Petroleum Hydrocarbon Removal from Wastewaters: A Review
Abstract
1. Introduction
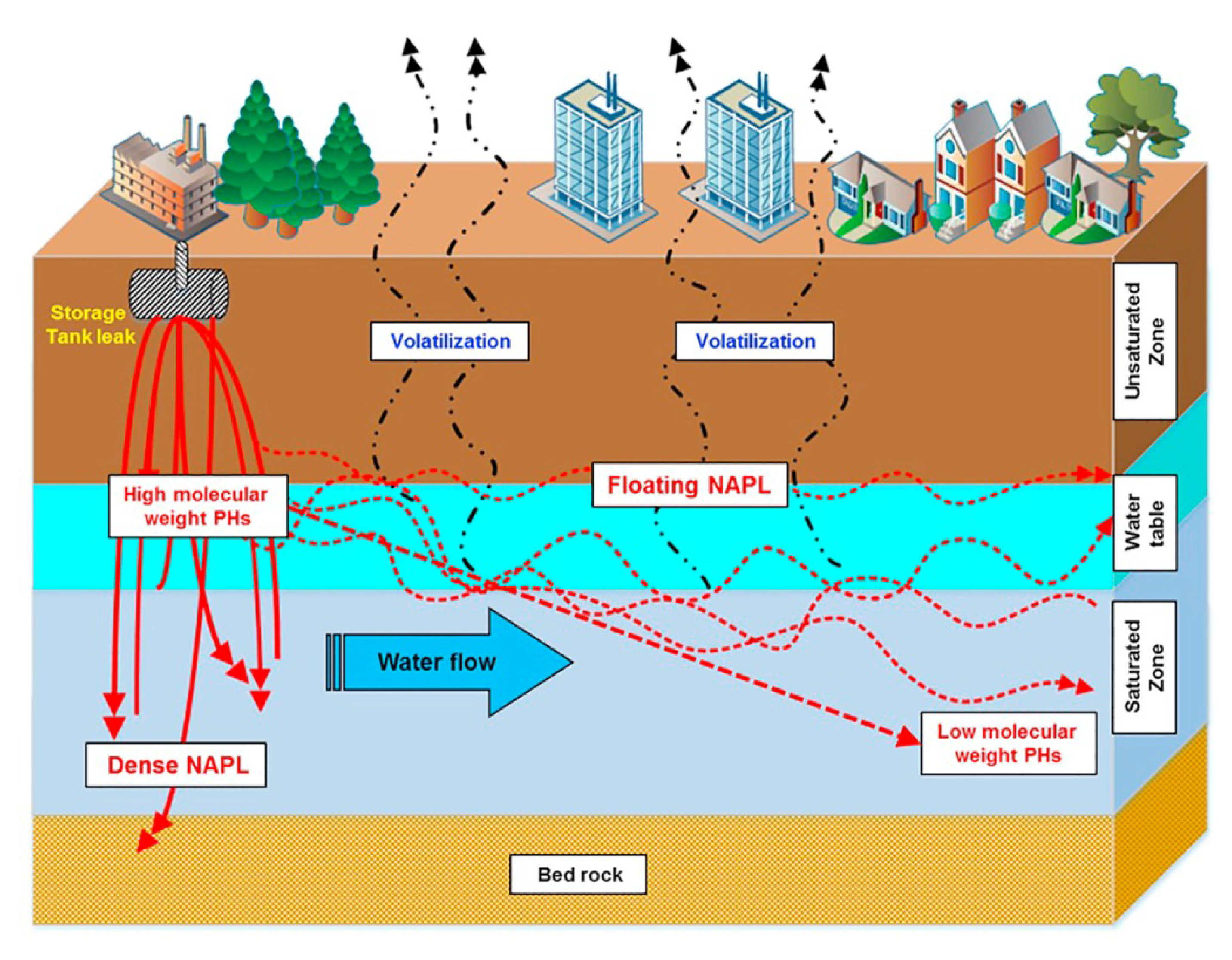
Environmental Problems/Scenarios/Fate
2. Removal and Purification Methods
2.1. Canvas
2.2. Chemicals
2.3. Microorganisms
2.4. Adsorption Method
2.5. Soil Vapor Extraction (SVE)
2.6. Plasma Treatment Method
2.7. Photocatalytic Oxidation
2.8. Nanostructure Materials
2.9. Advanced and Chemical Oxidation

2.10. Coagulation and Electrocoagulation
3. Effect of Factors on Efficiency
3.1. Effect of Solution pH
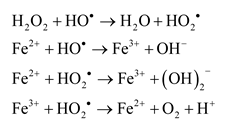
3.2. Effect of Temperature
3.3. Effect of Reaction Time
3.4. Effect of Ionic Strength
4. Conclusions
- Conventional refining techniques are not able to affect the effective elimination of oil compounds, and the high concentrations of these pollutants may affect the activity and efficiency of the treatment plant due to the high toxicity of these compounds that affects the activity of the active sludge pool and creates layers in the film which can cause blockage of the tubes.
- Purification and removal of oil pollutants are necessary, especially in industries; the output of sewage chemical purification (coagulation, DAF flocculation) can be transmitted to the biologic reactor for further purification.
- Electromethods are more advanced than conventional physical and chemical methods, such as electrocoagulation and flotation.
- Physical methods can separate large amounts of petroleum compounds, and, in some cases, these compounds can be recycled with a number of processes.
- Third-party refinement can provide water reuse targets with methods such as nanofiltration, reverse osmosis, and advanced oxidation.
- Adsorption is an emergency technology for petrochemical wastewater treatment that can be applied by using minerals and organic materials. By using low-cost adsorbent materials and combining the adsorption process with one of the advanced methods, sludge production may be lowered and can reduce the cost of the process.
Author Contributions
Funding
Conflicts of Interest
References
- Daraei, H.; Mittal, A.; Noorisepehr, M.; Daraei, F. Kinetic and equilibrium studies of adsorptive removal of phenol onto eggshell waste. Environ. Sci. Pollut. Res. 2013, 20, 4603–4611. [Google Scholar] [CrossRef] [PubMed]
- Durmusoglu, E.; Taspinar, F.; Karademir, A. Health risk assessment of BTEX emissions in the landfill environment. J. Hazard. Mater. 2010, 176, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Song, Y.; Shen, Z.; Zhou, Y.; Wang, K.; Jin, X.; Han, Z.; Liu, T. A comprehensive review on toxic petrochemical wastewater pretreatment and advanced treatment. J. Clean. Prod. 2020, 245, 118692. [Google Scholar] [CrossRef]
- Garoma, T.; Gurol, M.D.; Osibodu, O.; Thotakura, L. Treatment of groundwater contaminated with gasoline components by an ozone/UV process. Chemosphere 2008, 73, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Skodras, A.; Christopoulos, C.; Ebrahimi, T. The JPEG 2000 still image compression standard. IEEE Signal Process. Mag. 2001, 18, 36–58. [Google Scholar] [CrossRef]
- Singh, J.G.; Chang-Yen, I.; Stoute, V.A.; Chatergoon, L. Hydrocarbon levels in edible fish, crabs and mussels from the marine environment of Trinidad. Mar. Pollut. Bull. 1992, 24, 270–272. [Google Scholar] [CrossRef]
- Shim, H.; Shin, E.; Yang, S.-T. A continuous fibrous-bed bioreactor for BTEX biodegradation by a co-culture of Pseudomonas putida and Pseudomonas fluorescens. Adv. Environ. Res. 2002, 7, 203–216. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Sutherland, J.B. Relative roles of bacteria and fungi in polycyclic aromatic hydrocarbon biodegradation and bioremediation of contaminated soils. In Fungi in Biogeochemical Cycles; Gadd, G.M., Ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 182–211. [Google Scholar]
- Schneider, P.; Lorinci, G.; Gebefugi, I.L.; Heinrich, J.; Kettrup, A.; Wichmann, H.E. Vertical and horizontal variability of volatile organic compounds in homes in Eastern Germany. J. Expo. Sci. Environ. Epidemiol. 1999, 9, 282–292. [Google Scholar] [CrossRef][Green Version]
- Sairat, T.; Homwuttiwong, S.; Homwutthiwong, K.; Ongwandee, M. Investigation of gasoline distributions within petrol stations: Spatial and seasonal concentrations, sources, mitigation measures, and occupationally exposed symptoms. Environ. Sci. Pollut. Res. 2015, 22, 13870–13880. [Google Scholar] [CrossRef]
- El-Din, N.M.; Abdel-Moati, M.A.R. Accumulation of Trace Metals, Petroleum Hydrocarbons, and Polycyclic Aromatic Hydrocarbons in Marine Copepods from the Arabian Gulf. Bull. Environ. Contam. Toxicol. 2001, 66, 110–117. [Google Scholar] [CrossRef]
- Veellu, R. Petroleum hydrocarbon along the coastal areas of Port Dickson. Petranika 1989, 12, 349–355. [Google Scholar]
- Quinlan, M. The Implications of Labour Market Restructuring in Industrialized Societies for Occupational Health and Safety. Econ. Ind. Democr. 1999, 20, 427–460. [Google Scholar] [CrossRef]
- Shaughnessy, P.D. Population Assessment of New Zealand Fur Seals and Australian Sea Lions in Some South Australian Breeding Colonies and Haul-Out Sites 2003–2004. Rep. Dep. Environ. Herit. 2004, 13, 87–98. [Google Scholar]
- Gkorezis, P.; Daghio, M.; Franzetti, A.; Van Hamme, J.D.; Sillen, W.; Vangronsveld, J. The Interaction between Plants and Bacteria in the Remediation of Petroleum Hydrocarbons: An Environmental Perspective. Front. Microbiol. 2016, 7, 1836. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Du, Q.; Li, H.; Zhou, G.; Chen, S.; Jiao, Z.; Ren, J. Catalytic combustion of volatile aromatic compounds over CuO-CeO2 catalyst. Korean J. Chem. Eng. 2017, 34, 1944–1951. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- El Samra, M.I.; Emara, H.I.; Shunbo, F. Dissolved petroleum hydrocarbon in the northwestern Arabian Gulf. Mar. Pollut. Bull. 1986, 17, 65–68. [Google Scholar] [CrossRef]
- Payne, J.R.; McNabb, G.D., Jr.; Clayton, J.R., Jr. Oil-weathering behavior in Arctic environments. Polar Res. 1991, 10, 631–662. [Google Scholar] [CrossRef]
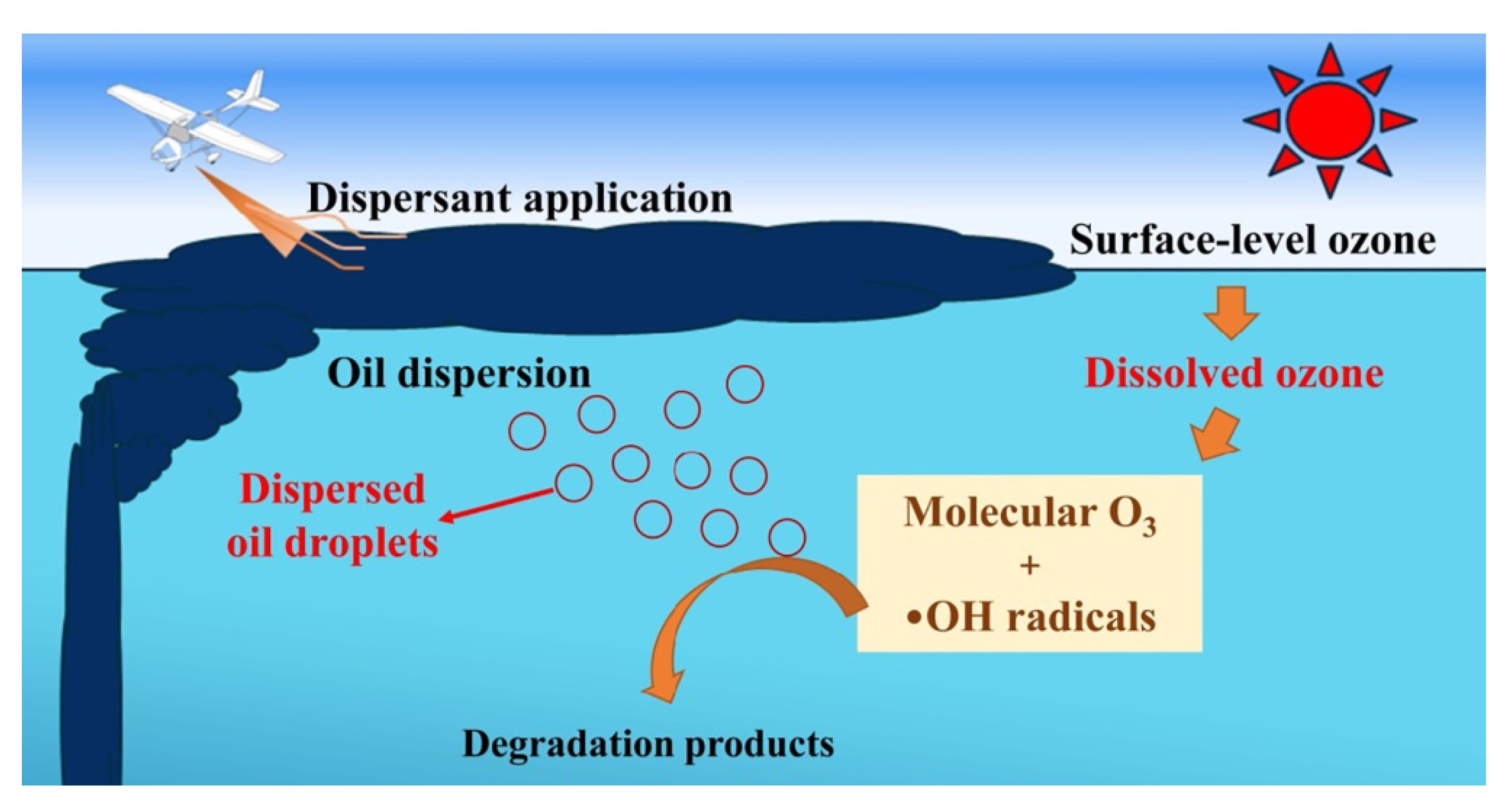
- Ji, H.; Gong, Y.; Duan, J.; Zhao, D.; Liu, W. Degradation of petroleum hydrocarbons in seawater by simulated surface-level atmospheric ozone: Reaction kinetics and effect of oil dispersant. Mar. Pollut. Bull. 2018, 135, 427–440. [Google Scholar] [CrossRef]
- Mango, F.D. The light hydrocarbons in petroleum: A critical review. Org. Geochem. 1997, 26, 417–440. [Google Scholar] [CrossRef]
