of chemically modified palm oil by adding CuO and MoS2 nanoparticles. Tribol. Int. 2015, 88, 271–279. [Google Scholar] [CrossRef]
- Jamison, W.E. Structure and bonding effects on the lubricating properties of crystalline solids. ASLE Trans. 1972, 15, 296–305. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Lovell, M.R.; Jen, T.C. The size effect of boron nitride particles on the tribological performance of biolubricants for energy conservation and sustainability. Tribol. Lett. 2013, 51, 437–452. [Google Scholar] [CrossRef]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. A Review on the science and technology of natural and synthetic biolubricants. J. Bio. Tribo. Corros. 2017, 3, 11. [Google Scholar] [CrossRef]
- Liu, W.; Ye, C.; Gong, Q.; Wang, H.; Wang, P. Tribological performance of room-temperature ionic liquids as lubricant. Tribol. Lett. 2002, 13, 81–85. [Google Scholar] [CrossRef]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2286. [Google Scholar] [CrossRef]
- Yao, M.; Fan, M.; Liang, Y.; Zhou, F.; Xia, Y. Imidazolium hexafluorophosphate ionic liquids as high temperature lubricants for steel–steel contacts. Wear 2010, 268, 67–71. [Google Scholar] [CrossRef]
- Wang, A.; Chen, L.; Jiang, D.; Zeng, H.; Yan, Z. Vegetable oil-based ionic liquid microemulsion biolubricants: Effect of integrated surfactants. Ind. Crops Prod. 2014, 62, 515–521. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Sharma, B.K.; Hwang, H.S.; Erhan, S.Z.; Perez, J.M. Development of biobased synthetic fluids: Application of molecular modeling to structure-physical property relationship. Ind. Eng. Chem. Res. 2006, 45, 928–933. [Google Scholar] [CrossRef]
- Totten, G.E.; Westbrook, S.R.; Shah, R.J. Fuels and Lubricants Handbook Technology, Properties, Performance, and Testing; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Hart, H.; Schuetz, R.D. Organic Chemistry: A Short Course; Houghton Mifflin: Boston, MA, USA, 1978. [Google Scholar]
- Haase, K.D.; Heynen, A.J.; Laane, N.L.M. Composition and application of isostearic acid. Fat Sci. Technol. 1989, 91, 350–353. [Google Scholar]
- Perin, G.; Alvaro, G.; Westphal, E.; Jacob, R.G.; Lenardao, E.J.; Viana, L.H.; D’Oca, M.G.M. Transesterification of castor oil assisted by microwave irradiation. Fuel 2008, 87, 2838–2841. [Google Scholar] [CrossRef]
- Battersby, N.S.; Morgan, P. A note on the use of the CEC L-33-A-93 test to predict the potential biodegradation of mineral oil based lubricants in soil. Chemosphere 1997, 35, 1773–1779. [Google Scholar] [CrossRef]
- Willing, A. Lubricants based on renewable resources-an environmentally compatible alternative to mineral oil products. Chemosphere 2001, 43, 89–98. [Google Scholar] [CrossRef]
- Klamann, D.; Rost, R.R.; Esso, A.G.R.C. Lubricants and Related Products: Synthesis, Properties, Applications, International Standards; Verlag Chemie: Weinheim, Germany, 1984. [Google Scholar]
- Igartua, A.; Fernández, X.; Areitioaurtena, O.; Luther, R.; Seyfert, C.; Rausch, J.; Illarramendi, I.; Berg, M.; Schultheiß, H.; Duffau, B.; et al. Biolubricants and triboreactive materials for automotive applications. Tribol. Int. 2009, 42, 561–568. [Google Scholar] [CrossRef]
- Singh, A.K. Castor oil-based lubricant reduces smoke emission in two-stroke engines. Ind. Crops Prod. 2011, 33, 287–295. [Google Scholar] [CrossRef]
