Malus baccata var. gracilis and Malus toringoides Bark Polyphenol Studies and Antioxidant, Antimicrobial and Anticancer Activities
Abstract
:1. Introduction
2. Results
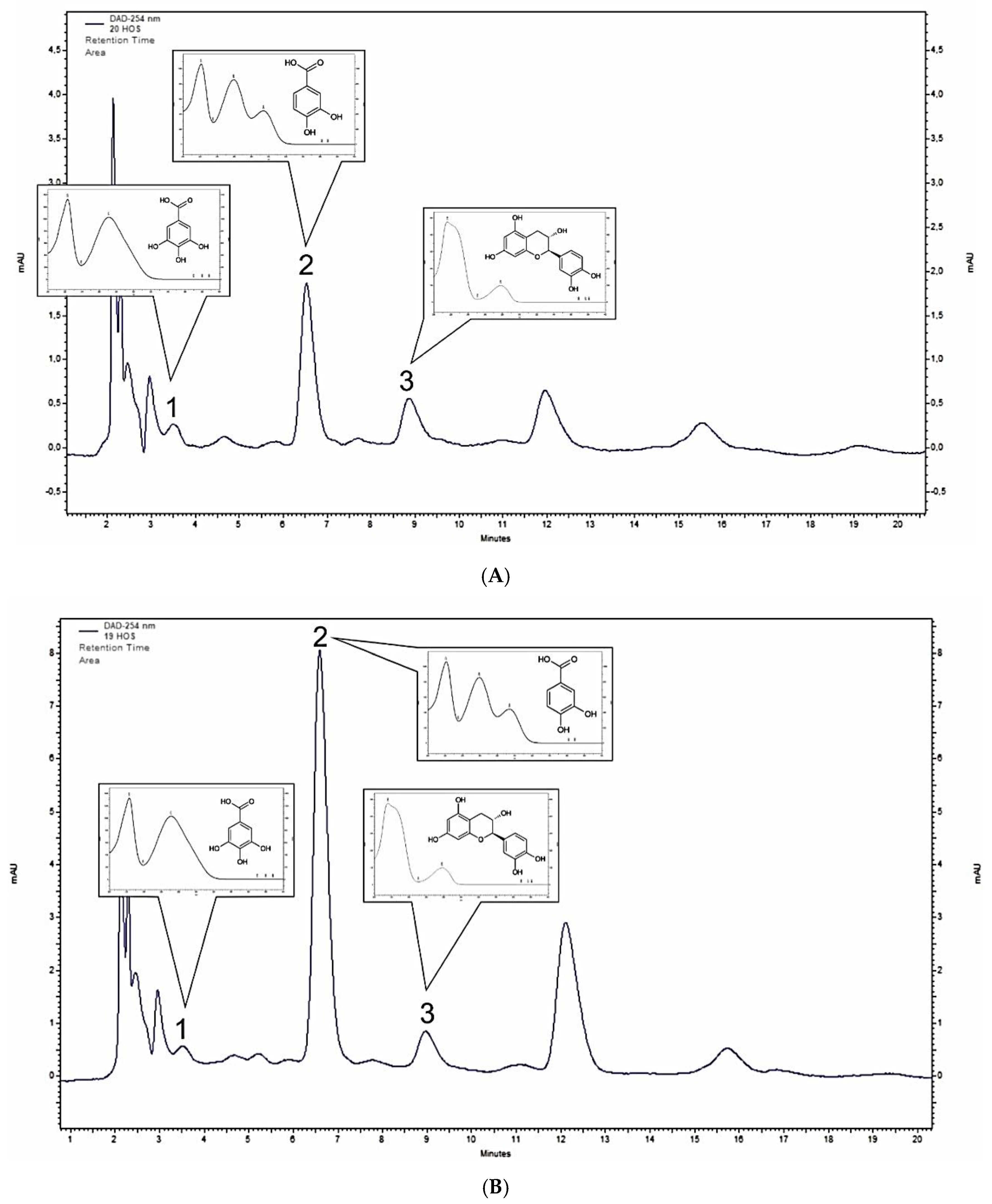
2.1. Polyphenol Profiling of M. baccata and M. toringoides Bark
2.2. Antioxidant Effects
2.3. Anticancer Effects
2.4. Antibacterial Activities
2.5. Antifungal Effects
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation
4.2. Chemicals
4.3. Analyses of Phenolic Compounds
4.4. Anticancer Activities
4.5. Antioxidant Activity
4.6. Antibacterial Activity
4.7. Antifungal Activity
4.8. Research Ethics
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ebrahimi, A.; Schluesener, H. Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls. Ageing Res. Rev. 2012, 11, 329–345. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; El-Abedin, T.K.A.Z.; Mattar, M.A.; Ekiert, H. Phenolic Compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have Antioxidant and Anticancer Activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elansary, H.O. Tree Bark Phenols Regulate the Physiological and Biochemical Performance of Gladiolus Flowers. Processes 2020, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Szopa, A.; Klimek-Szczykutowicz, M.; Jafernik, K.; Ekiert, H.; Mahmoud, E.A.; Barakat, A.A.; El-Ansary, D.O. Mammillaria Species—Polyphenols Studies and Anti-Cancer, Anti-Oxidant, and Anti-Bacterial Activities. Molecules 2019, 25, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, A.M.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- El-Sabrout, M.A.; Salem, Z.M.M.; Bin-Jumah, M.; Allam, A.A. Toxicological Activity of Some Plant Essential Oils Against Tribolium castaneum and Culex pipiens Larvae. Processes 2019, 7, 933. [Google Scholar] [CrossRef] [Green Version]
- Okla, K.M.; Alamri, A.S.; Salem, Z.M.M.; Ali, M.H.; Behiry, I.S.; Nasser, A.R.; Alaraidh, A.I.; Al-Ghtani, M.S.; Soufan, W. Yield, Phytochemical Constituents, and Antibacterial Activity of Essential Oils from the Leaves/Twigs, Branches, Branch Wood, and Branch Bark of Sour Orange (Citrus aurantium L.). Processes 2019, 7, 363. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.Z.M.; Elansary, H.O.; Ali, H.M.; El-Settawy, A.A.; Elshikh, M.S.; Abdel-Salam, E.M.; Skalicka-Wozniak, K. Bioactivity of essential oils extracted from Cupressus macrocarpa branchlets and Corymbia citriodora leaves grown in Egypt. BMC Complement. Altern. Med. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant Activity and Phenolic Profile of Selected Organic and Conventional Honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Siddiqui, S.A. Concurrent chemoradiotherapy with or without induction chemotherapy for the management of cervical lymph node metastasis from unknown primary tumor. J. Cancer Res. Ther. 2018, 14, 1117–1120. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Choi, H.Y.; Yang, G.-M.; Kim, K.; Saha, S.K.; Cho, S.-G. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef]