- Najafi, A.R.; Rahimpour, M.R.; Jahanmiri, A.H.; Roostaazad, R.; Arabian, D.; Ghobadi, Z. Enhancing biosurfactant production from an indigenous strain of Bacillus mycoides by optimizing the growth conditions using a response surface methodology. Chem. Eng. J. 2010, 163, 188–194. [Google Scholar] [CrossRef]
- Jones, K.C.; de Voogt, P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Silliman, B.R.; van de Koppel, J.; McCoy, M.W.; Diller, J.; Kasozi, G.N.; Earl, K.; Adams, P.N.; Zimmerman, A.R. Degradation and resilience in Louisiana salt marshes after the BP—Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 11234. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Anm, F.; Ahmed, F.; Anm, F. A Review on Environmental Contamination of Petroleum Hydrocarbons and its Biodegradation. Int. J. Environ. Sci. Nat. Resour. 2018, 11, 63–69. [Google Scholar]
- Logeshwaran, P.; Megharaj, M.; Chadalavada, S.; Bowman, M.; Naidu, R. Petroleum hydrocarbons (PH) in groundwater aquifers: An overview of environmental fate, toxicity, microbial degradation and risk-based remediation approaches. Environ. Technol. Innov. 2018, 10, 175–193. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Peng, S.; Zhou, Q.; Ma, L.Q. Temporal and spatial trends of total petroleum hydrocarbons in the seawater of Bohai Bay, China from 1996 to 2005. Mar. Pollut. Bull. 2010, 60, 238–243. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. A review of indoor air treatment technologies. Rev. Environ. Sci. Bio/Technol. 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Sah, J.; Kao, C.-M. Application of iron electrode corrosion enhanced electrokinetic-Fenton oxidation to remediate diesel contaminated soils: A laboratory feasibility study. J. Hydrol. 2010, 380, 4–13. [Google Scholar] [CrossRef]
- Vilve, M.; Vilhunen, S.; Vepsäläinen, M.; Kurniawan, T.A.; Lehtonen, N.; Isomäki, H.; Sillanpää, M. Degradation of 1,2-dichloroethane from wash water of ion-exchange resin using Fenton’s oxidation. Environ. Sci. Pollut. Res. 2010, 17, 875–884. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Lu, B.; Jiang, A.; Wan, C. Study on the removal of indoor VOCs using biotechnology. J. Hazard. Mater. 2010, 182, 204–209. [Google Scholar] [CrossRef]
- Jo, W.-K.; Yang, C.-H. Granular-activated carbon adsorption followed by annular-type photocatalytic system for control of indoor aromatic compounds. Sep. Purif. Technol. 2009, 66, 438–442. [Google Scholar] [CrossRef]
- Campo, R.; Di Bella, G. Petrochemical slop wastewater treatment by means of aerobic granular sludge: Effect of granulation process on bio-adsorption and hydrocarbons removal. Chem. Eng. J. 2019, 378. [Google Scholar] [CrossRef]
- de Abreu Domingos, R.; da Fonseca, F.V. Evaluation of adsorbent and ion exchange resins for removal of organic matter from petroleum refinery wastewaters aiming to increase water reuse. J. Environ. Manag. 2018, 214, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Saremnia, B. Comparison study of adsorption and nanofiltration methods for removal of total petroleum hydrocarbons from oil-field wastewater. J. Pet. Sci. Eng. 2018, 171, 403–413. [Google Scholar] [CrossRef]
- Filatova, E.G.; Soboleva, V.G. Extraction of oil and petroleum products from water solutions by natural adsorbents. Izv. Vyss. Uchebnykh Zaved. Seriya Khimiya Khimicheskaya Tekhnologiya 2019, 62, 131–137. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sedighi, M.; Hemati, M. Removal of petroleum asphaltenes by improved activity of NiO nanoparticles supported on green AlPO-5 zeolite: Process optimization and adsorption isotherm. Petroleum 2019. [Google Scholar] [CrossRef]
- Nazifa, T.H.; Hadibarata, T.; Yuniarto, A.; Elshikh, M.S.; Syafiuddin, A. Equilibrium, Kinetic and Thermodynamic Analysis Petroleum Oil Adsorption from Aqueous Solution by Magnetic Activated Carbon. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Sadare, O.O.; Masitha, M.; Yoro, K.O.; Daramola, M.O. Removal of sulfur (e.g., DBT) from petroleum distillates using activated carbon in a continuous packed-bed adsorption column. In Proceedings of the World Congress on Engineering and Computer Science, WCECS 2018, San Francisco, CA, USA, 23–25 October 2018; pp. 509–513. [Google Scholar]
- Salehi, E.; Shafie, M. Adsorptive removal of acetaldehyde from water using strong anionic resins pretreated with bisulfite: An efficient method for spent process water recycling in petrochemical industry. J. Water Process Eng. 2020, 33. [Google Scholar] [CrossRef]
- Shahat, A.; Hassan, H.M.A.; El-Shahat, M.F.; El Shahawy, O.; Awual, M.R. Visual nickel(II) ions treatment in petroleum samples using a mesoporous composite adsorbent. Chem. Eng. J. 2018, 334, 957–967. [Google Scholar] [CrossRef]
- Yurmazova, T.A.; Shakhova, N.B.; Tuan, H.T.; Plankina, M.V. Adsorption of petroleum substances and inorganic ions from aqueous solutions using mineral sorbent. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2018, 329, 125–134. [Google Scholar]
- Alcántara-Garduño, M.E.; Okuda, T.; Nishijima, W.; Okada, M. Ozonation of trichloroethylene in acetic acid solution with soluble and solid humic acid. J. Hazard. Mater. 2008, 160, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, W.F.L.M.; Beckers, F.J.C.M.; Pemen, A.J.M.; van Heesch, E.J.M.; Kling, W.L. Oxidative degradation of toluene and limonene in air by pulsed corona technology. J. Phys. D Appl. Phys. 2012, 45, 055202. [Google Scholar] [CrossRef]
- Shiraishi, F.; Ishimatsu, T. Toluene removal from indoor air using a miniaturized photocatalytic air purifier including a preceding adsorption/desorption unit. Chem. Eng. Sci. 2009, 64, 2466–2472. [Google Scholar] [CrossRef]
- Yokosuka, Y.; Oki, K.; Nishikiori, H.; Tatsumi, Y.; Tanaka, N.; Fujii, T. Photocatalytic degradation of trichloroethylene using N-doped TiO2 prepared by a simple sol–gel process. Res. Chem. Intermed. 2009, 35, 43–53. [Google Scholar] [CrossRef]
- Hubbard, H.; Coleman, B.; Sarwar, G.; Corsi, R. Abstract. Indoor Air 2005, 15, 432–444. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef]
- Derwent, R.G.; Jenkin, M.E.; Saunders, S.M.; Pilling, M.J. Photochemical ozone creation potentials for organic compounds in northwest Europe calculated with a master chemical mechanism. Atmos. Environ. 1998, 32, 2429–2441. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Feron, V.J.; Til, H.P.; de Vrijer, F.; van Bladeren, P.J. Toxicology of Volatile Organic Compounds in Indoor Air and Strategy for Further Research. Indoor Environ. 1992, 1, 69–81. [Google Scholar]
