Industrial Production of Poly-β-hydroxybutyrate from CO2: Can Cyanobacteria Meet this Challenge?
Abstract
1. Introduction
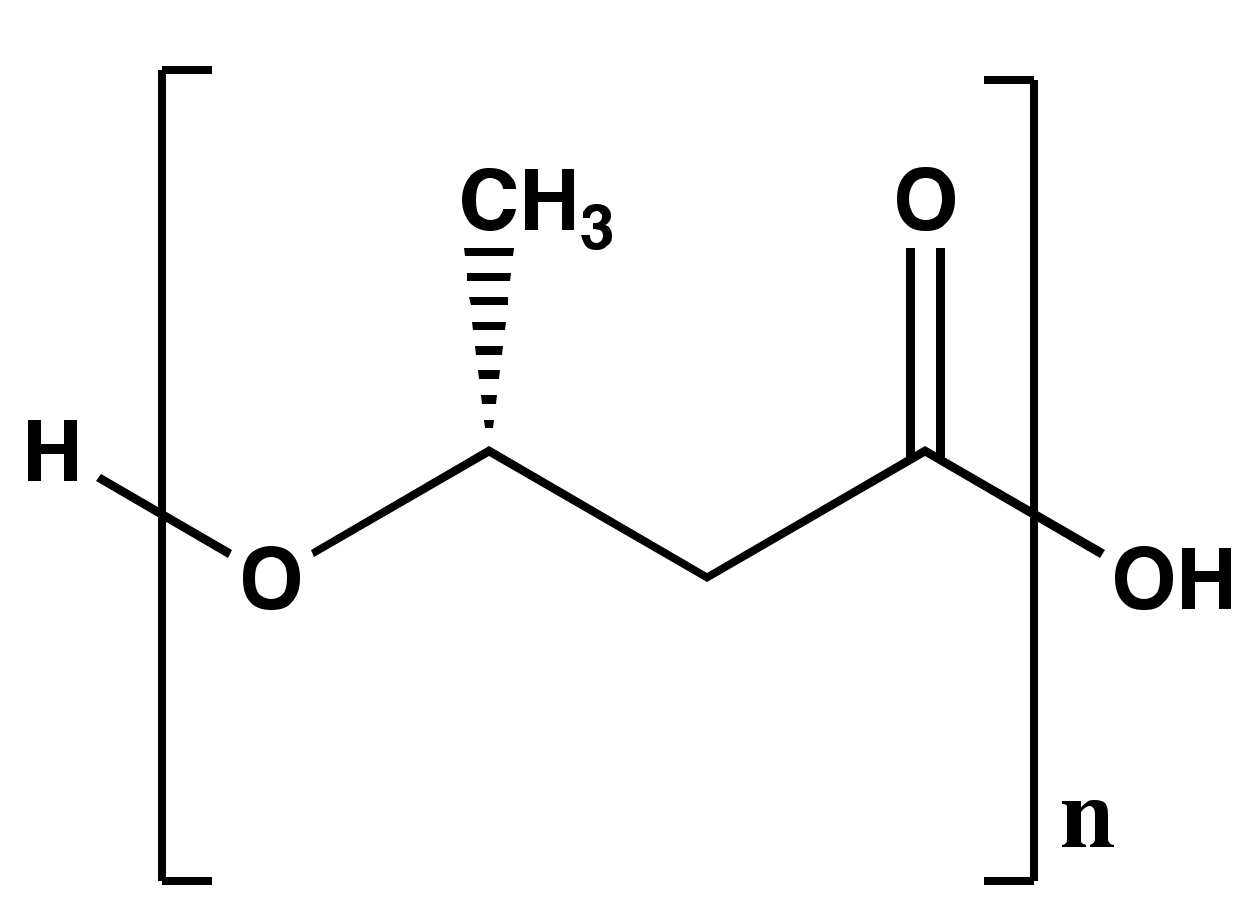
2. Poly-β-hydroxybutyrate (PHB) Structure
3. Commercial Status of PHB
4. Conventional Production System of PHB
5. Cyanobacteria and PHB
6. Improvement of PHB Production in Cyanobacteria
6.1. Optimization of Growth Conditions
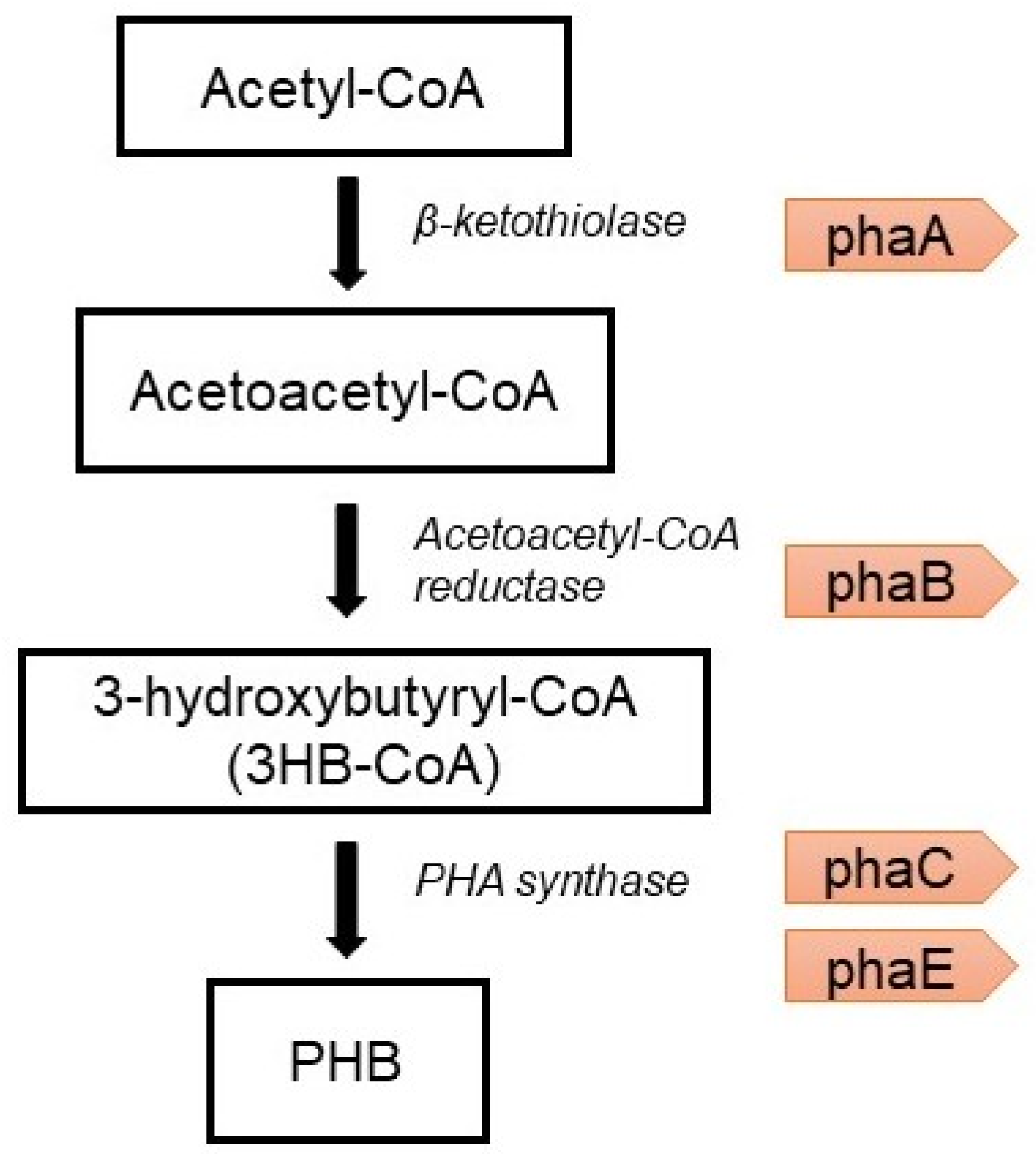
6.2. Genetic Engineering Approach
- The condensation of two molecules of acetyl-CoA into acetoacetyl-CoA by PHA-specific beta-ketothiolase (phaA) [75];
- The reduction of acetoacetyl-CoA to (R)-3-hydroxybutyryl-CoA by acetoacetyl-CoA reductase (phaB);
6.3. Continuous PHB Production
- The possibility to separate the biomass growth phase from the PHB production phase. The two phases are characterized by different optimal operating conditions;
- Minimization of equipment downtime and time loss due to the lag phase of the microbial cultures [82];
- The continuous cultivation can guarantee the growth of microorganisms and their long-term genetic stability under defined nutrient limitations for prolonged time periods, resulting in both high productivities and constant product quality [82];
- The possibility to harvest biomass at a desired PHB-mass fraction;
- The applied dilution rate D may significantly influence the molar mass of PHB. The PHB productivity/concentration may be affected by the dilution; indeed, alteration of the dilution rate could affect both utilization of the substrate and the growth of cells [83].
6.4. Mathematical Modeling
- Strains characterized by distinct phases: biomass growth phase and PHA production phase, typically induced by N or P limitation (prototype organisms: Pseudomonas sp. 2F, Methylmonas extorquens) [8];
- Strains characterized by the accumulation of PHB during cell growth phase under balanced nutritional conditions and by the increase of the PHB fraction under the non-growth phase (usually induced by N or P limitation) (prototype organism: Cupriavidus necator) [85];
7. PHB Recovery
- Recovery of PHB accumulated in cyanobacteria using ionic liquids to dissolve cyanobacteria and retain PHB [102]. Ionic liquids (ILs) have emerged as a potential solvent for several applications. ILs are characterized by a melting point below 100 °C. They are characterized by interesting properties—e.g., high ionic conductivity and high thermal stability—which are difficult to achieve in general organic solvents [102]. Furthermore, ILs are characterized by negligible vapor pressure. They are gaining attention as novel green solvents;
- Spontaneous liberation of intracellular PHB granules using a genetic engineering approach. Jung et al. [103] manipulated the initial inoculum size and the composition of the medium and obtained an Escherichia coli strain that was able to produce PHB at a very high fraction [103]. After spontaneous cell lysis, PHB granules were released into the medium;
- Extraction of PHB with a solvent-free approach using enzyme digestion in an aqueous medium [106]. Martino et al. [106] used biomass of Cupriavidus necator DSM 428 grown on used cooking oil (UCO) for extraction of the PHB granules using sodium dodecyl sulphate (SDS), ethylenediaminetetraacetic acid (EDTA), and the enzyme alcalase in an aqueous medium. The recovered PHB granules showed >90% purity and no crystallization.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, L.; Kumar Singh, A.; Panda, B.; Mallick, N. Process optimization for poly-beta-hydroxybutyrate production in a nitrogen fixing cyanobacterium, Nostoc muscorum using response surface methodology. Bioresour. Technol. 2007, 98, 987–993. [Google Scholar] [PubMed]
- Hempel, F.; Bozarth, A.S.; Lindenkamp, N.; Klingl, A.; Zauner, S.; Linne, U.; Steinbüchel, A.; Maier, U.G. Microalgae as bioreactors for bioplastic production. Microb. Cell Fact. 2011, 10, 81. [Google Scholar] [PubMed]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as source of polyhydroxyalkanoates (PHAs)—A review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar]
- Singh, A.K.; Sharma, L.; Mallick, N.; Mala, J. Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J. Appl. Phycol. 2017, 29, 1213–1232. [Google Scholar]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Pseudomonas oleovorans as a source of poly (beta-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 1988, 54, 1977–1982. [Google Scholar] [PubMed]
- Nomura, C.; Taguchi, K.; Gan, Z.; Kuwabara, K.; Tanaka, T.; Takase, K.; Koyama, N.; Doi, Y. Expression of 3-ketoacyl-acyl carrier protein reductase (fabG) genes enhances production of polyhydroxyalkanoate copolymer from glucose in recombinant Escherichia coli JM109. Appl. Environ. Microbiol. 2005, 71, 4297–4306. [Google Scholar] [PubMed]
