Outdoor Large-Scale Cultivation of the Acidophilic Microalga Coccomyxa onubensis in a Vertical Close Photobioreactor for Lutein Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga and Culture Medium
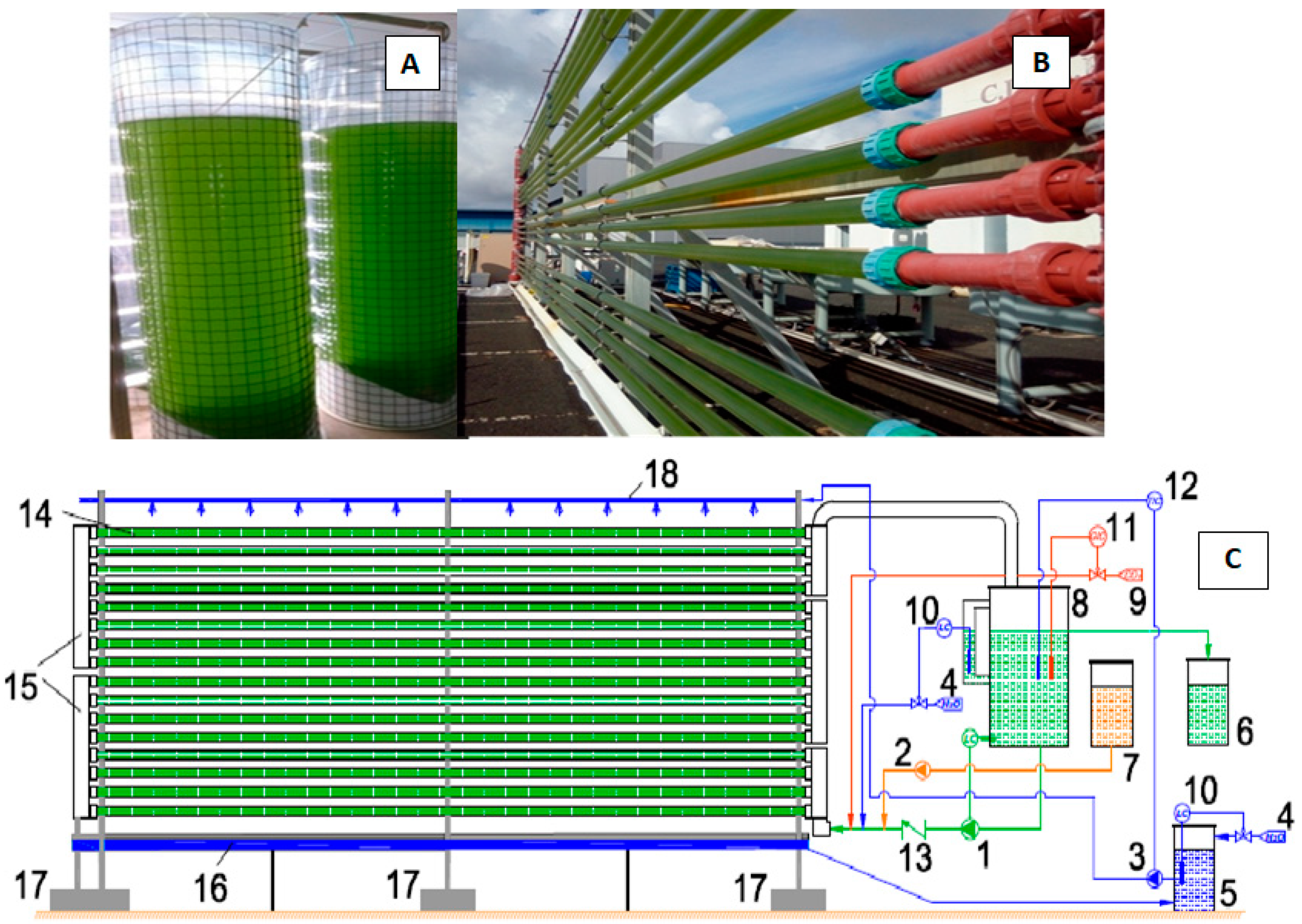
2.2. Cultivation of C. onubensis in Indoor Air Fluidized-Bed Plastic Bags
2.3. Cultivation of C. onubensis in an Outdoor Vertical Tubular Photobioreactor
2.4. Harvesting of Microalgal Biomass
2.5. Growth Measurements and Productivity
2.6. Maximum Photosystem II Quantum Yield (Fv/Fm)
2.7. Pigment Analysis
2.8. Statistics
3. Results and Discussion
3.1. Batch Cultivation of C. onubensis in Plastic Bags Indoors
3.2. Cultivation of C. onubensis in a Tubular Photobioreactor Outdoors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forján, E.; Navarro, F.; Cuaresma, M.; Vaquero, I.; Ruíz-Domínguez, M.C.; Gojkovic, Z.; Vázquez, M.; Márquez, M.; Mogedas, B.; Bermejo, E.; et al. Microalgae: Fast-growth sustainable green factories. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1705–1755. [Google Scholar] [CrossRef]
- Holdmann, C.; Schmid-Staiger, U.; Hirth, T. Outdoor microalgae cultivation at different biomass concentrations—Assessment of different daily and seasonal light scenarios by modeling. Algal Res. 2019, 38, 101405. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Norsker, N.H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production: A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Acién-Fernández, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Photobioreactors for the production of microalgae. Rev. Environ. Sci. Biotechnol. 2013, 12, 131–151. [Google Scholar] [CrossRef]
- Molina, E.; Fernández, J.; Acién, F.G.; Chisti, Y. Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- Madhubalaji, C.K.; Ajam, S.; Sijil, P.V.; Mudliar, S.; Chauhan, V.S.; Sarada, R.; Rao, A.R.; Ravishankar, G.A. Open Cultivation Systems and Closed Photobioreactors for Microalgal Cultivation and Biomass Production. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 178–202. [Google Scholar]
- Ben-Amotz, A. Industrial Production of Microalgal Cell-Mass and Secondary Products-Major Industrial Species: Dunaliella. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 273–280. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q. Chlorella: Industrial Production of Cell Mass and Chemicals. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; John Wiley & Sons: Oxford, UK, 2013; pp. 329–338. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién-Fernández, F.G.; Molina-Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- McClure, D.D.; Nightingale, J.K.; Sachin Black, A.L.; Zhu, J.; Kavanagh, J.M. Pilot-scale production of lutein using Chlorella vulgaris. Algal Res. 2019, 44. [Google Scholar] [CrossRef]
- Vaquero, I.; Ruiz-Domínguez, M.C.; Mogedas, B.; Vilchez, C.; Vega, J.M. Production of lutein-enriched biomass by growing Coccomyxa sp. (strain onubensis; Chlorophyta) under spring outdoor conditions of southwest Spain. J. Adv. Biotechnol. 2016, 6, 828–834. [Google Scholar] [CrossRef]
- Navarro, F.; Forján, E.; Vázquez, M.; Toimil, A.; Montero, Z.; Ruiz-Domínguez, M.C.; Garbayo, I.; Castaño, M.A.; Vílchez, C.; Vega, J.M. Antimicrobial activity of the acidophilic eukaryotic microalga Coccomyxa sp. (strain onubensis). Phycol. Res. 2017, 65, 38–43. [Google Scholar] [CrossRef]