- Nemecek-Marshall, M.; Wojciechowski, C.; Kuzma, J.; Silver, G.M.; Fall, R. Marine Vibrio species produce the volatile organic compound acetone. Appl. Environ. Microbiol. 1995, 61, 44–47. [Google Scholar] [CrossRef]
- Mitsui, T.; Tsutsui, K.; Matsui, T.; Kikuchi, R.; Eguchi, K. Catalytic abatement of acetaldehyde over oxide-supported precious metal catalysts. Appl. Catal. B Environ. 2008, 78, 158–165. [Google Scholar] [CrossRef]
- Patnaik, P. A Comprehensive Guide to the Hazardous Properties of Chemical Substances; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Gaunt, J.L.; Lehmann, J. Energy Balance and Emissions Associated with Biochar Sequestration and Pyrolysis Bioenergy Production. Environ. Sci. Technol. 2008, 42, 4152–4158. [Google Scholar] [CrossRef]
- Agnihotri, S.; Zheng, Y.; Mota, J.P.B.; Ivanov, I.; Kim, P. Practical Modeling of Heterogeneous Bundles of Single-Walled Carbon Nanotubes for Adsorption Applications: Estimating the Fraction of Open-Ended Nanotubes in Samples. J. Phys. Chem. C 2007, 111, 13747–13755. [Google Scholar] [CrossRef]
- Ding, Z.; Wan, Y.; Hu, X.; Wang, S.; Zimmerman, A.R.; Gao, B. Sorption of lead and methylene blue onto hickory biochars from different pyrolysis temperatures: Importance of physicochemical properties. J. Ind. Eng. Chem. 2016, 37, 261–267. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef]
- Sun, K.; Keiluweit, M.; Kleber, M.; Pan, Z.; Xing, B. Sorption of fluorinated herbicides to plant biomass-derived biochars as a function of molecular structure. Bioresour. Technol. 2011, 102, 9897–9903. [Google Scholar] [CrossRef]
- Tang, D.; Zheng, Z.; Lin, K.; Luan, J.; Zhang, J. Adsorption of p-nitrophenol from aqueous solutions onto activated carbon fiber. J. Hazard. Mater. 2007, 143, 49–56. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and Applications of Biochar for Environmental Remediation: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Nicodem, D.E.; Guedes, C.L.B.; Correa, R.J.; Fernandes, M.C.Z. Photochemical processes and the environmental impact of petroleum spills. Biogeochemistry 1997, 39, 121–138. [Google Scholar] [CrossRef]
- Snape, I.; Harvey, P.M.; Ferguson, S.H.; Rayner, J.L.; Revill, A.T. Investigation of evaporation and biodegradation of fuel spills in Antarctica I. A chemical approach using GC–FID. Chemosphere 2005, 61, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef]
- Ge, M.; Xi, Z.; Zhu, C.; Liang, G.; Yang, Y.; Hu, G.; Jamal, L.; Alam, S.M.J. Adsorption process and properties analyses of a pure magadiite and a modified magadiite on rhodamine-B from an aqueous solution. Processes 2019, 7, 565. [Google Scholar] [CrossRef]
- Jung, K.; Oh, S.; Bak, H.; Song, G.H.; Kim, H.T. Adsorption of arsenic and lead onto stone powder and chitosan-coated stone powder. Processes 2019, 7, 599. [Google Scholar] [CrossRef]
- Nicolaou, E.; Philippou, K.; Anastopoulos, I.; Pashalidis, I. Copper adsorption by magnetized pine-needle biochar. Processes 2019, 7, 903. [Google Scholar] [CrossRef]
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.A.; AbdEl-Salam, N.M.; Ghaffar, A. CVD synthesis, functionalization and CO2 adsorption attributes of multiwalled carbon nanotubes. Processes 2019, 7, 634. [Google Scholar] [CrossRef]
- Ucán, C.A.; Abatal, M.; Romero, C.M.; Franseschi, F.A.; Elias, M.A.R.; Lozano, D.C. Removal of an ethoxylated alkylphenol by adsorption on zeolites and photocatalysis with TiO2/Ag. Processes 2019, 7, 889. [Google Scholar] [CrossRef]
- Yu, D.; Morisada, S.; Kawakita, H.; Ohto, K.; Inoue, K.; Song, X.; Zhang, G. Selective cesium adsorptive removal on using crosslinked tea leaves. Processes 2019, 7, 412. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, R.; Ren, B.; Yaseen, M.; Hursthouse, A. Enhancing the removal of Sb (III) from water: A Fe3O4@HCO composite adsorbent caged in sodium alginate microbeads. Processes 2020, 8, 44. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Bikiaris, D.N.; Mitropoulos, A.C. Graphene composites as dye adsorbents: Review. Chem. Eng. Res. Des. 2018, 129, 75–88. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Kyzas, G.Z.; Triantafyllidis, K.S.; Matis, K.A. Activated carbons for the removal of heavy metal ions: A systematic review of recent literature focused on lead and arsenic ions. Open Chem. 2015, 13, 699–708. [Google Scholar] [CrossRef]
- DesMarais, T.A.; Stone, K.J.; Dyer, J.C.; Hird, B.; Goldman, S.A.; Seiden, P. Absorbent Foam Materials for Aqueous Fluids Made from High Internal Phase Emulsions Having Very High Water-to-oil Ratios. U.S. Patent 5,650,222, 1997. [Google Scholar]
- Choi, H.M.; Cloud, R.M. Natural sorbents in oil spill cleanup. Environ. Sci. Technol. 1992, 26, 772–776. [Google Scholar] [CrossRef]
- Schrader, E. A practical composition of organic, synthetic and inorganic sorbents. Clean Gulf. 1993, 93, 17–22. [Google Scholar]
- Choi, H.-M.; Kwon, H.-J.; Moreau, J.P. Cotton Nonwovens as Oil Spill Cleanup Sorbents. Text. Res. J. 1993, 63, 211–218. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R.; Sanchirico, R. Advanced oxidation processes for the treatment of mineral oil-contaminated wastewaters. Water Res. 2000, 34, 620–628. [Google Scholar] [CrossRef]
- Boudouch, O.; Daoud, E.; Mariem, K.; Benadda, B. Influence of Soil Air Permeability Change on Soil Vapour Extraction Systems Design. CLEAN–Soil Air Water 2012, 40, 461–471. [Google Scholar] [CrossRef]
- Farhan, S.; Holsen, T.M.; Budiman, J. Interaction of Soil Air Permeability and Soil Vapor Extraction. J. Environ. Eng. 2001, 127, 32–37. [Google Scholar] [CrossRef]
- García-Herruzo, F.; Gómez-Lahoz, C.; Rodríguez-Jiménez, J.J.; Wilson, D.J.; García-Delgado, R.A.; Rodríguez-Maroto, J.M. Influence of water evaporation on soil vapor extraction (SVE). Water Sci. Technol. 1994, 30, 115–118. [Google Scholar] [CrossRef]
- Huon, G.; Simpson, T.; Holzer, F.; Maini, G.; Will, F.; Kopinke, F.-D.; Roland, U. In Situ Radio-Frequency Heating for Soil Remediation at a Former Service Station: Case Study and General Aspects. Chem. Eng. Technol. 2012, 35, 1534–1544. [Google Scholar] [CrossRef]
- Preis, S.; Klauson, D.; Gregor, A. Potential of electric discharge plasma methods in abatement of volatile organic compounds originating from the food industry. J. Environ. Manag. 2013, 114, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Revah, S.; Morgan-Sagastume, J.M. Methods of Odor and VOC Control. In Biotechnology for Odor and Air Pollution Control; Shareefdeen, Z., Singh, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 29–63. [Google Scholar]