- Steinbüchel, A.; Wiese, S.A. Pseudomonas strain accumulating polyesters of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids. Appl. Microbiol. Biotechnol. 1992, 37, 691–697. [Google Scholar]
- Braunegg, G.; Lefebvre, G.; Genser, K.F. Polyhydroxyalkanoates, biopolyesters from renewable resources: Physiological and engineering aspects. J. Biotechnol. 1998, 65, 127–161. [Google Scholar]
- Balaji, S.; Gopi, K.; Muthuvelan, B. A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res. 2013, 2, 278–285. [Google Scholar]
- Amaro, T.M.M.M.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA) Production. Front Microbiol. 2019, 10, 992. [Google Scholar]
- Roland-Holst, D.; Heft-Neal, S. Bioplastics in California. Economic Assessment of Market. Conditions for PHA/PHB Bioplastics Produced from Waste Methane; Department of Resources Recycling and Recovery: Sacramento, CA, USA, 2013. [Google Scholar]
- Chandani, N.; Mazumder, P.B.; Bhattacharjee, A. Production of polyhydroxybutyrate (biopolymer) by Bacillus tequilensis NCS-3 Isolated from municipal waste areas of silchar, assam. Int. J. Sci. Res. 2014, 3, 198–203. [Google Scholar]
- Jiang, Y.; Marang, L.; Kleerebezem, R.; Muyzer, G.; van Loosdrecht, M.C.M. Polyhydroxybutyrate production from lactate using a mixed microbial culture. Biotechnol. Bioeng. 2011, 108, 2022–2035. [Google Scholar]
- Budde, C.F.; Riedel, S.L.; Hübner, F.; Risch, S.; Popovi, M.K.; Rha, C.; Sinskey, A.J. Growth and polyhydroxybutyrate production by Ralstonia eutropha in emulsified plant oil medium. Appl. Microbiol. Biotechnol. 2011, 89, 1611–1619. [Google Scholar]
- Han, J.; Qiu, Y.Z.; Liu, D.C.; Chen, G.Q. Engineered Aeromonas hydrophila for enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with alterable monomers composition. FEMS Microbiol. Lett. 2004, 239, 195–201. [Google Scholar]
- Wong, P.A.L.; Cheung, M.K.; Lo, W.; Chua, H.; Hoi, P.; Yu, F. Investigation of the effects of the types of food waste utilized as carbon source on the molecular weight distributions and thermal properties of polyhydroxy- butyrate produced by two strains of microorganisms. e-Polymers 2004, 31, 1–11. [Google Scholar]
- Pozo, C.; Martínez-Toledo, M.V.; Rodelas, B.; González-López, J. Effects of culture conditions on the production of polyhydroxyalkanoates by azotobacter chroococcum H23 in media containing a high concentration of alpechín (wastewater from olive oil mills) as primary carbon source. J. Biotechnol. 2002, 97, 125–131. [Google Scholar]
- ŁAbuzek, S.; Radecka, I. Biosynthesis of PHB tercopolymer by Bacillus cereus UW85. J. Appl. Microbiol. 2001, 90, 353–357. [Google Scholar]
- Chaijamrus, S.; Udpuay, N. Poly(3-Hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of alcaligenes latus under nitrogen limitation. Agric. Eng. Int. CIGRE J. 2008, 27, 10. [Google Scholar]
- Thirumala, M.; Reddy, S.V.; Mahmood, S.K.; Sultanpuram, M.T.; Reddy, V.; Mahmood, S.K. Production and characterization of PHB from two novel strains of Bacillus spp. isolated from soil and activated sludge. J. Ind. Microbiol. Biotechnol. 2010, 37, 271–278. [Google Scholar]
- Brämer, C.O.; Vandamme, P.; Da Silva, L.F.; Gomez, J.G.C.; Steinbüchel, A. Burkholderia sacchari sp. nov., a polyhydroxyalkanoate-accumulating bacterium isolated from soil of a sugar-cane plantation in Brazil. Int. J. Syst. Evol. Microbiol. 2001, 51, 1709–1713. [Google Scholar]
- Silva, L.F.; Taciro, M.K.; Michelin Ramos, M.E.; Carter, J.M.; Pradella, J.G.C.; Gomez, J.G.C. Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 2004, 31, 245–254. [Google Scholar]
- Qi, Q.; Rehm, B.H.A. Polyhydroxybutyrate biosynthesis in caulobacter crescentus: Molecular characterization of the polyhydroxybutyrate synthase. Microbiology 2001, 147, 3353–3358. [Google Scholar]
- Daneshi, A.; Younesi, H.; Ghasempouri, S.M.; Sharifzadeh, M. Production of poly-3-hydroxybutyrate by Cupriavidus necator from corn syrup: Statistical modeling and optimization of biomass yield and volumetric productivity. J. Chem. Technol. Biotechnol. 2010, 85, 1528–1539. [Google Scholar]
- Cavalheiro, J.M.B.T.; Catarina, M.; de Almeida, M.D.; Grandfils, C.; da Fonseca, M.M.R. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process. Biochem. 2009, 44, 509–515. [Google Scholar]
- Ceyhan, N.; Ozdemir, G. Poly-β-hydroxybutyrate (PHB) production from domestic wastewater using enterobacter aerogenes. Afr. J. Microbiol. Res. 2011, 5, 690–702. [Google Scholar]
- Nikel, P.I.; Pettinari, M.J.; Galvagno, M.A.; Méndez, B.S. Poly(3-hydroxybutyrate) synthesis by recombinant Escherichia coli arcA mutants in microaerobiosis. Appl. Environ. Microbiol. 2006, 72, 2614–2620. [Google Scholar]
- Quillaguamán, J.; Hashim, S.; Bento, F.; Mattiasson, B.; Hatti-Kaul, R. Poly(beta-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate. J. Appl. Microbiol. 2005, 99, 151–157. [Google Scholar]