- Costea, T.; Nagy, P.; Ganea, C.; Szöllősi, J.; Mocanu, M.-M. Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1062. [Google Scholar] [CrossRef] [Green Version]
- Alvarado-Sansininea, J.J.; Sánchez-Sánchez, L.; López-Muñoz, H.; Escobar, M.L.; Flores-Guzmán, F.; Tavera-Hernández, R.; Jiménez-Estrada, M. Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines. Molecules 2018, 23, 2579. [Google Scholar] [CrossRef] [Green Version]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, Through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mansour, M.M.A.; Elansary, H.O. Evaluation of the effect of inner and outer bark extracts of sugar maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J. Wood Chem. Technol. 2019, 39, 136–147. [Google Scholar] [CrossRef]
- Saleh, A.; ElFayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. N-S Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef]
- Andrade, C.; Ferreres, F.; Gomes, G.M.N.; Duangsrisai, S.; Srisombat, N.; Vajrodaya, S.; Pereira, M.D.; Gil-Izquierdo, A.; Andrade, B.P.; Valentão, P. Phenolic Profiling and Biological Potential of Ficus curtipes Corner Leaves and Stem Bark: 5-Lipoxygenase Inhibition and Interference with NO Levels in LPS-Stimulated RAW 264.7 Macrophages. Biomolecules 2019, 9, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dróżdż, P.; Pyrzynska, K.J. Assessment of polyphenol content and antioxidant activity of oak bark extracts. Eur. J. Wood Prod. 2018, 76, 793–795. [Google Scholar] [CrossRef] [Green Version]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R.J. Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, S.M.; Amorós, A.; Carbonell-Barrachina, A.Á; Hernández, F. Polyphenol Compounds and Biological Activity of Caper (Capparis spinosa L.) Flowers Buds. Plants 2019, 8, 539. [Google Scholar]
- Fejér, J.; Kron, I.; Pellizzeri, V.; Pľuchtová, M.; Eliašová, A.; Campone, L.; Gervasi, T.; Bartolomeo, G.; Cicero, N.; Babejová, A.; et al. First Report on Evaluation of Basic Nutritional and Antioxidant Properties of Moringa Oleifera Lam. from Caribbean Island of Saint Lucia. Plants 2019, 8, 537. [Google Scholar] [CrossRef] [Green Version]