- Hackam, R.; Aklyama, H. Air pollution control by electrical discharges. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 654–683. [Google Scholar] [CrossRef]
- Urashima, K.; Jen-Shih, C. Removal of volatile organic compounds from air streams and industrial flue gases by non-thermal plasma technology. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 602–614. [Google Scholar] [CrossRef]
- Satoh, K.; Matsuzawa, T.; Itoh, H. Decomposition of benzene in a corona discharge at atmospheric pressure. Thin Solid Films 2008, 516, 4423–4429. [Google Scholar] [CrossRef]
- Schmid, S.; Jecklin, M.C.; Zenobi, R. Degradation of volatile organic compounds in a non-thermal plasma air purifier. Chemosphere 2010, 79, 124–130. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef]
- Singh, P.; Ojha, A.; Borthakur, A.; Singh, R.; Lahiry, D.; Tiwary, D.; Mishra, P.K. Emerging trends in photodegradation of petrochemical wastes: A review. Environ. Sci. Pollut. Res. 2016, 23, 22340–22364. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.-H. Sonochemical Synthesis of Ce-doped TiO2 Nanostructure: A Visible-Light-Driven Photocatalyst for Degradation of Toluene and O-Xylene. Materials 2019, 12, 1265. [Google Scholar] [CrossRef]
- Laokiat, L.; Khemthong, P.; Grisdanurak, N.; Sreearunothai, P.; Pattanasiriwisawa, W.; Klysubun, W. Photocatalytic degradation of benzene, toluene, ethylbenzene, and xylene (BTEX) using transition metal-doped titanium dioxide immobilized on fiberglass cloth. Korean J. Chem. Eng. 2011, 29, 377–383. [Google Scholar] [CrossRef]
- Mahanty, B.; Jesudas, S.; Padmaprabha, A. Toxicity of surface functionalized iron oxide nanoparticles toward pure suspension culture and soil microcosm. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100235. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering Treatment and Reuse; McGraw-Hill Higher Education: Boston, MA, USA, 2003. [Google Scholar]
- Lim, H.-N.; Choi, H.; Hwang, T.-M.; Kang, J.-W. Characterization of ozone decomposition in a soil slurry: Kinetics and mechanism. Water Res. 2002, 36, 219–229. [Google Scholar] [CrossRef]
- Chen, T.; Delgado, A.G.; Yavuz, B.M.; Maldonado, J.; Zuo, Y.; Kamath, R.; Westerhoff, P.; Krajmalnik-Brown, R.; Rittmann, B.E. Interpreting Interactions between Ozone and Residual Petroleum Hydrocarbons in Soil. Environ. Sci. Technol. 2017, 51, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodríguez, J.J. Chemical Pathway and Kinetics of Phenol Oxidation by Fenton’s Reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.D.; Parker, B.L.; Al, T.A.; Cherry, J.A.; Loomer, D. Geochemical Reactions Resulting from In Situ Oxidation of PCE-DNAPL by KMnO4 in a Sandy Aquifer. Environ. Sci. Technol. 2001, 35, 1266–1275. [Google Scholar] [CrossRef]
- Bajagain, R.; Gautam, P.; Jeong, S.-W. Degradation of petroleum hydrocarbons in unsaturated soil and effects on subsequent biodegradation by potassium permanganate. Environ. Geochem. Health 2019. [Google Scholar] [CrossRef]
- Arslan, İ.; Balcioǧlu, I.A.; Bahnemann, D.W. Advanced chemical oxidation of reactive dyes in simulated dyehouse effluents by ferrioxalate-Fenton/UV-A and TiO2/UV-A processes. Dyes Pigment. 2000, 47, 207–218. [Google Scholar] [CrossRef]
- Mohammadi, L.; Bazrafshan, E.; Noroozifar, M.; Ansari-Moghaddam, A.; Barahuie, F.; Balarak, D. Adsorptive removal of Benzene and Toluene from aqueous environments by cupric oxide nanoparticles: Kinetics and isotherm studies. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Mohammadi, L.; Bazrafshan, E.; Noroozifar, M.; Ansari-Moghaddam, A.; Barahuie, F.; Balarak, D. Removing 2,4-dichlorophenol from aqueous environments by heterogeneous catalytic ozonation using synthesized MgO nanoparticles. Water Sci. Technol. 2017, 76, 3054–3068. [Google Scholar] [CrossRef]
- Mohammadi, L.; Bazrafshan, E.; Noroozifar, M.; Ansari-Moghaddama, A.R.; Khazaei Feizabad, A.R.; Mahvi, A.H. Optimization of the catalytic ozonation process using copper oxide nanoparticles for the removal of benzene from aqueous solutions. Glob. J. Environ. Sci. Manag. 2017, 3, 403–416. [Google Scholar]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar light induced and TiO2 assisted degradation of textile dye reactive blue 4. Chemosphere 2002, 46, 1173–1181. [Google Scholar] [CrossRef]
- Pirkanniemi, K.; Sillanpää, M. Heterogeneous water phase catalysis as an environmental application: A review. Chemosphere 2002, 48, 1047–1060. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Mota, A.L.; Albuquerque, L.; Beltrame, L.; Chiavone-Filho, O.; Machulek, A.; Nascimento, C. Advanced oxidation processes and their application in the petroleum industry: A review. Braz. J. Pet. Gas 2009, 2. [Google Scholar]
- Santos, M.S.F.; Alves, A.; Madeira, L.M. Paraquat removal from water by oxidation with Fenton’s reagent. Chem. Eng. J. 2011, 175, 279–290. [Google Scholar] [CrossRef]
- Peyton, G.R. Effect of bicarbonate alkalinity on performance of advanced oxidation processes. Water Res. Found. 1998. [Google Scholar]
- Bhatnagar, A.; Cheung, H.M. Sonochemical Destruction of Chlorinated C1 and C2 Volatile Organic Compounds in Dilute Aqueous Solution. Environ. Sci. Technol. 1994, 28, 1481–1486. [Google Scholar] [CrossRef]
- Shirgaonkar, I.Z.; Pandit, A.B. Sonophotochemical destruction of aqueous solution of 2,4,6-trichlorophenol. Ultrason. Sonochem. 1998, 5, 53–61. [Google Scholar] [CrossRef]
- Compton, R.G.; Eklund, J.C.; Marken, F.; Rebbitt, T.O.; Akkermans, R.P.; Waller, D.N. Dual activation: Coupling ultrasound to electrochemistry—An overview. Electrochim. Acta 1997, 42, 2919–2927. [Google Scholar] [CrossRef]
- Johnson, O.A.; Affam, A.C. Petroleum sludge treatment and disposal: A review. Environ. Eng. Res. 2019, 24, 191–201. [Google Scholar] [CrossRef]
- Moussavi, G.; Khosravi, R.; Farzadkia, M. Removal of petroleum hydrocarbons from contaminated groundwater using an electrocoagulation process: Batch and continuous experiments. Desalination 2011, 278, 288–294. [Google Scholar] [CrossRef]
- Islam, B. Petroleum sludge, its treatment and disposal: A review. Int. J. Chem. Sci. 2015, 13, 1584–1602. [Google Scholar]
- Kong, S.-H.; Watts, R.J.; Choi, J.-H. Treatment of petroleum-contaminated soils using iron mineral catalyzed hydrogen peroxide. Chemosphere 1998, 37, 1473–1482. [Google Scholar] [CrossRef]