- James, B.W.; Mauchline, W.S.; Dennis, P.J.; Keevil, C.W.; Wait, R. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl. Environ. Microbiol. 1999, 65, 822–827. [Google Scholar]
- Wendlandt, K.D.; Geyer, W.; Mirschel, G.; Al-Haj Hemidi, F. Possibilities for controlling PHB accumulation using various analytical methods. J. Biotechnol. 2005, 117, 119. [Google Scholar]
- Akar, A.; Akkaya, E.U.; Yesiladali, S.K.; Çelikyilmaz, G.; Çokgor, E.U.; Tamerler, C.; Orhon, D.; Çakar, Z.P. Accumulation of polyhydroxyalkanoates by Microlunatus phosphovorus under various growth conditions. J. Ind. Microbiol. Biotechnol. 2006, 33, 215–220. [Google Scholar]
- De Paula, F.C.; Kakazu, S.; de Paula, C.B.C.; Gomez, J.G.C.; Contiero, J. Polyhydroxyalkanoate production from crude glycerol by newly isolated Pandoraea sp. J. King Saud Univ. 2017, 29, 166–173. [Google Scholar]
- Fernández, D.; Rodríguez, E.; Bassas, M.; Viñas, M.; Solanas, A.M.; Llorens, J.; Marqués, A.M.; Manresa, A. Agro-industrial oily wastes as substrates for PHA production by the new strain Pseudomonas aeruginosa NCIB 40045: Effect of culture conditions. Biochem. Eng. J. 2005, 26, 159–167. [Google Scholar]
- Koller, M.; Bona, R.; Chiellini, E.; Fernandes, E.G.; Horvat, P.; Kutschera, C.; Hesse, P.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour. Technol. 2008, 99, 4854–4863. [Google Scholar] [PubMed]
- Goff, M.; Ward, P.G.; O’Connor, K.E. Improvement of the conversion of polystyrene to polyhydroxyalkanoate through the manipulation of the microbial aspect of the process: A nitrogen feeding strategy for bacterial cells in a stirred tank reactor. J. Biotechnol. 2007, 132, 283–286. [Google Scholar]
- Jiang, Y.; Song, X.; Gong, L.; Li, P.; Dai, C.; Shao, W. High poly(b-hydroxybutyrate) production by Pseudomonas fluorescens A2a5 from inexpensive substrates. Enzym. Microb. Technol. 2008, 42, 167–172. [Google Scholar]
- Mukhopadhyay, M.; Patra, A.; Paul, A.K. Production of poly(3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Rhodopseudomonas palustris SP5212. World J. Microbiol. Biotechnol. 2005, 21, 765–769. [Google Scholar]
- Bonatto, D.; Matias, F.; Lisbôa, M.P.; Bogdawa, H.M.; Henriques, J.A.P. Production of short side chain-poly[hydroxyalkanoate] by a newly isolated Ralstonia pickettii strain. World J. Microbiol. Biotechnol. 2004, 20, 395–403. [Google Scholar]
- Lopez-Arenas, T.; González-Contreras, M.; Anaya-Reza, O.; Sales-Cruz, M. Analysis of the fermentation strategy and its impact on the economics of the production process of PHB (polyhydroxybutyrate). Comput. Chem. Eng. 2017, 107, 140–150. [Google Scholar]
- Mittendorf, V.; Robertson, E.J.; Leech, R.M.; Krüger, N.; Steinbüchel, A.; Poirier, Y. Synthesis of medium-chain-length polyhydroxyalkanoates in arabidopsis thaliana using intermediates of peroxisomal fatty acid beta-oxidation. Proc. Natl. Acad. Sci. USA 1998, 95, 13397–13402. [Google Scholar]
- Poirier, Y.; Dennis, D.E.; Klomparens, K.; Somerville, C. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science 1992, 256, 520–523. [Google Scholar]
- Gopi, K.; Balaji, S.; Muthuvelan, B. Isolation purification and screening of biodegradable polymer PHB producing cyanobacteria from marine and fresh water resources. Iran. J. Energy Environ. 2014, 5, 94–100. [Google Scholar]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar]
- Wijffels, H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar] [PubMed]
- Blankenship, R.E. Early evolution of photosynthesis. Plant. Physiol. 2010, 154, 434–438. [Google Scholar] [PubMed]
- Vermaas, W.F.J. Photosynthesis and respiration in cyanobacteria. Encycl. Life Sci. 2001, 1–7. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar]
- Rezanka, T.; Dembitsky, V.M. Metabolites produced by cyanobacteria belonging to several species of the family Nostocaceae. Folia Microbiol. 2006, 51, 159–182. [Google Scholar]
- Costa, J.A.V.; Moreira, J.B.; Lucas, B.F.; Da Silva Braga, V.; Cassuriaga, A.P.A.; De Morais, M.G. Recent advances and future perspectives of PHB production by cyanobacteria. Ind. Biotechnol. 2018, 14, 249–256. [Google Scholar]
- Wu, G.F.; Wu, Q.Y.; Shen, Z.Y. Accumulation of poly-beta-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC 6803. Bioresour. Technol. 2001, 76, 85–90. [Google Scholar]
- Angermayr, S.A.; Hellingwerf, K.J.; Lindblad, P.; de Mattos, M.J.T. Energy biotechnology with cyanobacteria. Curr. Opin. Biotechnol. 2009, 20, 257–263. [Google Scholar]
- Bhati, R.; Mallick, N. Production and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer by a N2-fixing cyanobacterium, nostoc muscorum agardh. J. Chem. Technol. Biotechnol. 2012, 87, 505–512. [Google Scholar]
- Samantaray, S.; Mallick, N. Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer by the diazotrophic cyanobacterium Aulosira fertilissima CCC 444. J. Appl. Phycol. 2014, 26, 237–245. [Google Scholar]
