Electrolytic Oxidation as a Sustainable Method to Transform Urine into Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Urine Composition
2.2. Chemicals
2.3. Analytical Methods
2.4. Electrolytic Treatment
3. Results and Discussion
4. Conclusions
- −
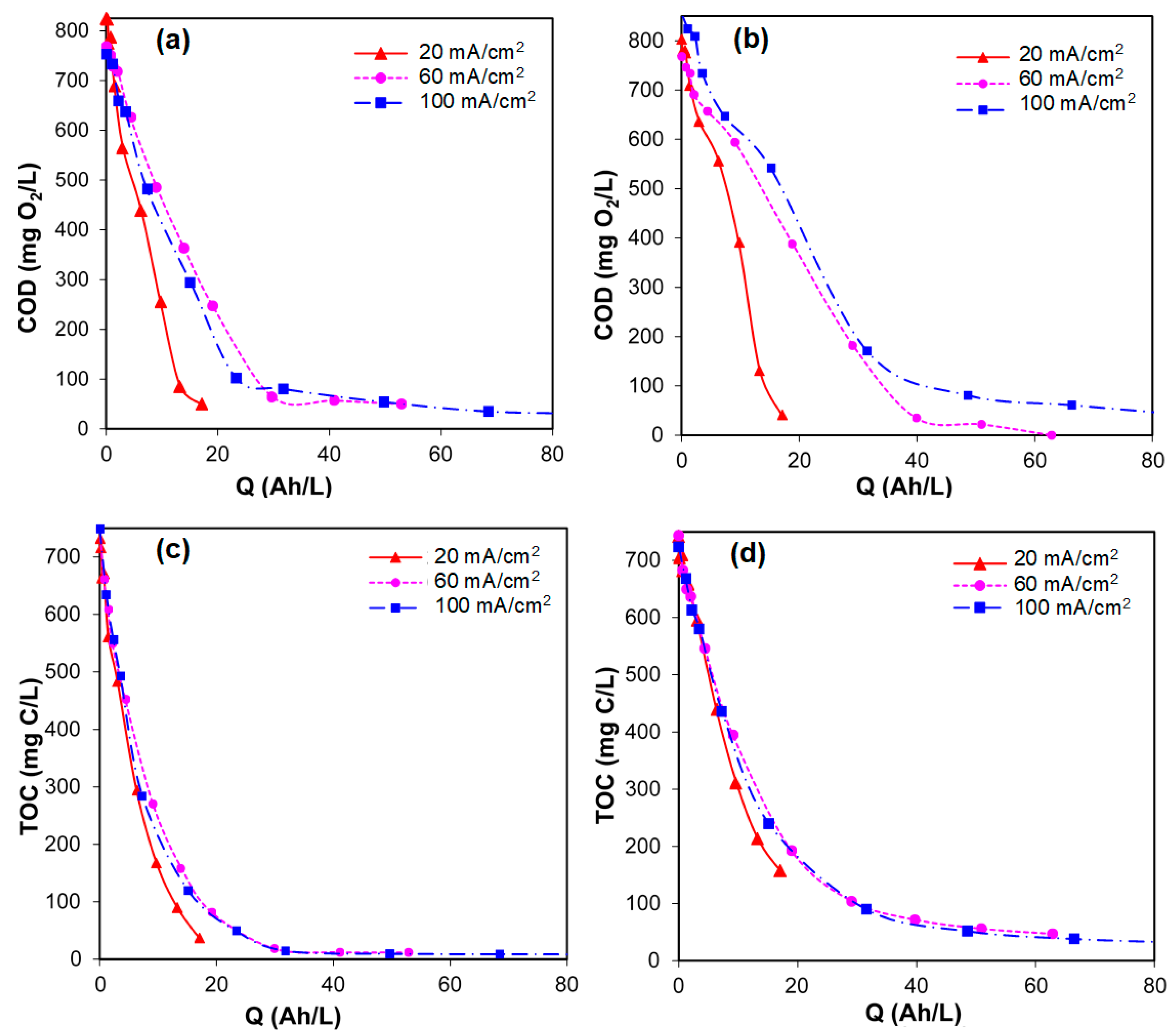
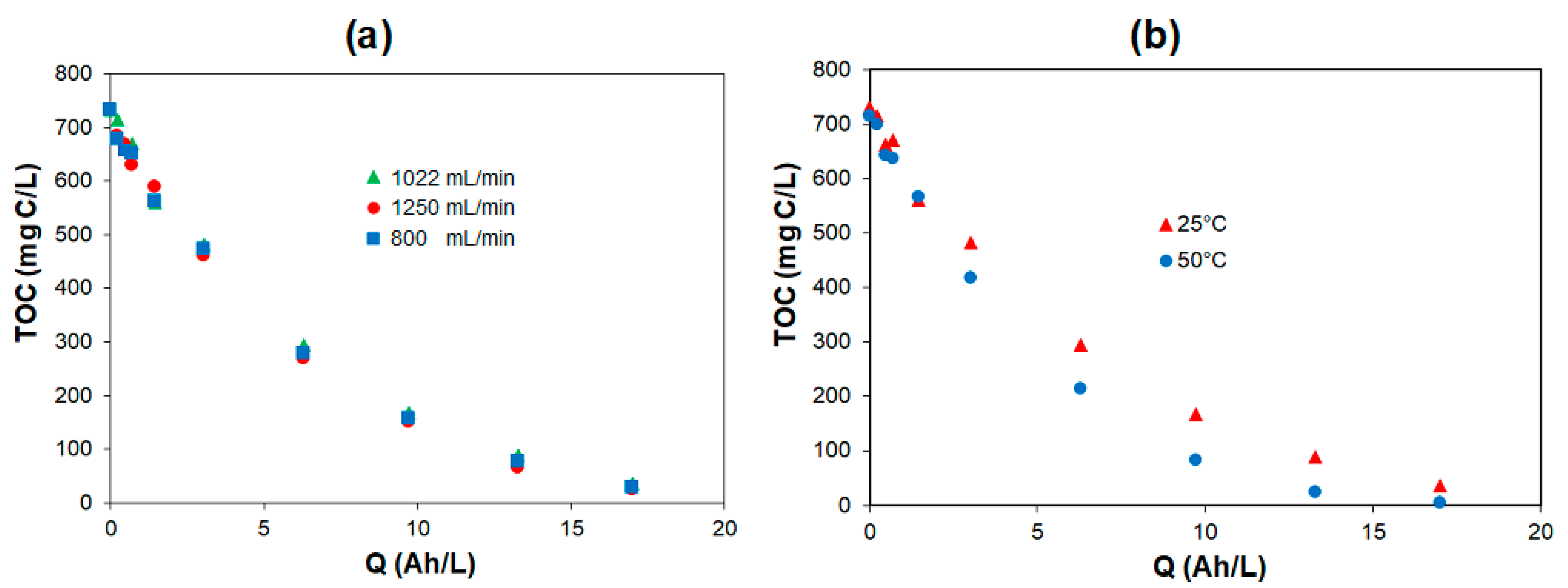
- Electrolytic oxidation using BDD and DSA anodes achieved the almost complete mineralization of the organic carbon initially contained in synthetic urine, regardless of the current density, flow rate, and temperature. Higher current densities were less efficient because the oxygen evolution becomes competitive with the degradation of organic pollution.