- Yeh, C.K.-J.; Wu, H.-M.; Chen, T.-C. Chemical oxidation of chlorinated non-aqueous phase liquid by hydrogen peroxide in natural sand systems. J. Hazard. Mater. 2003, 96, 29–51. [Google Scholar] [CrossRef]
- Verma, S.; Prasad, B.; Mishra, I.M. Pretreatment of petrochemical wastewater by coagulation and flocculation and the sludge characteristics. J. Hazard. Mater. 2010, 178, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- El Ashtoukhy, E.S.Z.; El-Taweel, Y.A.; Abdelwahab, O.; Nassef, E. Treatment of Petrochemical Wastewater Containing Phenolic Compounds by Electrocoagulation Using a Fixed Bed Electrochemical Reactor. Int. J. Electrochem. Sci. 2013, 8, 1534–1550. [Google Scholar]
- Saeedi, M.; Khalvati-Fahlyani, A. Treatment of Oily Wastewater of a Gas Refinery by Electrocoagulation Using Aluminum Electrodes. Water Environ. Res. 2011, 83, 256–264. [Google Scholar] [CrossRef]
- Wang, G.-D.; Li, Q.-J.; Luo, B.; Chen, X.-Y. Ex planta phytoremediation of trichlorophenol and phenolic allelochemicals via an engineered secretory laccase. Nat. Biotechnol. 2004, 22, 893–897. [Google Scholar] [CrossRef]
- Singh, H.B.; Tabazadeh, A.; Evans, M.J.; Field, B.D.; Jacob, D.J.; Sachse, G.; Crawford, J.H.; Shetter, R.; Brune, W.H. Oxygenated volatile organic chemicals in the oceans: Inferences and implications based on atmospheric observations and air-sea exchange models. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Comba, M.E.; Kaiser, K.L.E. Suspended particulate concentrations in the St. Lawrence river (1985–1987) determined by centrifugation and filtration. Sci. Total Environ. 1990, 97–98, 191–206. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Kar, D.D.; De, S. A study on recovery of oil from sludge containing oil using froth flotation. J. Environ. Manag. 2007, 85, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; O’Shea, K.E.; Cooper, W.J. Oxidative degradation of alternative gasoline oxygenates in aqueous solution by ultrasonic irradiation: Mechanistic study. Sci. Total Environ. 2012, 430, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Haigh, S.D. A review of the interaction of surfactants with organic contaminants in soil. Sci. Total Environ. 1996, 185, 161–170. [Google Scholar] [CrossRef]
- Petts, G.E.; Thoms, M.C.; Brittan, K.; Atkin, B. A freeze-coring technique applied to pollution by fine sediments in gravel-bed rivers. Sci. Total Environ. 1989, 84, 259–272. [Google Scholar] [CrossRef]
- Azzouz, A.; Ballesteros, E. Combined microwave-assisted extraction and continuous solid-phase extraction prior to gas chromatography–mass spectrometry determination of pharmaceuticals, personal care products and hormones in soils, sediments and sludge. Sci. Total Environ. 2012, 419, 208–215. [Google Scholar] [CrossRef]
- Lima, A.T.; Ottosen, L.M.; Heister, K.; Loch, J.P.G. Assessing PAH removal from clayey soil by means of electro-osmosis and electrodialysis. Sci. Total Environ. 2012, 435–436, 1–6. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Lin, K.-H.; Lai, N.; Shieh, Z.-X. Element and PAH constituents in the residues and liquid oil from biosludge pyrolysis in an electrical thermal furnace. Sci. Total Environ. 2014, 481, 533–541. [Google Scholar] [CrossRef]
- Rijkenberg, M.J.A.; Depree, C.V. Heavy metal stabilization in contaminated road-derived sediments. Sci. Total Environ. 2010, 408, 1212–1220. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Clutterbuck, B.; Yallop, A.R. Land management as a factor controlling dissolved organic carbon release from upland peat soils 2: Changes in DOC productivity over four decades. Sci. Total Environ. 2010, 408, 6179–6191. [Google Scholar] [CrossRef]
- Lisk, D.J. Environmental effects of landfills. Sci. Total Environ. 1991, 100, 415–468. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Weber, J.; Stevenson, G.; Slee, D.; Gancarz, D.; Rofe, A.; Smith, E. In vivo measurement, in vitro estimation and fugacity prediction of PAH bioavailability in post-remediated creosote-contaminated soil. Sci. Total Environ. 2014, 473–474, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Wang, S.; Li, F.; Guo, G. Effect of biostimulation on community level physiological profiles of microorganisms in field-scale biopiles composed of aged oil sludge. Bioresour. Technol. 2012, 111, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Chen, X.; Konsolakis, M.; Psarras, A.C.; Tavares, P.B.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic oxidation of toluene on Ce–Co and La–Co mixed oxides synthesized by exotemplating and evaporation methods. Catal. Today 2015, 244, 161–171. [Google Scholar] [CrossRef]
- Dziembaj, R.; Molenda, M.; Chmielarz, L.; Drozdek, M.; Zaitz, M.M.; Dudek, B.; Rafalska-Łasocha, A.; Piwowarska, Z. Nanostructured Cu-Doped Ceria Obtained by Reverse Microemulsion Method as Catalysts for Incineration of Selected VOCs. Catal. Lett. 2010, 135, 68–75. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Liu, G.; Li, S.; Li, D.; Li, W.; Chen, Y. Preparation of hierarchical layer-stacking Mn-Ce composite oxide for catalytic total oxidation of VOCs. J. Rare Earths 2015, 33, 62–69. [Google Scholar] [CrossRef]
- Ward, O.; Singh, A.; Van Hamme, J. Accelerated biodegradation of petroleum hydrocarbon waste. J. Ind. Microbiol. Biotechnol. 2003, 30, 260–270. [Google Scholar] [CrossRef]
- Aivalioti, M.; Vamvasakis, I.; Gidarakos, E. BTEX and MTBE adsorption onto raw and thermally modified diatomite. J. Hazard. Mater. 2010, 178, 136–143. [Google Scholar] [CrossRef]
- Sangkhun, W.; Laokiat, L.; Tanboonchuy, V.; Khamdahsag, P.; Grisdanurak, N. Photocatalytic degradation of BTEX using W-doped TiO2 immobilized on fiberglass cloth under visible light. Superlattices Microstruct. 2012, 52, 632–642. [Google Scholar] [CrossRef]
- Hubbard, H.F.; Coleman, B.K.; Sarwar, G.; Corsi, R.L. Effects of an ozone-generating air purifier on indoor secondary particles in three residential dwellings. Indoor Air 2005, 15, 432–444. [Google Scholar] [CrossRef]
- Doran, G.F.; Carini, F.H.; Fruth, D.A.; Drago, J.A.; Leong, L.Y.C. Evaluation of Technologies to Treat Oil Field Produced Water to Drinking Water or Reuse Quality. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: San Antonio, TX, USA, 1997; p. 12. [Google Scholar]
- Mousa, K.M.; Hadi, H.J. Coagulation/Flocculation Process for Produced Water Treatment. 2016. Available online: http://inpressco.com/category/ijcet (accessed on 15 February 2020).