- Takahashi, H.; Miyake, M.; Tokiwa, Y.; Asada, Y. Improved accumulation of poly- 3-hydroxybutyrate by a recombinant cyanobacterium. Biotechnol. Lett. 1998, 20, 183–186. [Google Scholar]
- Coelho, V.C.; Klasener, C.; Terra, A.L.; Alberto, J.; Costa, V.; Morais, M.G. De Polyhydroxybutyrate production by Spirulina sp. LEB 18 grown under different nutrient concentrations. Afr. J. Microbiol. Res. 2015, 9, 1586–1594. [Google Scholar]
- Panda, B.; Mallick, N. Enhanced poly-beta-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 2007, 44, 194–198. [Google Scholar]
- Carpine, R.; Raganati, F.; Olivieri, G.; Hellingwerf, K.J.; Pollio, A.; Salatino, P.; Marzocchella, A. Poly-β-hydroxybutyrate (PHB) production by Synechocystis PCC 6803 from CO2: Model development. Algal Res. 2018, 29, 49–60. [Google Scholar]
- Haase, S.M.; Huchzermeyer, B.; Rath, T. PHB accumulation in Nostoc muscorum under different carbon stress situations. J. Appl. Phycol. 2012, 24, 157–162. [Google Scholar]
- Smith, A.J. Modes of cyanobacterial carbon metabolism. In The Biology of Cyanobacteria; Carr, N.G., Whitton, B.A., Eds.; Blackwell: London, UK, 1982; pp. 47–85. [Google Scholar]
- Kaewbai-ngam, A.; Incharoensakdi, A.; Monshupanee, T. Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 2016, 212, 342–347. [Google Scholar]
- Di Pippo, F.; Ellwood, N.T.W.; Gismondi, A.; Bruno, L.; Rossi, F.; Magni, P.; de Philippis, R. Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J. Appl. Phycol. 2013, 25, 1697–1708. [Google Scholar]
- Panda, B.; Jain, P.; Sharma, L.; Mallick, N. Optimization of cultural and nutritional conditions for accumulation of poly-beta-hydroxybutyrate in Synechocystis sp. PCC 6803. Bioresour. Technol. 2006, 97, 1296–301. [Google Scholar]
- Campbell, J.; Stevens, S.E.; Balkwill, D.L. Accumulation of poly-beta-hydroxybutyrate in Spirulina platensis. J. Bacteriol. 1982, 149, 361–363. [Google Scholar] [PubMed]
- Lama, L.; Nicolaus, B.; Calandrelli, V.; Manca, M.C.; Romano, I.; Gambacorta, A. Effect of growth conditions on endo- and exopolymer biosynthesis in Anabaena cylindrica 10 C. Phytochemistry 1996, 42, 655–650. [Google Scholar]
- Samantaray, S.; Mallick, N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2012, 24, 803–814. [Google Scholar]
- Stal, L.; Heyer, H.; Jacobs, G. Occurrence and role of poly-hydroxyalkanoates in the cyanobacterium Oscillatoria limosa. In Novel Biodegradable Microbial Polymers; Kluwer Academic: Dordrecht, The Netherlands, 1990; pp. 435–438. [Google Scholar]
- Singh, M.K.; Rai, P.K.; Rai, A.; Singh, S.; Singh, J.S. Poly-β-hydroxybutyrate production by the cyanobacterium scytonema geitleri bharadwaja under varying environmental conditions. Biomolecules 2019, 9, 198. [Google Scholar]
- Toh, Y.; Jau, M.-H.; Yew, S.-P.; Abed, R.M.M.; Sudesh, K. Comparison of polyhydroxyalkanoates biosynthesis, mobilization and the effects on cellular morphology in Spirulina platensis and Synechocystis sp. uniwg. J. Biosci. 2008, 19, 21–38. [Google Scholar]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar]
- Ruffing, A.M. Engineered cyanobacteria: Teaching an old bug new tricks. Bioeng. Bugs 2011, 2, 136–149. [Google Scholar]
- Koksharova, O.A.; Wolk, C.P. Genetic tools for cyanobacteria. Appl. Microbiol. Biotechnol. 2002, 58, 123–137. [Google Scholar]
- Angermayr, S.A.; Gorchs Rovira, A.; Hellingwerf, K.J. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 2015, 33, 352–361. [Google Scholar]
- Miyake, M.; Takase, K.; Narato, M.; Khatipov, E.; Schnackenberg, J.; Shirai, M.; Kurane, R.; Asada, Y. Polyhydroxybutyrate production from carbon dioxide by cyanobacteria. Appl. Biochem. Biotechnol. 2000, 84, 991–1002. [Google Scholar]
- Carpine, R.; Du, W.; Olivieri, G.; Pollio, A.; Hellingwerf, K.J.; Marzocchella, A.; Branco dos Santos, F. Genetic engineering of Synechocystis sp. PCC 6803 for poly-β-hydroxybutyrate overproduction. Algal Res. 2017, 25, 117–127. [Google Scholar]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Bioresource technology enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [PubMed]
- Sudesh, K.; Taguchi, K.; Doi, Y. Effect of increased PHA synthase activity on polyhydroxyalkanoates biosynthesis in Synechocystis sp. PCC 6803. Int. J. Biol. Macromol. 2002, 30, 97–104. [Google Scholar] [PubMed]
- Wu, G.F.; Shen, Z.Y.; Wu, Q.Y. Modification of carbon partitioning to enhance PHB production in Synechocystis sp. PCC 6803. Enzym. Microb. Technol. 2002, 30, 710–715. [Google Scholar]
- Hondo, S.; Takahashi, M.; Osanai, T.; Matsuda, M.; Hasunuma, T.; Tazuke, A.; Nakahira, Y.; Chohnan, S.; Hasegawa, M.; Asayama, M. Genetic engineering and metabolite profiling for overproduction of polyhydroxybutyrate in cyanobacteria. J. Biosci. Bioeng. 2015, 120, 510–517. [Google Scholar]