- Bazzicalupo, M.; Burlando, B.; Denaro, M.; Barreca, D.; Trombetta, D.; Smeriglio, A.; Cornara, L. Polyphenol Characterization and Skin-Preserving Properties of Hydroalcoholic Flower Extract from Himantoglossum robertianum (Orchidaceae). Plants 2019, 8, 502. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Missiakas, D.; Schneewind, O. Mouse models for infectious diseases caused by Staphylococcus aureus. J. Immunol. Methods 2014, 410, 88–99. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; Elansary, H.O.; El-Ansary, D.O.; Al-Mana, F.A. Elevated Bioactivity of Ruta graveolens against Cancer Cells and Microbes Using Seaweeds. Processes 2020, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.N.; Anyoha, A.; Madoff, L.C.; Lassmann, B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: A review of ProMED reports from 1996 to 2018. Int. J. Infect. Dis. 2019, 84, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Aguirre-Joya, J.A.; Pastrana-Castro, L.; Nieto-Oropeza, D.; Ventura-Sobrevilla, J.; Rojas-Molina, R.; Aguilar, C.N. The physicochemical, antifungal and antioxidant properties of a mixed polyphenol based bioactive film. Heliyon 2018, 4, e00942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yessoufou, K.; Elansary, H.O.; Mahmoud, E.A.; Skalicka-Wozniak, K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind. Crop. Prod. 2015, 74, 752–758. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of Traditional Medicinal Plants in Alexandria. Evid. Based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elansary, H.O.; Salem, M.Z.M.; Ashmawy, N.A.; Yessoufou, K.; El-Settawy, A.A.A. In vitro antibacterial, antifungal and antioxidant activities of Eucalyptus spp. leaf extracts related to phenolic composition. Nat. Prod. Res. 2017, 31, 2927–2930. [Google Scholar] [CrossRef] [PubMed]
- Cornille, A.; Giraud, T.; Smulders, M.J.M.; Roldán-Ruiz, I.; Gladieux, P. The domestication and evolutionary ecology of apples. Trends Genet. 2014, 30, 57–65. [Google Scholar] [CrossRef]
- Volk, G.M.; Chao, C.T.; Norelli, J.; Brown, S.K.; Fazio, G.; Peace, C.; McFerson, J.; Zhong, G.-Y.; Bretting, P. The vulnerability of US apple (Malus) genetic resources. Genet. Resour. Crop. Evol. 2015, 62, 765–794. [Google Scholar] [CrossRef]
- Dadwal, V.; Agrawal, H.; Sonkhla, K.; Joshi, R.; Gupta, M. Characterization of phenolics, amino acids, fatty acids and antioxidant activity in pulp and seeds of high altitude Himalayan crab apple fruits (Malus baccata). J. Food Sci. Technol. 2018, 55, 2160–2169. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, S.; Zhang, Z.; Fang, H.; Xu, H.; Wang, Y.; Chen, X. Malus sieversii: The origin, flavonoid synthesis mechanism, and breeding of red-skinned and red-fleshed apples. Hortic. Res. Engl. 2018, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.H. Chemical evidence from the flavonoids relevant to the classification of Malus species. Bot. J. Linn. Soc. 1982, 84, 31–39. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 9, 952943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. J. Oxid. Med. Cel. Longevity. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekar, V.; Belur, P.D.; Regupathi, I. Effect of hydroxybenzoic acids antioxidants on the oxidative stability of sardine oil. Resource Effic. Technol. 2016, 2, S114–S118. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-H.; Chen, J.-H.; Chou, F.-P.; Wang, C.-J. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.T.S.; Švajdlenka, E. Biological Evaluation and Molecular Docking of Protocatechuic Acid from Hibiscus sabdariffa L. as a Potent Urease Inhibitor by an ESI-MS Based Method. Molecules 2017, 22, 1696. [Google Scholar] [CrossRef] [Green Version]
- Miklasińska, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Zdebik, A.; Orlewska, K.; Wąsik, T.J. Antibacterial Activity of Protocatechuic Acid Ethyl Ester on Staphylococcus aureus Clinical Strains Alone and in Combination with Antistaphylococcal Drugs. Molecules 2015, 20, 13536–13549. [Google Scholar] [CrossRef] [Green Version]
- Heleno, S.A.; Barros, L.; Martins, A.; Morales, P.; Fernández-Ruiz, V.; Glamoclija, J.; Sokovic, M.; Ferreira, I.C.F.R. Nutritional value, bioactive compounds, antimicrobial activity and bioaccessibility studies with wild edible mushrooms. LWT Food Sci. Technol. 2015, 63, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Yessoufou, K.; Daru, B.H.; Tafirei, R.; Elansary, H.O.; Rampedi, I. Integrating biogeography, threat and evolutionary data to explore extinction crisis in the taxonomic group of cycads. Ecol. Evol. 2017, 7, 2735–2746. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, X.H.; Naing, K.W.; Lee, Y.S.; Moon, J.H.; Lee, J.H.; Kim, K.Y. Isolation and characteristics of protocatechuic acid from Paenibacillus elgii HOA73 against Botrytis cinerea on strawberry fruits. J. Basic Microbiol. 2015, 55, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.Z.M.; El-Hefny, M.; Nasser, R.A.; Ali, H.M.; El-Shanhorey, N.A.; Elansary, H.O. Medicinal and biological values of Callistemon viminalis extracts: history, current situation and prospects. Asian Pac. J. Trop. Med. 2017, 10, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sulkowska-Ziaja, K.; Maslanka, A.; Szewczyk, A.; Muszynska, B. Physiologically Active Compounds in Four Species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD- HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krośniak, M.; Marzec-Wróblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A. ×prunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed Extracts Enhance Salam Turfgrass Performance during Prolonged Irrigation Intervals and Saline Shock. Front. Plant. Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.P.A.; Miranda, I.; Sousa, V.B.; Pereira, H. Chemical composition of barks from Quercus faginea trees and characterization of their lipophilic and polar extracts. PLoS ONE 2018, 13, e0197135. [Google Scholar] [CrossRef]