- −
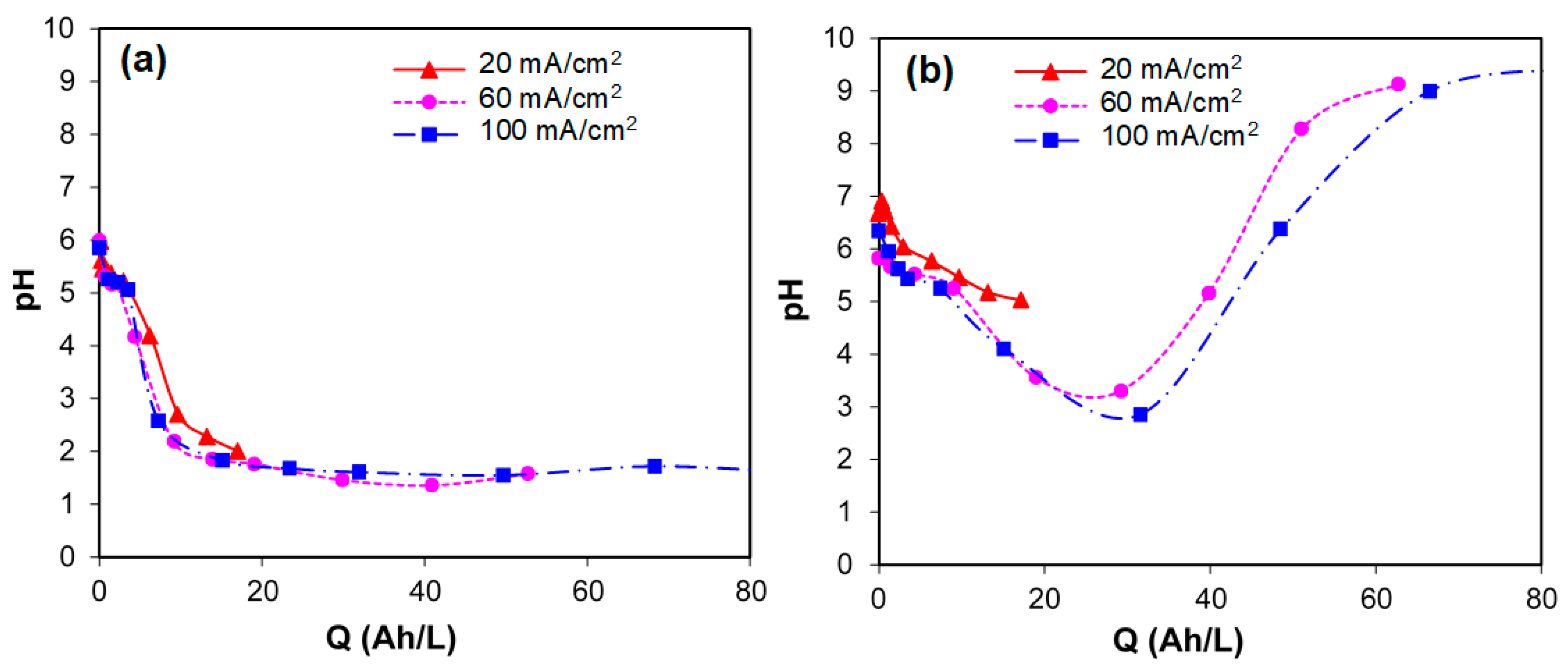
- Nitrates, ammonium, and other volatile nitrogen forms were released during the electrolytic treatment of synthetic urine. Higher amounts of inorganic nitrogen were produced with BDD anodes than DSA. This is in good correlation with the pH changes observed during the electrolytic treatment with BDD (acid pH) and DSA (basic pH).