- Osanai, T.; Numata, K.; Oikawa, A.; Kuwahara, A.; Iijima, H.; Doi, Y.; Tanaka, K.; Saito, K.; Hirai, M.Y. Increased bioplastic production with an RNA polymerase sigma factor SigE during nitrogen starvation in Synechocystis sp. PCC 6803. DNA Res. 2013, 20, 525–535. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar]
- Koller, M.; Braunegg, G. Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2015, 2, 94–121. [Google Scholar]
- Braunegg, G.; Lefebvre, G.; Renner, G.; Zeiser, A.; Haage, G.; Loidl-Lanthaler, K. Kinetics as a tool for polyhydroxyalkanoate production optimization. Can. J. Microbiol 1995, 41, 239–248. [Google Scholar]
- Koyama, N.; Doi, Y. Continuous production of poly (3-hydroxybutyrate-co-3-hyhroxyvalerate) by Alcaligenes eutrophus. Biotechnol. Lett. 1995, 17, 281–284. [Google Scholar]
- Luengo, J.M.; García, B.; Sandoval, A.; Naharro, G.; Olivera, E.R. Bioplastics from microorganisms. Curr. Opin. Microbiol. 2003, 6, 251–260. [Google Scholar] [PubMed]
- Novak, M. Mathematical modelling as a tool for optimized PHA production. Chem. Biochem. Eng. Q. 2015, 29, 183–220. [Google Scholar]
- Hartmann, R.; Hany, R.; Pletscher, E.; Ritter, A.; Witholt, B.; Zinn, M. Tailor-made olefinic medium-chain-length poly[(R)-3-hydroxyalkanoates] by Pseudomonas putida GPo1: Batch versus chemostat production. Biotechnol. Bioeng. 2006, 93, 737–746. [Google Scholar] [PubMed]
- Koller, M.; Salerno, A.; Dias, M.; Reiterer, A.; Braunegg, G. Modern biotechnological polymer synthesis: A review. Food Technol. Biotechnol. 2010, 48, 255–269. [Google Scholar]
- Islam Mozumder, M.S.; Garcia-Gonzalez, L.; De Wever, H.; Volcke, E.I.P. Poly (3-hydroxybutyrate) (PHB) production from CO2: Model development and process optimization. Biochem. Eng. J. 2015, 98, 107–116. [Google Scholar]
- Faccin, D.J.L.; Corrêa, M.P.; Rech, R.; Ayub, M.A.Z.; Secchi, A.R.; Cardozo, N.S.M. Modeling P(3HB) production by Bacillus megaterium. J. Chem. Technol. Biotechnol. 2012, 87, 325–333. [Google Scholar]
- Khanna, S.; Srivastava, A.K. A Simple structured mathematical model for biopolymer (PHB) production. Biotechnol.Prog. 2005, 21, 830–838. [Google Scholar]
- Aramvash, A.; Gholami-Banadkuki, N.; Moazzeni-Zavareh, F.; Hajizadeh-Turchi, S. An environmentally friendly and efficient method for extraction of PHB biopolymer with non-halogenated solvents. J. Microbiol. Biotechnol. 2015, 25, 1936–1943. [Google Scholar]
- López-Abelairas, M.; García-Torreiro, M.; Lú-Chau, T.; Lema, J.M.; Steinbüchel, A. Comparison of several methods for the separation of poly(3-hydroxybutyrate) from Cupriavidus necator H16 cultures. Biochem. Eng. J. 2015, 93, 250–259. [Google Scholar]
- Jendrossek, D.; Pfeiffer, D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly (3-hydroxybutyrate). Environ. Microbiol. 2014, 16, 2357–2373. [Google Scholar]
- Gerngross, T.U.; Reilly, P.; Stubbe, J.; Sinskey, A.J.; Peoples, O.P. Immunocytochemical analysis of poly-hydroxybutyrate (PHB) synthase in alcaligenes eutrophus H16: Localization of the synthase enzyme at the surface of PHB granules. J. Bacteriol. 2001, 173, 5289–5293. [Google Scholar]
- Stubbe, J.; Tian, J.; He, A.; Sinskey, A.J.; Lawrence, A.G.; Liu, P. Nontemplate-dependent polymerization processes: Polyhydroxyalkanoate synthases as a paradigm. Annu. Rev. Biochem. 2005, 74, 433–480. [Google Scholar] [PubMed]
- Das, S.; Lengweiler, U.D.; Seebach, D.; Reusch, R.N. Proof for a nonproteinaceous calcium-selective channel in Escherichia coli by total synthesis from (R)-3-hydroxybutanoic acid and inorganic polyphosphate. Proc. Natl. Acad. Sci. USA 1997, 94, 9075–9079. [Google Scholar] [PubMed]
- Asada, Y.; Miyake, M.; Miyake, J.; Kurane, R.; Tokiwa, Y. Photosynthetic accumulation of poly-(hydroxybutyrate) by cyanobacteria--the metabolism and potential for CO2 recycling. Int. J. Biol. Macromol. 1999, 25, 37–42. [Google Scholar]
- Zhang, D.; Dechatiwongse, P.; Hellgardt, K. Modelling light transmission, cyanobacterial growth kinetics and fluid dynamics in a laboratory scale multiphase photo-bioreactor for biological hydrogen production. Algal Res. 2015, 8, 99–107. [Google Scholar]
- Kunasundari, B.; Sudesh, K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. [Google Scholar]
- Jacquel, N.; Lo, C.; Wei, Y.; Wu, H.; Wang, S.S. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar]
- Ramsay, J.A.; Berger, E.; Voyer, R.; Chavarie, C.; Ramsay, B.A. Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnology 1994, 8, 589–594. [Google Scholar]
- Kobayashi, D.; Fujita, K.; Nakamura, N.; Ohno, H. A simple recovery process for biodegradable plastics accumulated in cyanobacteria treated with ionic liquids. Appl. Microbiol. Biotechnol. 2014, 99, 1647–1653. [Google Scholar]