- El-Esawi, A.M.; Elkelish, A.; Soliman, M.; Elansary, O.H.; Zaid, A.; Shabir, W.H. Serratia marcescens BM1 Enhances Cadmium Stress Tolerance and Phytoremediation Potential of Soybean Through Modulation of Osmolytes, Leaf Gas Exchange, Antioxidant Machinery, and Stress-Responsive Genes Expression. Antioxidants 2020, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Szopa, A.; Klimek-Szczykutowicz, M.; Ekiert, H.; Barakat, A.A.; Al-Mana, F.A. Antiproliferative, Antimicrobial, and Antifungal Activities of Polyphenol Extracts from Ferocactus Species. Processes 2020, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Mahmoud, E.A. Basil cultivar identification using chemotyping still favored over genotyping using core barcodes and possible resources of antioxidants. J. Essent. Oil Res. 2015, 27, 82–87. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E.A.; Skaicka-Wozniak, K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crop. Prod. 2016, 92, 50–56. [Google Scholar] [CrossRef]
- Abd El-Kareem, M.S.M.; Mohamed, A.R.; Elansary, H.O.; Al-Mana, F.A. Mass Spectral Fragmentation of Pelargonium graveolens Essential Oil Using GC–MS Semi-Empirical Calculations and Biological Potential. Processes 2020, 8, 128. [Google Scholar] [CrossRef] [Green Version]


| Protocatechuic Acid | Gallic Acid | Catechin | |
|---|---|---|---|
| M. baccata | 3.16 ± 0.44 | 0.29 ± 0.07 | 5.55 ± 0.91 |
| M. toringoides | 7.15 ± 0.40 | 0.44 ± 0.13 | 6.80 ± 1.12 |
| β-Carotene-Bleaching Assay (IC50, µg mL−1) | DPPH (IC50, µg mL−1) | FRAP (IC50, mM TEAC/g Extract) | |
|---|---|---|---|
| M. baccata | 7.5 ± 0.3c | 5.8 ± 0.2c | 9.3 ± 0.1e |
| M. toringoides | 6.2 ± 0.2d | 5.2 ± 0.1cd | 7.5 ± 0.3e |
| protocatechuic acid | 9.1 ± 0.3d | 7.3 ± 0.1d | 11.5 ± 0.7f |
| BHT | 3.3 ± 0.1e | 2.7 ± 0.1e | - |
| Trolox | - | - | 3.3 ± 0.1g |
| HeLa | HT-29 | MCF-7 | Jurkat | HEK-293 | |
|---|---|---|---|---|---|
| M. baccata | 64.2 ± 0.2 | 104.62 ± 4.8 | 41.3 ± 2.1 | 38.3 ± 1.1 | ˃400 |
| M. toringoides | 55.31 ± 1.3 | 89.59 ± 2.5 | 30.35 ± 1.2 | 31.25 ± 1.4 | ˃400 |
| protocatechuic acid | 40.58 ± 2.3 | 97.54 ± 4.9 | 19.65 ± 1.7 | 45.32 ± 3.1 | ˃400 |
| vinblastine sulfate | 2.3 ± 0.08 | 18.23 ± 0.7 | ‒ | 0.1 ± 0.01 | 45.5 ± 0.9 |
| taxol | ‒ | ‒ | 0.07 ± 0.009 | ‒ | ‒ |
| B. Cereus MIC MBC | P. Aeruginosa MIC MBC | L. Monocytogenes MIC MBC | E. Coli MIC MBC | M. Flavus MIC MBC | S. Aureus MIC MBC | |
|---|---|---|---|---|---|---|
| M. baccata | 0.21 ± 0.03 | 0.36 ± 0.02 | 0.32 ± 0.03 | 0.41 ± 0.05 | 0.79 ± 0.05 | 0.47 ± 0.03 |
| 0.56 ± 0.05 | 0.87 ± 0.03 | 0.92 ± 0.05 | 1.29 ± 0.11 | 2.13 ± 0.27 | 1.12 ± 0.17 | |