- Kriipsalu, M.; Marques, M.; Maastik, A. Characterization of oily sludge from a wastewater treatment plant flocculation-flotation unit in a petroleum refinery and its treatment implications. J. Mater. Cycles Waste Manag. 2008, 10, 79–86. [Google Scholar] [CrossRef]
- Bellamy, W.D.; Hickman, G.T.; Mueller, P.A.; Ziemba, N. Treatment of VOC-Contaminated Groundwater by Hydrogen Peroxide and Ozone Oxidation. Res. J. Water Pollut. Control Fed. 1991, 63, 120–128. [Google Scholar]
- Fadaei, S.; Moghadam, F.N.; Hashemi, M.H.P. BTEX Removal from Aqueous Solution by Modified Multi-Walled Carbon Nanotubes With Ozone. Anu. Inst. Geocienc. 2017, 40, 235–242. [Google Scholar] [CrossRef]
- Mohammadi, L.; Bazrafshan, E.; Noroozifar, M.; Ansari-Moghaddam, A. Application of Heterogeneous Catalytic Ozonation Process with Magnesium Oxide Nanoparticles for Toluene Degradation in Aqueous Environments. Health Scope 2016, 5, e40439. [Google Scholar] [CrossRef]
- Asgari, G.; Mohammadi, A.S.; Mortazavi, S.B.; Ramavandi, B. Investigation on the pyrolysis of cow bone as a catalyst for ozone aqueous decomposition: Kinetic approach. J. Anal. Appl. Pyrolysis 2013, 99, 149–154. [Google Scholar] [CrossRef]
- Veiga, M.C.; Fraga, M.; Amor, L.; Kennes, C. Biofilter performance and characterization of a biocatalyst degrading alkylbenzene gases. Biodegradation 1999, 10, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Lin, M.-R.; Chu, C. Effects of pH, moisture, and flow pattern on trickle-bed air biofilter performance for BTEX removal. Adv. Environ. Res. 2002, 6, 99–106. [Google Scholar] [CrossRef]
- Berenjian, A.; Chan, N.; Malmiri, H.J. Volatile organic compounds removal methods, a review. Am. J. Biochem. Biotechnol. 2012, 8, 220–229. [Google Scholar]
- Benatti, C.T.; Tavares, C.R.G.; Guedes, T.A. Optimization of Fenton’s oxidation of chemical laboratory wastewaters using the response surface methodology. J. Environ. Manag. 2006, 80, 66–74. [Google Scholar] [CrossRef]
- Kang, Y.W.; Hwang, K.-Y. Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res. 2000, 34, 2786–2790. [Google Scholar] [CrossRef]
- Yang, C.; Chen, H.; Zeng, G.; Yu, G.; Luo, S. Biomass accumulation and control strategies in gas biofiltration. Biotechnol. Adv. 2010, 28, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Deeb, R.A.; Alvarez-Cohen, L. Temperature effects and substrate interactions during the aerobic biotransformation of BTEX mixtures by toluene-enriched consortia and Rhodococcus rhodochrous. Biotechnol. Bioeng. 1999, 62, 526–536. [Google Scholar] [CrossRef]
- Hwang, S.-C.J.; Wu, S.-J.; Lee, C.-M. Water Transformation in the Media of Biofilters Controlled by Rhodococcus fascians in Treating an Ethyl Acetate-Contaminated Airstream. J. Air Waste Manag. Assoc. 2002, 52, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Leili, M.; Mousavi, S.R.; Nadafi, K.; Ghaffari, M. The investigation of single ozonation process, catalytic ozonation process and single adsorption on activated carbon efficienciesfor removal of furfural from aqueous solution. J. Sabzevar Univ. Med. Sci. 2013, 20, 51–61. [Google Scholar]
- Church, J.; Lundin, J.G.; Diaz, D.; Mercado, D.; Willner, M.R.; Lee, W.H.; Paynter, D.M. Identification and characterization of bilgewater emulsions. Sci. Total Environ. 2019, 691, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Ríos, G.; Pazos, C.; Coca, J. Destabilization of cutting oil emulsions using inorganic salts as coagulants. Colloids Surf. A Physicochem. Eng. Asp. 1998, 138, 383–389. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.; Chanamai, R.; McClements, D.J. Influence of pH and CaCl2 on the stability of dilute whey protein stabilized emulsions. Food Res. Int. 2000, 33, 15–20. [Google Scholar] [CrossRef]
- Mitra, R.K.; Paul, B.K. Effect of temperature and salt on the phase behavior of nonionic and mixed nonionic-ionic microemulsions with fish-tail diagrams. J. Colloid Interface Sci. 2005, 291, 550–559. [Google Scholar] [CrossRef]
- Asselin, M.; Drogui, P.; Brar, S.K.; Benmoussa, H.; Blais, J.F. Organics removal in oily bilgewater by electrocoagulation process. J. Hazard. Mater. 2008, 151, 446–455. [Google Scholar] [CrossRef]
- Santisi, S.; Gentile, G.; Volta, A.; Bonsignore, M.; Mancini, G.; Quatrini, P.; Capello, S. Isolation and characterization of oil degrading bacteria from bilge water. Int. J. Microbiol. Appl. 2015, 2, 45–49. [Google Scholar]
- McMillen, S.J.; Magaw, R.I.; Kerr, J.M.; Sweeney, R.E.; Nakles, D.V.; Geiger, S.C. A new risk based approach to establish clean up levels for total petroleum hydrocarbon. In Proceedings of the 6th International Petroleum Environmental Conference, Houston, TX, USA, 16–18 November 1999; Sublette, K.L., Ed.; SCG, Inc.: Tulsa, OK, USA, 2000; pp. 438–459. [Google Scholar]
- Kuyukina, M.S.; Krivoruchko, A.V.; Ivshina, I.B. Advanced Bioreactor Treatments of Hydrocarbon-Containing Wastewater. Appl. Sci. 2020, 10, 831. [Google Scholar] [CrossRef]
- Gomes, H.T.; Samant, P.V.; Serp, P.; Kalck, P.; Figueiredo, J.L.; Faria, J.L. Carbon nanotubes and xerogels as supports of well-dispersed Pt catalysts for environmental applications. Appl. Catal. B Environ. 2004, 54, 175–182. [Google Scholar] [CrossRef]
- Lu, C.; Su, F.; Hu, S. Surface modification of carbon nanotubes for enhancing BTEX adsorption from aqueous solutions. Appl. Surf. Sci. 2008, 254, 7035–7041. [Google Scholar] [CrossRef]
- Aivalioti, M.; Papoulias, P.; Kousaiti, A.; Gidarakos, E. Adsorption of BTEX, MTBE and TAME on natural and modified diatomite. J. Hazard. Mater. 2012, 207–208, 117–127. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S. Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 181–193. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Hu, S. Adsorption of benzene, toluene, ethylbenzene and p-xylene by NaOCl-oxidized carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 83–91. [Google Scholar] [CrossRef]
- Ji, G.D.; Sun, T.H.; Ni, J.R.; Tong, J.J. Anaerobic baffled reactor (ABR) for treating heavy oil produced water with high concentrations of salt and poor nutrient. Bioresour. Technol. 2009, 100, 1108–1114. [Google Scholar] [CrossRef]
- Rastegar, S.O.; Mousavi, S.M.; Shojaosadati, S.A.; Sheibani, S. Optimization of petroleum refinery effluent treatment in a UASB reactor using response surface methodology. J. Hazard. Mater. 2011, 197, 26–32. [Google Scholar] [CrossRef]
- Hayder, G.; Kutty, S.R.M.; Isa, M.H.; Lemma, T.A. Optimization of Anaerobic Treatment of Petroleum Refinery Wastewater Using Artificial Neural Networks. Res. J. Appl. Sci. Eng. Technol. 2013, 6, 2077–2082. [Google Scholar]
- Zheng, T. A compact process for treating oilfield wastewater by combining hydrolysis acidification, moving bed biofilm, ozonation and biologically activated carbon techniques. Environ. Technol. 2016, 37, 1171–1178. [Google Scholar] [CrossRef]
- Huo, S.; Zhu, F.; Zou, B.; Xu, L.; Cui, F.; You, W. A two-stage system coupling hydrolytic acidification with algal microcosms for treatment of wastewater from the manufacture of acrylonitrile butadiene styrene (ABS) resin. Biotechnol. Lett. 2018, 40, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Chen, J.; Chen, X.; Wang, F.; Xu, L.; Zhu, F.; Guo, D.; Li, Z. Advanced treatment of the low concentration petrochemical wastewater by Tribonema sp. microalgae grown in the open photobioreactors coupled with the traditional Anaerobic/Oxic process. Bioresour. Technol. 2018, 270, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ghimire, N.; Xin, G.; Wakjera, E.; Bakke, R. Efficient high strength petrochemical wastewater treatment in a hybrid vertical anaerobic biofilm (HyVAB) reactor: A pilot study. Water Pract. Technol. 2017, 12, 501–513. [Google Scholar] [CrossRef][Green Version]
- Janka, E.; Carvajal, D.; Wang, S.; Bakke, R.; Dinamarca, C. Treatment of Metformin-Containing Wastewater by a Hybrid Vertical Anaerobic Biofilm-Reactor (HyVAB). Int. J. Environ. Res. Public Health 2019, 16, 4125. [Google Scholar] [CrossRef]

| Benzene | Toluene | m-Xylene | o-Xylene | p-Xylene | Ethylbenzene | |