- Jung, I.L.; Phyo, K.H.; Kim, K.C.; Park, H.K.; Kim, I.G. Spontaneous liberation of intracellular polyhydroxybutyrate granules in escherichia coli. Res. Microbiol. 2005, 156, 865–873. [Google Scholar]
- Rosengart, A.; Cesário, M.T.; de Almeida, M.C.M.D.; Raposo, R.S.; Espert, A.; de Apodaca, E.D.; da Fonseca, M.M.R. Efficient P(3HB) extraction from Burkholderia sacchari cells using non-chlorinated solvents. Biochem. Eng. J. 2015, 103, 39–46. [Google Scholar]
- Wang, G.; Gislum, M.; Filippov, G.; Montgomery, S. An environment-friendly and efficient method for extraction of PHB biopolymer by non-halogenated solvents. J. Microbiol. Biotechnol. 2015, 31, 785–794. [Google Scholar]
- Martino, L.; Cruz, M.V.; Scoma, A.; Freitas, F.; Bertin, L.; Scandola, M.; Reis, M.A.M. Recovery of amorphous polyhydroxybutyrate granules from Cupriavidus necator cells grown on used cooking oil. Int. J. Biol. Macromol. 2014, 71, 117–123. [Google Scholar] [PubMed]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Keshavarz, T.; Bucke, C.; Roy, I. Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. J. Biotechnol. 2007, 132, 251–258. [Google Scholar] [PubMed]
- Fiorese, M.L.; Freitas, F.; Pais, J.; Ramos, A.M.; De Aragão, G.M.F.; Reis, M.A.M. Recovery of polyhydroxybutyrate (PHB) from Cupriavidus necator biomass by solvent extraction with 1,2-propylene carbonate. Eng. Life Sci. 2009, 9, 454–461. [Google Scholar]
- Wampfler, B.; Ramsauer, T.; Rezzonico, S.; Hischier, R.; Köhling, R.; Thöny-Meyer, L.; Zinn, M. Isolation and purification of medium chain length poly (3-hydroxyalkanoates) (mcl-PHA) for medical applications using nonchlorinated solvents. Biomacromolecules 2010, 11, 2716–2723. [Google Scholar] [PubMed]
- Fei, T.; Cazeneuve, S.; Wen, Z.; Wu, L.; Wang, T. Effective recovery of poly-b-hydroxybutyrate (PHB) biopolymer from Cupriavidus necator using a novel and environmentally friendly solvent system. Am. Inst. Chem. Eng. 2016, 32, 678–685. [Google Scholar]
- Elbahloul, Y.; Steinbüchel, A. Large-scale production of poly (3-hydroxyoctanoic acid) by Pseudomonas putida GPo1 and a simplified downstream process. Appl. Environ. Microbiol. 2009, 75, 643–651. [Google Scholar]
- Choi, J.; Lee, S.Y. Efficient and economical recovery of poly (3-Hydroxybutyrate) from recombinant escherichia coli by simple. Biotechnol. Bioeng. 1999, 62, 546–553. [Google Scholar]
- Lee, K.M.; Chang, H.N.; Chang, Y.K.; Kim, B.S.; Hahn, S.K. The lysis of gram-negative Alcaligenes eutrophus and Alcaligenes latus by palmitoyl carnitine. Biotechnol. Tech. 1993, 7, 295–300. [Google Scholar]
- Dong, Z.; Sun, X.; Zhaolin, D.; Xuenan, S.U.N. A new method of recovering polyhydroxyalkanoate from Azotobacter chroococcum. Chin. Sci. Bull. 2000, 45, 252–256. [Google Scholar]
- Ramsay, J.A.; Berger, E.; Ramsay, B.A.; Chavarie, C. Recovery of poly-3-hydroxyalkanoic acid granules by a surfactant-hypochlorite treatment. Biotechnol. Tech. 1990, 4, 221–226. [Google Scholar]
- Hahn, S.K.; Chang, Y.K.; Lee, S.Y. Recovery and characterization of poly (3-hydroxybutyric acid) synthesized in Alcaligenes eutrophus and recombinant Escherichia coli. Recovery and characterization of poly (3-Hydroxybutyric Acid) synthesized in Alcaligenes eutrophus and recombinant Es. Appl. Environ. Microbiol. 1995, 61, 34–39. [Google Scholar] [PubMed]
- Marjadi, D.; Dharaiya, N. Recovery and characterization of poly (3-Hydroxybutyric acid) synthesized in Staphylococcus epidermidis. Afr. J. Environ. Sci. Technol. 2015, 8, 319–329. [Google Scholar]
- Lakshman, K.; Shamala, T.R. Extraction of polyhydroxyalkanoate from Sinorhizobium meliloti cells using Microbispora sp. culture and its enzymes. Enzym. Microb. Technol. 2006, 39, 1471–1475. [Google Scholar]
- Chen, Y.; Yang, H.; Zhou, Q.; Chen, J.; Gu, G. Cleaner recovery of poly (3-hydroxybutyric acid) synthesized in Alcaligenes eutrophus. Process. Biochem. 2001, 36, 501–506. [Google Scholar]
- Yu, J.; Chen, L.X.L. Cost-effective recovery and purification of polyhydroxyalkanoates by selective dissolution of cell mass. Biotechnol. Prog. 2006, 22, 547–553. [Google Scholar]
- Kathiraser, Y.; Aroua, M.K.; Ramachandran, K.B.; Kit, I.; Tan, P. Chemical characterization of polyhydroxyalkanoates (PHAs) recovered by enzymatic treatment and ultrafiltration. J. Chem. Technol. Biotechnol. 2007, 82, 847–855. [Google Scholar]
- Tamer, I.M.; Moo-Young, M.; Christi, Y. Disruption of alcaligenes latus for recovery of poly (-hydroxybutyric acid): Comparison of high-pressure homogenization, bead milling, and chemically induced lysis. Ind. Eng. Chem. Res. 1998, 37, 1807–1814. [Google Scholar]
- Ghatnekar, M.S.; Pai, J.S.; Ganesh, M. Production and recovery of poly-3-hydroxy-butyrate from Methylobacterium sp. V49. J. Chem. Technol. Biotechnol. 2002, 77, 444–448. [Google Scholar]




| Types of Biodegradable Plastics | Examples |
|---|---|