| M. toringoides | 0.15 ± 0.03 | 0.24 ± 0.03 | 0.30 ± 0.03 | 0.37 ± 0.03 | 0.98 ± 0.07 | 0.31 ± 0.03 |
| 0.43 ± 0.05 | 0.73 ± 0.05 | 0.83 ± 0.05 | 1.13 ± 0.15 | 2.01 ± 0.31 | 1.03 ± 0.13 | |
| protocatechuic acid | 0.23 ± 0.02 | 0.35 ± 0.03 | 0.25 ± 0.01 | 0.27 ± 0.01 | 0.55± 0.03 | 0.26± 0.03 |
| 0.65 ± 0.05 | 0.83 ± 0.04 | 0.67 ± 0.05 | 0.71 ± 0.03 | 1.37 ± 0.13 | 0.79 ± 0.05 | |
| streptomycin | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.02 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.16 ± 0.02 |
| 0.19 ± 0.02 | 0.17 ± 0.01 | 0.27 ± 0.03 | 0.21 ± 0.02 | 0.21 ± 0.01 | 0.31 ± 0.03 |
| A. Flavus MIC MFC | A. Ochraceus MIC MFC | A. Niger MIC MFC | C. Albicans MIC MFC | P. Funiculosum MIC MFC | P. Ochrochloron MIC MFC | |
|---|---|---|---|---|---|---|
| M. baccata | 0.43 ± 0.03 | 0.37± 0.03 | 0.39 ± 0.03 | 0.47 ± 0.05 | 0.51± 0.01 | 1.89 ± 0.13 |
| 0.95 ± 0.05 | 0.69 ± 0.03 | 0.75 ± 0.05 | 1.34 ± 0.21 | 1.23 ± 0.23 | N.D. | |
| M. toringoides | 0.29 ± 0.03 | 0.25 ± 0.02 | 0.29 ± 0.05 | 0.34 ± 0.05 | 0.45 ± 0.05 | 1.13 ± 0.02 |
| 0.57 ± 0.05 | 0.49 ± 0.03 | 0.69 ± 0.03 | 1.23 ± 0.13 | 1.11 ± 0.09 | N.D. | |
| protocatechuic acid | 0.37 ± 0.03 | 0.29 ± 0.03 | 0.33 ± 0.02 | 0.59 ± 0.7 | 0.59 ± 0.01 | 1.37 ± 0.15 |
| 0.86 ± 0.05 | 0.53 ± 0.03 | 0.61 ± 0.03 | 1.11 ± 0.16 | 1.73 ± 0.31 | N.D. | |
| KTZ | 0.19 ± 0.01 | 0.20 ± 0.01 | 0.11± 0.01 | 0.21± 0.01 | 2.04 ± 0.15 | 0.20 ± 0.03 |
| 0.41 ± 0.03 | 0.43 ± 0.03 | 0.21 ± 0.03 | 0.42 ± 0.03 | 3.73 ± 0.09 | 0.41 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elansary, H.O.; Szopa, A.; Kubica, P.; O. El-Ansary, D.; Ekiert, H.; A. Al-Mana, F. Malus baccata var. gracilis and Malus toringoides Bark Polyphenol Studies and Antioxidant, Antimicrobial and Anticancer Activities. Processes 2020, 8, 283. https://doi.org/10.3390/pr8030283
Elansary HO, Szopa A, Kubica P, O. El-Ansary D, Ekiert H, A. Al-Mana F. Malus baccata var. gracilis and Malus toringoides Bark Polyphenol Studies and Antioxidant, Antimicrobial and Anticancer Activities. Processes. 2020; 8(3):283. https://doi.org/10.3390/pr8030283
Chicago/Turabian StyleElansary, Hosam O., Agnieszka Szopa, Paweł Kubica, Diaa O. El-Ansary, Halina Ekiert, and Fahed A. Al-Mana. 2020. "Malus baccata var. gracilis and Malus toringoides Bark Polyphenol Studies and Antioxidant, Antimicrobial and Anticancer Activities" Processes 8, no. 3: 283. https://doi.org/10.3390/pr8030283
APA StyleElansary, H. O., Szopa, A., Kubica, P., O. El-Ansary, D., Ekiert, H., & A. Al-Mana, F. (2020). Malus baccata var. gracilis and Malus toringoides Bark Polyphenol Studies and Antioxidant, Antimicrobial and Anticancer Activities. Processes, 8(3), 283. https://doi.org/10.3390/pr8030283