- Navarro, F.J.; Forján, E.; Vázquez, M.; Montero, Z.; Bermejo, E.; Castaño, M.A.; Toimil, A.; Chagüaceda, E.; García-Sevillano, M.A.; Sánchez, M.; et al. Microalgae as a safe food source for animals: Nutritional characteristics of the acidophilic microalga Coccomyxa onubensis. Food Nutr. Res. 2016, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, J.L.; Huss, V.; Montero, Z.; Torronteras, R.; Cuaresma, M.; Garbayo, I.; Vílchez, C. Phylogenetic characterization and morphological and physiological aspects of a novel acidophilic and halotolerant microalga Coccomyxa onubensis sp. nov. (Chlorophyta, Trebouxiophyceae). J. Appl. Phycol. 2016, 28, 3269–3279. [Google Scholar] [CrossRef]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Casal, C.; Cuaresma, M.; Vega, J.M.; Vílchez, C. Enhanced productivity of a lutein-enriched novel acidophile microalga grown on urea. Mar. Drugs 2011, 9, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquero, I.; Vázquez, M.; Ruiz-Domínguez, M.C.; Vílchez, C. Enhanced production of a lutein-rich acidic environment microalga. J. Appl. Microbiol. 2014, 116, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Cuaresma, M.; Janssen, M.; Vílchez, C.; Wijffels, R. Horizontal or vertical photobioreactors? How to improve microalgae photosynthetic efficiency. Bioresour. Technol. 2011, 102, 5129–5137. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Vaquero, I.; Obregón, V.; de la Morena, B.; Vílchez, C.; Vega, J.M. Lipid accumulation and antioxidant activity in the eukaryotic acidophilic microalga Coccomyxa sp. (strain onubensis) under nutrient starvation. J. Appl. Phycol. 2015, 27, 1099–1108. [Google Scholar] [CrossRef]
- Spinola, M.V.; Díaz-Santos, E. Microalgae Nutraceuticals: The Role of Lutein in Human Health. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 243–263. [Google Scholar]
- Hirooka, S.; Miyagishima, S.Y. Cultivation of acidophilic algae Galdieria sulfuraria and Pseudochlorella sp. YKT1 in media derived from acidic hot springs. Front. Microbiol. 2016, 7, 2022. [Google Scholar] [CrossRef] [Green Version]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.; Garcia-Guerrero, M. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef]
- Blanco, A.M.; Moreno, J.; Del Campo, J.A.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007, 73, 1259–1266. [Google Scholar] [CrossRef]
- De Vree, J.H.; Bosma, R.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Comparison of four outdoor pilot-scale photobioreactors. Biotechnol. Biofuels 2015, 8, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquero, I.; Ruiz-Domínguez, M.C.; Márquez, M.; Vílchez, C. Cu-mediated biomass productivity enhancement and lutein enrichment of the novel microalga Coccomyxa onubensis. Process. Biochem. 2012, 47, 694–700. [Google Scholar] [CrossRef]
- Gross, W. Ecophysiology of algae living in highly acidic environments. Hydrobiologia 2000, 433, 31–37. [Google Scholar] [CrossRef]
- De Farias-Neves, F.; Hoinaski, L.; Rubi-Rörig, L.; Bianchini-Derner, R.; de Melo-Lisboa, H. Carbon biofixation and lipid composition of an acidophilic microalga cultivated on treated wastewater supplied with different CO2 levels. Environ. Technol. 2019, 3308–3317. [Google Scholar] [CrossRef]