| Bioplastics that are biobased (biomass products). | This group includes polysaccharides, such as starches or animal protein (e.g., casein, whey and gelatin). |
| Bioplastics that are biobased (from microorganisms). | This group includes biodegradable biopolymer extracted from microorganisms (e.g., polyhydroxyalkanoates (PHA), polyhydroxybutyrate (PHB), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)). |
| Bioplastics that are biobased (from biotechnology). | This group includes biodegradable biopolymer produced by a conventional synthesis of bio derived monomers (e.g., polylactic acid (PLA)). |
| Bioplastics that are fossil-based. | This group includes bioplastics from petrolchemical products obtained from conventional synthesis of synthetic monomers (e.g., polycaprolactone (PCL), poly(butylene adipate-co-terephthalate) (PBAT)). |
| Name of the Company | City/Country | Production (kt Year−1) | Raw Material | Brand Name |
|---|---|---|---|---|
| Biomer | Schwalbach am Taunus/Germany | - | PHB | Biomer |
| Biomatera | Toronto/Canada | - | PHA | Biomatera |
| Bio-On | Bologna/Italy | 10 | PHA | Minerv |
| Kaneka | Osaka/Japan | 10 | PHB | |
| Tianjin Green-Bio | Taijin/China | 10 | PHA | Green Bio |
| Imperial Chemical Industries (ICI) | London/UK | 0.3 | PHB | - |
| Danimer Scientific | Georgia/USA | 0.3 | PHA | - |
| PHB Industrial | Serrana/Brazil | 0.1 | PHB | Biocycle |
| TEPHA | Massachusetts/USA | - | PHA | Tephaflex/TephElas |
| Tinan | Zhejiang/China | 10 | PHB | Enmat |
| SIRIM | Selangor/Malaysia | 2 | PHA | - |
| Shenzhen Ecomann | Shandong/China | 5 | PHA | AmBio |
| Bacterial Strain | Carbon Source | Polymer (s) | PHB % | References |
|---|---|---|---|---|
| Aeromonas hydrophila 4AK4 mutant | Lauric acid, oleic acid | MCL-PHA 1 | 64 | [15] |
| Alcaligenes latus | Malt, soy waste, milk waste | PHB 2 | 70 | [16] |
| Azotobacter chroococcum H23 | Waste water from olive oil mills | PHB 2 | 80 | [17] |
| Bacillus cereus UW85 | Glucose | PHB 2 | 9 | [18] |
| Bacillus megaterium ATCC 6748 | Corn steep liquor and molasses | PHB 2 | 43 | [19] |
| Bacillus spp. 87I | Glucose | PHB 2 | 67 | [20] |
| Burkholderia sacchari sp. IPT101 | Glucose | PHB 2 PHBV 3 | 68 | [21] |
| Burkholderia cepacia IPT 048 | bagasse | PHB 2 | 62 | [22] |
| Caulobacter crescentus DSM 4727 | Glucose | PHB 2 | 18 | [23] |
| Cupriavidus necator DSM 545 | Corn syrup | PHB 2 | 30 | [24] |
| Cupriavidus necator DSM 545 | waste glycerol | PHB 2 | 62 | [25] |
| Enterobacter aerogenes 12Bi | wastewater | PHB 2 | 43 | [26] |
| Escherichia coli mutants | Xylose | PHB 2 | 27 | [27] |
| Halomonas boliviensis LC1 | Starch, hydolysate, maltose, maltotetraose, and maltohexaose | PHB 2 | 56 | [28] |
| Legionella pneumophila 74/81 | Nutrient broth | PHB 2 | 16 | [29] |
| Methylocystis sp. GB 25 DSM 7674 | Methane | PHB 2 | 51 | [30] |
| Microlunatus phosphovorus DSM 10555 | Glucose, acetate | PHB 2 | 30 | [31] |
| Mixed Microbial Culture | Lactate | PHB 2 | 90 | [11] |
| Pandoraea sp. | Crude glycerol | PHB 2 | 63 | [32] |
| Pseudomonas aeruginosa NCIB 40045 | Agro-industrial oily wastes | MCL-PHA 1 | 66 | [33] |
| Pseudomonas hydrogenovora DSM 1749 | Dairy whey | MCL-PHA 1 | 21 | [34] |
| Pseudomonas putida CA-3 | Petrochemical plastic waste | PHB 2 | 30 | [35] |
| Pseudomonas fluorescens A2a5 | Sugar cane liquor | PHB 2 | 70 | [36] |
| Rhodopseudomonas palustris SP5212 | Acetate | PHB 2 | 34 | [37] |
| Ralstonia pickettii 61A6 | Sugar cane liquor | PHB 2 | 10 | [38] |
| Cyanobacteria Strain | Culture Conditions | Polymer | Polymer Content (%) | References |
|---|---|---|---|---|
| Arthrospira (Spirulina) platensis | Photoautotrophic | PHB | 6 | [63] |
| Anabaena cylindrica 10C | Propionate | P(3HB-co-3HV) | 2 | [64] |
| Aulosira fertilissima CCC 444 | Citrate + acetate and K2HPO4 | PHB | 85 | [65] |
| Aulosira fertilissima CCC 444 | Fructose + valerate | P(3HB-co-3HV) | 77 | [26] |
| Gloeothece sp. PCC 6909 | Acetate | PHB | 9 | [66] |
| Nostoc muscorum Agardh | Nitrogen deficiency + acetate + glucose + valerate | P(3HB-co-3HV) | 71 | [25] |
| Nostoc muscorum CCAP 1453/9 | CO2 | PHB | 22 | [31] |
| Scytonema geitleri Bharawaja | Acetate 30 mM | PHB | 7 | [67] |
| Synechococcus sp. PCC7942 | Acetate + nitrogen deficiency | PHB | 26 | [27] |
| Synechocystis sp. PCC 6803 | CO2; Nitrate concentration is the half of the optimal concentration | PHB | 8 | [30] |
| Synechocystis sp. PCC 6803 | CO2; Nitrate deficiency | PHB | 4 | [23] |
| Synechocystis sp. PCC 6803 | Phosphate-deficiency + gas exchange limitation + acetate | PHB | 38 | [35] |
| Spirulina sp. LEB 18 | sodium bicarbonate + nitrogen and phosphorus deficiency | PHB | 31 | [28] |
| Spirulina platensis UMACC 161 | Acetate and CO2 | PHB | 10 | [68] |