- Ye, S.H.; Zhou, W.; Song, J.; Peng, B.C.; Yuan, D.; Lu, Y.M.; Qi, P.P. Toxicity and health effects of vehicle emissions in Shanghai. Atmos. Environ. 2000, 34, 419–429. [Google Scholar] [CrossRef]
- Masjuki, H.H.; Maleque, M.A.; Kubo, A.; Nonaka, T. Palm oil and mineral oil based lubricants-their tribological and emission performance. Tribol. Int. 1999, 32, 305–314. [Google Scholar] [CrossRef]
- Mannekote, J.K.; Kailas, S.V. Experimental investigation of coconut and palm oils as lubricants in four-stroke engine. Tribol. Online 2011, 6, 76–82. [Google Scholar] [CrossRef]
- Reddy, K.S.V.; Kabra, N.; Kunchum, U.; Vijayakumar, T. Experimental investigation on usage of palm oil as a lubricant to substitute mineral oil in CI engines. Chin. J. Eng. 2014, 643521. [Google Scholar] [CrossRef]
- Arumugam, S.; Sriram, G.; Ellappan, R. Bio-lubricant-biodiesel combination of rapeseed oil: An experimental investigation on engine oil tribology, performance, and emissions of variable compression engine. Energy 2014, 72, 618–627. [Google Scholar] [CrossRef]
- Regueira, T.; Lugo, L.; Fandiño, O.; López, E.R.; Fernández, J. Compressibilities and viscosities of reference and vegetable oils for their use as hydraulic fluids and lubricants. Green Chem. 2011, 13, 1293–1302. [Google Scholar] [CrossRef]
- Bronshteyn, L.A.; Kreiner, J.H. Energy efficiency of industrial oils. Tribol. Trans. 1999, 42, 771–776. [Google Scholar] [CrossRef]
- Mendoza, G.; Igartua, A.; Fernández-Díaz, B.; Urquiola, F.; Vivanco, S.; Arguizoniz, R. Vegetable oils as hydraulic fluids for agricultural applications. Grasas Aceites 2011, 62, 29–38. [Google Scholar]
- Yunus, R.; Fakhru’l-Razi, A.; Ooi, T.L.; Iyuke, S.E.; Perez, J.M. Lubrication properties of trimethylolpropane esters based on palm oil and palm kernel oils. Eur. J. Lipid Sci. Technol. 2004, 106, 52–60. [Google Scholar] [CrossRef]
- Silva, M.S.; Foletto, E.L.; Alves, S.M.; de Castro Dantas, T.N.; Dantas Neto, A.A. New hydraulic biolubricants based on passion fruit and moringa oils and their epoxy. Ind. Crops Prod. 2015, 69, 362–370. [Google Scholar] [CrossRef]
- Kamalakar, K.; Rajak, A.K.; Prasad, R.B.N.; Karuna, M.S.L. Rubber seed oil-based biolubricant base stocks: A potential source for hydraulic oils. Ind. Crops Prod. 2013, 51, 249–257. [Google Scholar] [CrossRef]
- Nagendramma, P.; Kaul, S.; Bisht, R.P.S. Study of synthesized ecofriendly and biodegradable esters: Fire resistance and lubricating properties. Lubr. Sci. 2010, 22, 103–110. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z. Epoxidized soybean oil as a potential source of high temperature lubricants. Ind. Crops Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Ting, C.C.; Chen, C.C. Viscosity and working efficiency analysis of soybean oil based bio-lubricants. Measurement 2011, 44, 1337–1341. [Google Scholar] [CrossRef]
- Bartz, W.J. Lubricants and the environment. Tribol. Int. 1998, 31, 35–47. [Google Scholar] [CrossRef]
- Raynor, P.C.; Kim, S.W.; Bhattacharya, M. Mist generation from metalworking fluids formulated using vegetable oils. Ann. Occup. Hyg. 2005, 49, 283–293. [Google Scholar] [PubMed]