- Molina-Grima, E.; Acién-Fernández, F.G.; García-Camacho, F.; Chisti, Y. Photobioreactors: Light regime, mass transfer, and scale-up. J. Biotechnol. 2009, 70, 231–248. [Google Scholar] [CrossRef]
- Zijffers, J.W.F.; Schippers, K.J.; Zheng, K.; Janssen, M.; Tramper, J.; Wijffels, R.H. Maximum Photosynthetic Yield of Green Microalgae in Photobioreactors. Mar. Biotechnol. 2010, 12, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Gerloff-Elias, A.; Spijkerman, E.; Pröschold, T. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acidic lake (pH 2.6). Plant Cell Environ. 2005, 28, 1218–1229. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Investigation of Chlorella vulgaris UTEX 265 cultivation under light and low temperature stressed conditions for lutein production in flasks and the coiled tree photo-bioreactor (CTPBR). Appl. Biochem. Biotechnol. 2017, 183, 652–671. [Google Scholar] [CrossRef]

| Microalga | Growth System | pH | Productivity (g L−1 d−1) | Lutein (mg g−1) | Reference |
|---|---|---|---|---|---|
| C. onubensis | Indoor plastic bag 400 L | 2.5 | 0.09 | 9.7 | Present work |
| C. onubensis | Outdoor PBL 800 L | 2.5 | 0.14 | 10.0 | Present work |
| C. onubensis | Laboratory conditions 1 L | 2.5 | 1.80 | 6.0 | [18] |
| C. onubensis | Outdoor PBR 6 L | 2.5 | 0.40 | 4.8 | [12] |
| Chlamydomonas acidophila | Laboratory conditions. High light | 2.5 | 0.75 | - | [21] |
| Chlamydomonas acidophila | Laboratory conditions. Low light | 2.5 | 0.08 | - | [21] |
| Galdieria sulphuraria | Laboratory conditions | 2.0 | 0.27 | - | [22] |
| Muriellopsis sp. | Laboratory conditions 1 L | 7.5 | 1.60 | 6.0 | [23] |
| Muriellopsis sp. | Outdoor tubular | 7.5 | 13.0 † | 5.0 | [24] |
| Nannocloropsis sp. CCAP 211/78 | Outdoor PBR 6 L | 7.5 | 0.71 | - | [25] |
| Scenedesmus almeriensis | Laboratory conditions. High light | 7.0 | 0.95 | 5.3 | [21] |
| Scenedesmus almeriensis | Outdoor tubular 4000 L | 7.0 | 0.29 | 4.5 | [10] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, J.-L.; Montero, Z.; Cuaresma, M.; Ruiz-Domínguez, M.-C.; Mogedas, B.; Nores, I.G.; González del Valle, M.; Vílchez, C. Outdoor Large-Scale Cultivation of the Acidophilic Microalga Coccomyxa onubensis in a Vertical Close Photobioreactor for Lutein Production. Processes 2020, 8, 324. https://doi.org/10.3390/pr8030324
Fuentes J-L, Montero Z, Cuaresma M, Ruiz-Domínguez M-C, Mogedas B, Nores IG, González del Valle M, Vílchez C. Outdoor Large-Scale Cultivation of the Acidophilic Microalga Coccomyxa onubensis in a Vertical Close Photobioreactor for Lutein Production. Processes. 2020; 8(3):324. https://doi.org/10.3390/pr8030324
Chicago/Turabian StyleFuentes, Juan-Luis, Zaida Montero, María Cuaresma, Mari-Carmen Ruiz-Domínguez, Benito Mogedas, Inés Garbayo Nores, Manuel González del Valle, and Carlos Vílchez. 2020. "Outdoor Large-Scale Cultivation of the Acidophilic Microalga Coccomyxa onubensis in a Vertical Close Photobioreactor for Lutein Production" Processes 8, no. 3: 324. https://doi.org/10.3390/pr8030324
APA StyleFuentes, J.-L., Montero, Z., Cuaresma, M., Ruiz-Domínguez, M.-C., Mogedas, B., Nores, I. G., González del Valle, M., & Vílchez, C. (2020). Outdoor Large-Scale Cultivation of the Acidophilic Microalga Coccomyxa onubensis in a Vertical Close Photobioreactor for Lutein Production. Processes, 8(3), 324. https://doi.org/10.3390/pr8030324