| Cyanobacterial Strain | Engineered Genes | Carbon Source | Culture Conditions | Reactor | Polymer Fraction (% dcw) | References |
|---|---|---|---|---|---|---|
| Synechocystis sp. PCC 6803 mutant strain | The agp gene was inserted | CO2 0.035%, Acetate 5 mM | Nitrogen deprived | flask | 18.6 | [43] |
| Synechococcus sp. PCC7942 mutant strain | A PHB synthesizing enzyme from A. eutrophus was expressed from a plasmid | CO2 5%, Acetate 10 mM | Nitrogen deprived | flask | 25 | [27] |
| Synechocystis sp. PCC 6803 mutant strain | PHA synthase from C. necator was expressed from a plasmid | CO2 1%, Acetate 10 mM | Nitrogen deprived | flask | 11 | [42] |
| Synechocystis sp. PCC 6803 mutant strain | The native sigE was expressed from the chromosome | CO2 1% | Nitrogen deprived | flask | 1.4 | [45] |
| Synechocystis sp. PCC 6803 mutant strain | A PHA biosynthetic operon from M. aeruginosa was expressed from a plasmid | CO2 2% | Nitrogen deprived | flask | 7 | [44] |
| Synechocystis sp. PCC 6803 mutant strain | The native phaAB was overexpressed from the chromosome | CO2 0.035%, Acetate 4 mM | Nitrogen deprived | flask | 35 | [41] |
| Synechocystis sp. PCC 6803 mutant strain | xfpk from B. breve was expressed in a double pta and ach knock out. | CO2 2% | BG11 | PBR | 12 | [46] |
| Microorganism | Growth Rate | Production Rate | References | |||||
|---|---|---|---|---|---|---|---|---|
| Bacillus megaterium | - | - | [56] | |||||
| Cupriavidus necator | where | - | [55] | |||||
| Ralstonia eutropha | - | - | [57] | |||||
| Synechocystis PCC6803 | - | where | where | [30] |
| Extraction Method | Materials | Strain | Results | References |
|---|---|---|---|---|
| Solvent extraction | Chloroform | Bacillus cereus SPV | Purity: 92%; Yield: 31% | [107] |
| Chloroform | Cupriavidus necator DSM 545 | Purity: 95%; Yield: 96% | [108] | |
| 1,2-Propylene carbonate | Cupriavidus necator DSM 545 | Purity: 84%; Yield: 95% | [108] | |
| Methyl tert-butyl ether | Pseudomonas putida KT2440 | Yield: 80–85% | [109] | |
| Butyl acetate | C. necator | Purity: 99; Yield: 96 | [91] | |
| Non halogenated acetone/ethanol/propylene carbonate | C. necator | Purity: 93%; Yield: 92% | [110] | |
| Acetone | P. putida GPo1 | Yield: 94% | [111] | |
| Digestion Method | ||||
| Surfactant | SDS | Recombinant Escherichia coli | Purity: 99%; Yield: 89% | [112] |
| Palmitoyl carnitine | C. necator, Alcaligenes latus | Degree of lysis: 56–78% | [113] | |
| Surfactant-sodium hypochlorite | SDS-Sodium hypochlorite | Azotobacter chroococcum G-3 | Purity: 98%; Yield: 87% | [114] |
| Triton X-100-sodium hypochlorite | C. necator DSM 545 | Purity: 98% | [115] | |
| Sodium hypochlorite | Sodium hypochlorite | C. necator Recombinant Escherichia coli | Purity: 86%; Purity: 93% | [116] |
| Sodium hypochlorite | Staphylococcus epidermidis | [117] | ||
| Surfactant Chelate | Triton X-100-EDTA | Sinorhizobium meliloti | Purity: 68% | [118] |
| Betaine-EDTA disodium salt | C. necator DSM 545 | Purity: >96%; Yield: 90% | [119] | |
| Dispersion of sodium hypochlorite and chloroform | Chloroform-sodium hypochlorite | B. cereus SPV | Purity: 95%; Yield: 30% | [107] |
| Chloroform-sodium hypochlorite | C. necator Recombinant Escherichia coli | Purity: >98% | [116] | |
| Selective dissolution by protons | Sulfuric acid | C. necator | Purity: >97%; Yield: >95% | [120] |
| Enzymatic digestion | Enzyme combined with SDS-EDTA | P. putida | Purity: 93% | [121] |
| Alcalase combined with SDS and (EDTA), | Cupriavidus necator DSM 428 | Purity: >90%; Yield: >90% | [71] | |
| Mechanical disruption | Bead mill | A. latus | [122] | |
| High pressure homogenization | A. latus | [122] | ||
| SDS-High pressure homogenization | Metylobacterium sp V49 | Purity: 95%; Yield: 98% | [123] | |
| Spontaneous liberation | E. coli | Autolysis of 80% | [68] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpine, R.; Olivieri, G.; Hellingwerf, K.J.; Pollio, A.; Marzocchella, A. Industrial Production of Poly-β-hydroxybutyrate from CO2: Can Cyanobacteria Meet this Challenge? Processes 2020, 8, 323. https://doi.org/10.3390/pr8030323
Carpine R, Olivieri G, Hellingwerf KJ, Pollio A, Marzocchella A. Industrial Production of Poly-β-hydroxybutyrate from CO2: Can Cyanobacteria Meet this Challenge? Processes. 2020; 8(3):323. https://doi.org/10.3390/pr8030323
Chicago/Turabian StyleCarpine, Roberta, Giuseppe Olivieri, Klaas J. Hellingwerf, Antonino Pollio, and Antonio Marzocchella. 2020. "Industrial Production of Poly-β-hydroxybutyrate from CO2: Can Cyanobacteria Meet this Challenge?" Processes 8, no. 3: 323. https://doi.org/10.3390/pr8030323
APA StyleCarpine, R., Olivieri, G., Hellingwerf, K. J., Pollio, A., & Marzocchella, A. (2020). Industrial Production of Poly-β-hydroxybutyrate from CO2: Can Cyanobacteria Meet this Challenge? Processes, 8(3), 323. https://doi.org/10.3390/pr8030323