- John, J.; Bhattacharya, M.; Raynor, P.C. Emulsions containing vegetable oils for cutting fluid application. Colloids Surf. A Physicochem. Eng. Asp. 2004, 237, 141–150. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, A.K. Metalworking fluids from vegetable oils. J. Synth. Lubr. 2006, 23, 167–176. [Google Scholar] [CrossRef]
- Lawal, S.A.; Choudhury, I.A.; Nukman, Y. Application of vegetable oil-based metalworking fluids in machining ferrous metals—A review. Int. J. Mach. Tools Manuf. 2012, 52, 1–12. [Google Scholar] [CrossRef]
- Belluco, W.; De Chiffre, L. Performance evaluation of vegetable-based oils in drilling austenitic stainless steel. J. Mater. Process. Technol. 2004, 148, 171–176. [Google Scholar] [CrossRef]
- Tazehkandi, A.H.; Shabgard, M.; Pilehvarian, F. On the feasibility of a reduction in cutting fluid consumption via spray of biodegradable vegetable oil with compressed air in machining Inconel 706. J. Clean. Prod. 2015, 104, 422–435. [Google Scholar] [CrossRef]
- Höhn, B.R.; Michaelis, K.; Doleschel, A. Frictional behaviour of synthetic gear lubricants. Tribol. Ser. 2001, 39, 759–768. [Google Scholar]
- Nagendramma, P.; Kaul, S. Study of synthetic complex esters as automotive gear lubricants. J. Synth. Lubr. 2008, 25, 131–136. [Google Scholar] [CrossRef]
- Rac, A.; Vencl, A. Performance investigation of chain saw lubricants based on new sunflower oil (NSO). Tribol. Schmier. 2009, 56, 51. [Google Scholar]
- Garrett, S. Vegetable Oil for Lubricating Chain Saws, Fire Management Tech Tips; United States Department of Agriculture Forest Service: St. Paul, MN, USA, 1998.
- Stanovský, M.; Schürger, J.; Jankovský, M.; Messingerova, V.; Hnilica, R.; Kucera, M. The effect of lubricating oil on temperature of chainsaw cutting system. Croat. J. For. Eng. 2013, 34, 83–90. [Google Scholar]
- Earle, C.E. Lubricating Containing Lithium Salts. U.S. Patent 2274676A, 3 March 1942. [Google Scholar]
- Lugt, P.M. Modern advancements in lubricating grease technology. Tribol. Int. 2016, 97, 467–477. [Google Scholar] [CrossRef]
- Sanchez, R.; Franco, J.M.; Delgado, M.A.; Valencia, C.; Gallegos, C. Effect of thermo-mechanical processing on the rheology of oleogels potentially applicable as biodegradable lubricating greases. Chem. Eng. Res. Des. 2008, 86, 1073–1082. [Google Scholar] [CrossRef]
- Sanchez, R.; Franco, J.M.; Delgado, M.A.; Valencia, C.; Gallegos, C. Development of new green lubricating grease formulations based on cellulosic derivatives and castor oil. Green Chem. 2009, 11, 686–693. [Google Scholar] [CrossRef]
- Sanchez, R.; Franco, J.M.; Delgado, M.A.; Valencia, C.; Gallegos, C. Thermal and mechanical characterization of cellulosic derivatives-based oleogels potentially applicable as bio-lubricating greases: Influence of ethyl cellulose molecular weight. Carbohydr. Polym. 2011, 83, 151–158. [Google Scholar] [CrossRef]
- Sanchez, R.; Stringari, G.B.; Franco, J.M.; Valencia, C.; Gallegos, C. Use of chitin, chitosan and acylated derivatives as thickener agents of vegetable oils for biolubricant applications. Carbohydr. Polym. 2011, 85, 705–714. [Google Scholar] [CrossRef]
- Abdulbari, H.A.; Rosli, M.Y.; Abdurrahman, H.N.; Nizam, M.K. Lubricating grease from spent bleaching earth and waste cooking oil: Tribology properties. Int. J. Phys. Sci. 2011, 6, 4695–4699. [Google Scholar]