|---|---|---|---|---|---|---|
| Chemical structure | 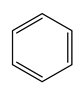 | 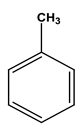 |  | 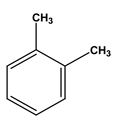 | 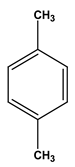 | 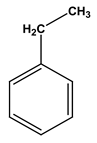 |
| Formula | C6H6 | C7H8 | C8H10 | C8H10 | C8H10 | C8H10 |
| Molecular weight (g/mol) | 78 | 92 | 106 | 106 | 106 | 106 |
| Solubility in water (mg/L) | 1700 | 515 | - | 175 | 198 | 152 |
| Steam pressure (mm Hg) at 20 °C | 95.2 | 28.4 | - | 6.6 | - | 9.5 |
| Special density (at 20 °C) | 0.8787 | 0.8669 | 0.8642 | 0.8802 | 0.8610 | 0.8670 |
| Octane coefficient (at 20 °C) | 2.13 | 2.69 | 3.15 | 3.15 | 2.77 | 3.20 |
| Fixed art law (in 25 °C) (kPam3/mol) | 0.55 | 0.67 | 0.7 | 0.5 | 0.71 | 0.8 |
| Maximum amount of contaminants (MCL) (mg/L) | 0.005 | 1 | 10 | 10 | 10 | 0.7 |
| Representatives | IDLH * | Sources | Health Effects | ||
|---|---|---|---|---|---|
| Australian and New Zealand Environmental Protection Regulations (ppb) | American National Drinking Water Standards (ppb) | WHO Drinking Water Regulations (ppb) | |||
| Benzene | 600 | 5 | 10 | Petroleum products | Carcinogen |
| Toluene | 180 | 1000 | 700 | Incomplete combustion of liquid fuels | Damage the ozone layer |
| Ethylbenzene | 50 | 700 | 300 | Adhesives Lacquers | Produce photochemical smog, and pose mutagenic hazards |
| Xylene | 200 | 1000 | 500 | Chemical industry, coal tar and oil, leak in oceans, forest fire | Neurological disorders, kidney and liver, skin problems |
| Methods | Reaction Conditions | Efficiency | Market Sales | Reuse | Waste Generation | Energy Consumption | BTEX Concentration | Ref. |
|---|---|---|---|---|---|---|---|---|
| Thermal incineration | Fixed-bed reactor; catalyst, MnOx/γ-Al2O3; temperature, 443–873 K. Rotary kiln incinerator; temperature, ~1273 K | >99% (40 min) | High | No | CO, NOx | Moderate | 20–25% | [28,29] |
| Fenton Oxidation | Wash water of ion-exchange resin, pH 3–4; CH2O2 = 1200 mg/L, CFe2+ = 300 mg/L. TCE-contaminated groundwater, pH 5.4, ORP = 465 mv; 10 g/L basic oxygen furnace, 1.0 g/L of H2O2 | 100% and 87% removal of 1,2-DCA and TOC in 90 min, respectively. 81% (1 h). | - | No | CO2; Cl−; Fe3+. CO2; Cl−; CE; DCE. | High | - | [30,31] |
| Condensation | Moderate | High | Yes | – | High | >5000 ppm | [29] | |
| Biological degradation | 100% (∼7 months) | Low | No | Acetaldehyde, Propanol, Acetone | Low | <5000 ppm | [32] | |
| Adsorption process | Activated carbon/photocatalytic oxidation hybrid system | >90% | High | Yes | Spent adsorbent | Moderate | 700–10,000 ppm | [33,34,35,36,37,38,39,40,41,42,43] |
| Ozonation process | Contaminated soil; flushing solvent, acetic acid; ozone concentration, 17 ± 2 mg/L; temperature, 20 ± 2 °C. | 100% (2 h) | High | - | High | 10% and 25% | [44] | |
| Plasma catalysis | A hybrid pulsed power corona reactor with adjustable energy density | 74–81% | High | No | Formic acid, Carboxylic acids, NOx, O3 | High | 70–100 ppm | [45] |
| Photocatalytic oxidation | Gas conditions; gas flow 200 mL/min for 30 min; infrared cell; UV irradiation, 150 W Xenon lamp; catalyst, N-doped TiO2. Gas conditions in O2 stream; low-pressure | 100% (5 min) | Low-moderate | No | Strong oxidant OH• radicals | Moderate | – | [46,47] |
| Catalytic ozonation process (COP) | ozone was generated using a commercial ozone generator marketed as an air purifier, and particle measurements were recorded before, during, and after the release of terpenes from a pine oil-based cleaning product. | 100% (2 h) | High | No | Secondary organic aerosols | High | 200–10 | [48] |
| Membrane separation | – | Low | Yes | Clogged membranes | High | <25% | [28,29] | |
| Coagulation/flocculation with sedimentation | 87 (as COD)- 98.8% | No | – | Moderate | >105 ppm | [49,50] | ||
| Flocculation with DAF | A pot trial was carried out to investigate the effect of biochar produced from green waste by pyrolysis on the yield of radish (Raphanussativus var. Long Scarlet) and the soil quality of an Alfisol | 90%, at higher rates of biochar application (>50 t/ha) | No | – | Moderate | >150 ppm | [51] | |
| Coagulation with foam separation | 95% | No | – | High | 0.9–58 | [52] | ||
| Coagulation/flocculation with MFC | screening aerobic, heterotrophic marine bacteria for production of volatile organic compounds | 94% | High | 50–100 | [53] | |||
| Demulsification with centrifugation | Catalytic combustion of acetaldehyde was investigated on various oxide-supported metal catalysts prepared by impregnation method | [54] | ||||||
| Electrocoagulation | Adsorption of phenol and its derivatives on activated carbons | 99% (as turbidity) | 80 | [49,55] | ||||
| Centrifugation | The implications for greenhouse gas emissions of optimizing a slow pyrolysis-based bioenergy system for Biochar and energy production rather than solely for energy production | 68.83% | [56] | |||||
| Coalescence on granular bed | Practical approach for adsorption modeling | 71–99% | 4–500 | [57,58,59] | ||||
| Membrane separation | The adsorption onto activated carbon | 97–99% | [60,61,62,63] |
| Method | UV Supported 1 | Advantages | Disadvantages |
|---|---|---|---|
| Thermal incineration | No | Efficient destruction within short time | High construction cost; potential formation of highly toxic byproducts. |
| Chemical coagulation/flocculation (with DAF/sedimentation/others) | No | High removal efficiency; Usually easy to operate | High consumption of reactants; Production of hazardous sludge; High operative costs for higher efficiencies (reactants, air diffusers) |
| Electrochemical coagulation/flotation | No | High removal efficiency; Low reactant consumption; Cheaper than chemical coagulation | Production of hazardous sludge (possibly larger than with chemical coagulation [116]); High installation cost |
| Fenton oxidation | Yes | Simplicity and efficiency | Costly chemical addition; acidic conditions; secondary pollutant formation. |
| Physical methods (DAF, centrifugation) | No | Low reactant consumption; No production of byproducts | High energy requirements; Lower removal efficiency |
| Coalescence on granular bed | No | No reactant consumption; Cheap and easy to operate | Slow BTEX removal; May not be possible for all types of wastewaters due to interference of other pollutants (e.g., suspended solids); Lower removal efficiency |
| Membrane separation | No | High removal efficiency; Low/no reactant consumption; No production of byproducts; Possibility of recovery of the oily retentate; Possibility of removing other pollutants simultaneously | High energy requirements; May require pretreatment (another upstream secondary treatment) of wastewater; High maintenance costs due to occurrence of membrane fouling |
| AOPs | Yes | Possibility of total elimination of organic pollution; Possibility of removing other pollutants simultaneously | Lower removal efficiency; Only adequate for low-pollution wastewaters; High reactant consumption |
| Biological treatment | No | High removal efficiency; Low reactant consumption; Cheap and easy to operate | Production of sludge; Only adequate for biodegradable wastewaters (may require pretreatment (another upstream secondary treatment) |
| Adsorption | No | High removal efficiency; No reactant consumption; Cheap (especially with low-cost materials); Easy to operate; No production of byproducts; Possibility of regeneration | May not be adequate for finely dispersed emulsions; May suffer interference of other pollutants |
| Phytoremediation | No | Economics, aesthetic and ecologic advantages | Time-consuming; incomplete metabolism and potential increase in bioavailability of contaminants [125] |
| Ozonation | Yes | Effective and fast removal of contaminants | Low solubility of ozone in water; ozone scavengers commonly found in environment; incomplete oxidation [44] |
| Plasma | No | Can simultaneously remove gas pollutants, airborne microbes, and even particles. | May produce O3, NOx, and other harmful by-products. High voltage and high energy consumption. |