- Núñez, N.; Martín-Alfonso, J.E.; Valencia, C.; Sanchez, M.C.; Franco, J.M. Rheology of new green lubricating grease formulations containing cellulose pulp and its methylated derivative as thickener agents. Ind. Crops Prod. 2012, 37, 500–507. [Google Scholar] [CrossRef]
- Boiko, M.; Lebedinsky, K. Biodegradable lubricant for railway transport. Transp. Probl. 2015, 10, 99–105. [Google Scholar] [CrossRef]
- Bertrand, Y.; Hoang, L.C. Vegetable Oils as Substitute for Mineral Insulating Oils in Medium-Voltage Equipments; CIGRE: Paris, France, 2004. [Google Scholar]
- Beroual, A.; Khaled, U.; Mbolo Noah, P.S.; Sitorus, H. Comparative study of breakdown voltage of mineral, synthetic and natural oils and based mineral oil mixtures under AC and DC voltages. Energies 2017, 10, 511. [Google Scholar] [CrossRef]
- Shah, Z.; Tahir, Q. Dielectric properties of vegetable oils. J. Sci. Res. 2011, 3, 481–492. [Google Scholar] [CrossRef]
- Oommen, T.V. Vegetable oils for liquid-filled transformers. IEEE Electr. Insul. Mag. 2002, 18, 6–11. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Happe, M.; Grand, P.; Farquet, S.; Aeby, S.; Héritier, J.C.; Corthay, F.; Mabillard, E.; Marti, R.; Vanoli, E.; Grogg, A.F.; et al. Microwave barrel reactor use in trimethylolpropane oleate synthesis by Candida antarctica lipase in a biphasic non-solvent process. Green Chem. 2012, 14, 2337–2345. [Google Scholar] [CrossRef]
- Cavalcanti da Silva, J.A.; Ferreira Soares, V.; Fernández-Lafuente, R.; Habert, A.C.; Freire, D.M.G. Enzymatic production and characterization of potential biolubricants from castor bean biodiesel. J. Mol. Catal. B 2015, 122, 323–329. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Dutt Sharma, C.; Gupta, P.; Kaul, S. Clean synthesis of biolubricant range esters using novel liquid lipase enzyme in solvent free medium. Springerplus 2015, 4, 165. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 12901-2. Nanotechnologies—Occupational Risk Management Applied to Engineered Nanomaterials—Part 2: Use of the Control Banding Approach; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Aguerre-Chariol, O.; Bressot, C. Stem imaging to characterize nanoparticle emissions and help to design nanosafer paints. Chem. Eng. Res. Des. 2018, 136, 663–674. [Google Scholar] [CrossRef]
- Bressot, C.; Aubry, A.; Pagnoux, C.; Aguerre-Chariol, O.; Morgeneyer, M. Assessment of functional nanomaterials in medical applications: Can time mend public and occupational health risks related to the products’ fate? J. Toxicol. Environ. Health A 2018, 81, 957–973. [Google Scholar] [CrossRef]
- Bressot, C.; Shandilya, N.; Jayabalan, T.; Fayet, G.; Voetz, M.; Meunier, L.; Le Bihan, O.; Aguerre-Chariol, O.; Morgeneyer, M. Exposure assessment of nanomaterials at production sites by a short time sampling (sts) approach strategy and first results of measurement campaigns. Process Saf. Environ. 2018, 116, 324–332. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Ramirez, A.; Smith, S.M.; Tweedie, R.; Heng, J.; Maass, S.; Bressot, C. Particle technology as a uniform discipline? Towards a holistic approach to particles, their creation, characterisation, handling and processing! Chem. Eng. Res. Des. 2019, 146, 162–165. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).