| Solvent extraction | No | Efficient method and very fast process | High cost and environmentally unfit, heavy metals cannot be removed by this process [126] |
| Centrifugation | No | Easy to process, no need for any solvent and environmentally safe | Large among of energy required, economically unfit and lower size molecules difficult to settle down [127] |
| Froth flotation | No | Easy to apply and less energy required | Highly viscous oily wastewater cannot be offered to this process [128] |
| Ultrasonic irradiation | Yes | Fast and effective, no need any chemicals | Heavy equipment cost, unable to treat heavy metals [129] |
| Surfactant EOR | Yes | Easy to process and limited application in heavy metals | High cost, surfactant should be toxic, alternate process required to remove surfactant and economically costly [130] |
| Freeze/thaw | No | Short treatment process and suitable for cold regions | Less effective and coastally process [131] |
| Microwave irradiation | Yes | Very fast and efficient and no need for chemical addition | Specially designed equipment, heavy costly and not effective for large scale process [132] |
| Electrokinetics | No | No need for chemical addition and fast process | Process is not easy and less effective [133] |
| Pyrolysis | No | Large treatment capacity, fast and effective | High capital, maintenance and operating cost [134] |
| Stabilization/solidification | No | Fast and efficient to produce PHC stabilized compounds, low cost and capture the heavy metals | Loss of recyclable energy and less effective in process [135] |
| Oxidation | Yes | Rapid and complete removal of PHCs in oily sludge | Large amount of chemical required, high cost and environmentally unfit [136] |
| Land farming | No | Low cost and do not need much maintenance and applicable to large quantity | Sand pollution and underground water pollution [137] |
| Landfill | No | Less cost and large treatment capacity | Very slow process and required more place [138] |
| Biopile/compositing | No | Large treatment capacity, low cost, faster and less area required for the process | Applicable in cold condition [139] |
| Bioslurry | No | Fastest degradation approach, great PHC removal | High cost and applicable to small scale [140] |
| Methods | pH | Time | Temperature (°F) | BTEX Concentration | Ref. |
|---|---|---|---|---|---|
| Thermal Incineration | – | 90 min | 700 | Higher than 20 but less than 25% of LEL | [141,142,143] |
| Condensation | – | – | Ambient | 5000–10,000 | [29] |
| Biological degradation | 7 | 7–24 d | 50–105 | >5000 | [32,144] |
| Absorption | 3–10 | >10 s | Normal | 500–15,000 | [32] |
| Adsorption | 6.5–9 | 0.5–240 min | <130 | 700–10,000 (but always less than 25% of LEL) | [33,145] |
| Plasma catalysis | – | 120 min | 500 | 1000 | [45] |
| Photocatalytic oxidation | – | 150–200 min | 450–500 | 250–900 | [46,47,146] |
| Ozone-catalytic oxidation | – | <45 min | 300 | 100–1000 but always less than 25% of LEL | [147] |
| Membrane separation | 9.5–10.0 | 60 min | Ambient | Very low concentration to 25% of LEL | [28,148] |
| Coagulation/flocculation withsedimentation | 2.0–4.5 | 36 min | Ambient | coagulant: 10–70, flocculent: 1–3 ppm | [149] |
| Flocculation with DAF | 7.7 ± 0.4 | 3–21 days | room temperature | 63.7–240 g/kg | [150] |
| Electrocoagulation | 9.5 | 3–20 h | 35 | 1500–2200 mg/L | [49,55] |
| Centrifugation | – | 6 months | 40–150 | 20–2000 mg/L | [56] |
| Ozonation | 6.3, 2.5, 1.9 | 0.45 s | Ambient | 50 mg/L | [151,152,153] |
| Equivalent Carbon Number (EC) | Water Solubility (mg/L) | Vapour Pressure (atm) | Log (Koc) (cm3/g) | H (cm3/cm3) | Retardation Factor, Rf # | Fugacity Level I Partitioning (%) | |
|---|---|---|---|---|---|---|---|
| EC > 12–16 aliphatic | 7.6 × 10−4 | 4.8 × 10−5 | 6.7 | 520 | 1.3 × 108 | Air | 1.8 × 10−2 |
| Water | 1.9 × 10−5 | ||||||
| NAPL | 70.0 | ||||||
| Soil | 29.7 | ||||||
| EC > 12–16 aromatic | 5.8 | 4.8 × 10−5 | 3.7 | 5.3 × 10−2 | 1.3 × 105 | Air | 4.7 × 10−4 |
| Water | 4.9 × 10−2 | ||||||
| NAPL | 24.2 | ||||||
| Soil | 75.8 | ||||||
| EC > 16–35 aliphatic | 2.5 × 10−6 | 9.8 × 10−7 | 8.8 | 4.9 × 103 | 1.6 × 1010 | Air | 5.4 × 10−4 |
| Water | 6.1 × 10−8 | ||||||
| NAPL | 88.0 | ||||||
| Soil | 12.0 | ||||||
| EC > 16–21 aromatic | 0.65 | 1.1 × 10−6 | 4.2 | 1.3 × 10−2 | 4.0 × 105 | Air | 3.4 × 10−4 |
| Water | 1.5 × 10−2 | ||||||
| NAPL | 28.6 | ||||||
| Soil | 71.4 | ||||||
| EC > 21–35 aromatic | 6.6 × 10−3 | 4.4 × 10−10 | 5.1 | 6.7 × 10−4 | 3.2 × 106 | Air | 1.9 × 10−6 |
| Water | 1.6 × 10−3 | ||||||
| NAPL | 38.9 | ||||||
| Soil | 61.1 | ||||||
| EC > 44 heavy hydrocarbon | 1.0 × 10−4 | 4.1 × 10−12 | 8.7 | 4.1 × 10−8 | 1.3 × 1010 | Air | 1.2 × 10−14 |
| Water | 1.7 × 10−7 | ||||||
| NAPL | 74.4 | ||||||
| Soil | 25.6 | ||||||
| Benzo [a] pyrene | 3.8 × 10−3 | 2.1 × 10−10 | 5.9 | 5.7 × 10−4 | 2.0 × 107 | Air | 1.4 × 10−7 |
| Water | 1.4 × 10−4 | ||||||
| NAPL | 4.6 | ||||||
| Soil | 95.4 | ||||||
| Wastewater Type | Bioreactor Configuration | Operational Conditions | Treatment Efficiency | Ref. |
|---|---|---|---|---|
| Oilfield produced water | ABR | Start-up and operational performance (total 212 days) with mixed acclimated oilfield and urban sewage sludges. | COD and oil removals of 65% and 88% | [177] |
| Petroleum refinery effluent | UASB | Mesophilic conditions (38 ± 1 °C) for over 120 days. Digested sludge from a dairy industry. | 76.3% COD removal, 0.25 L biogas/L feed d | [178] |
| Petroleum refinery wastewater | UASB | Treating under six different organic loads (from 0.58 to 4.14 kg COD/m3 d) during 180 days. | COD removal of 82% | [179] |
| Heavy oil wastewater | HA-MBBR O3-BAC | Sludge from anaerobic and aerobic tanks of petroleum refinery AS plant. Effluent concentrations of COD, oil and ammonia were 48, 1.3, and 3.5 mg/L. | 95.8, 98.9 and 94.4% removals of COD, oil, and ammonia | [180] |
| Acrylonitrile butadiene styrene resin-manufacturing wastewater | Stirred-tank HA with a series of algal photobioreactors | The wastewater was treated for 36 h in a batch process and the effluent was applied to the algal microcosm treatment using Chlorella sp. | COD, NH3-N and Phosphorus removal of 83%, 100% and 89% | [181] |
| Petrochemical wastewater | Open photobioreactors integrated with anaerobic/oxic process | Filamentous microalgae Tribonema sp. aeration and mixing by sparging air enriched with 1.5% CO2, gas flow rate 0.5 vvm, light intensity 300 µmol/m2·s, temperature 25 °C. | COD removal of 97.8% | [182] |
| Petroleum refinery wastewater | Pilot HyVAB | Granular sludge from paper and pulp wastewater treatment facility. Continuously operating at varying organic loading rates for 92 days. | 86% of the total COD and 91% of the soluble COD removal | [183] |
| Metformincontaining wastewater | Pilot HyVAB | Granular sludge from petrochemical wastewater treatment bioreactor. Co-digest pharmaceutical- containing wastewater with the wastewater rich in easily degradable organics. | 98% COD removal and 100% metformin removal | [184] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, L.; Rahdar, A.; Bazrafshan, E.; Dahmardeh, H.; Susan, M.A.B.H.; Kyzas, G.Z. Petroleum Hydrocarbon Removal from Wastewaters: A Review. Processes 2020, 8, 447. https://doi.org/10.3390/pr8040447
Mohammadi L, Rahdar A, Bazrafshan E, Dahmardeh H, Susan MABH, Kyzas GZ. Petroleum Hydrocarbon Removal from Wastewaters: A Review. Processes. 2020; 8(4):447. https://doi.org/10.3390/pr8040447
Chicago/Turabian StyleMohammadi, Leili, Abbas Rahdar, Edris Bazrafshan, Hamid Dahmardeh, Md. Abu Bin Hasan Susan, and George Z. Kyzas. 2020. "Petroleum Hydrocarbon Removal from Wastewaters: A Review" Processes 8, no. 4: 447. https://doi.org/10.3390/pr8040447
APA StyleMohammadi, L., Rahdar, A., Bazrafshan, E., Dahmardeh, H., Susan, M. A. B. H., & Kyzas, G. Z. (2020). Petroleum Hydrocarbon Removal from Wastewaters: A Review. Processes, 8(4), 447. https://doi.org/10.3390/pr8040447