- −
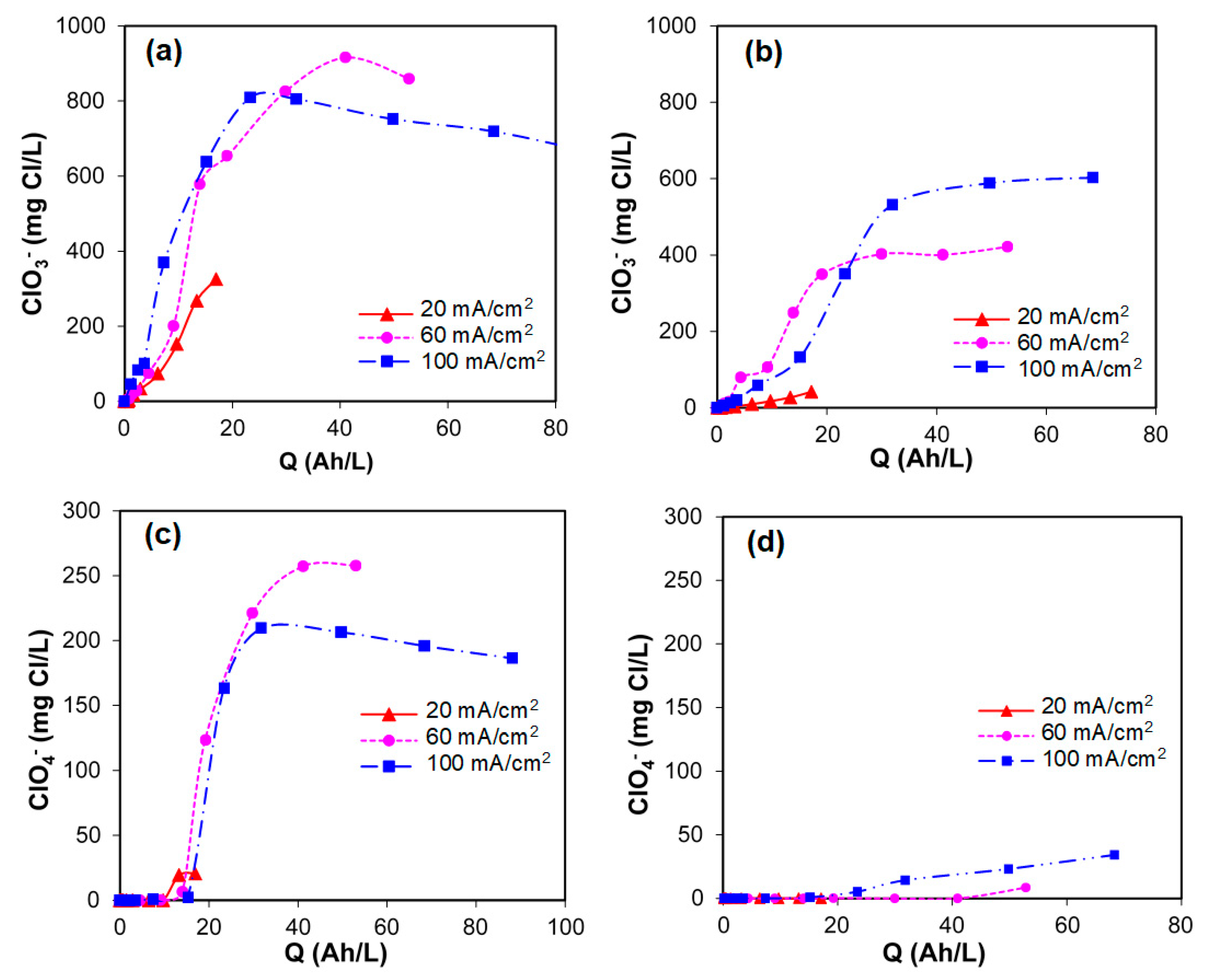
- Chlorides were transformed into hypochlorites that rapidly react with ammonium and/or ammonia to form chloramines. The formation of these species during the electrolytic treatment is very important because they deactivate the microorganisms and kill the pathogens.
- −
- Chlorates and perchlorates can be formed at high current densities with BDD and DSA. The control of the current density can avoid the formation of these toxic and hazardous chlorine species especially when DSA is used as an anode material.
- −
- Phosphates are affected by the pH of the medium and the nature of the anode material. The amount of phosphates measured at the end of the treatment provides the needed amount of phosphorus in liquid form, facilitating its assimilation by plants.
- −
- Electrolytic oxidation using DSA or BDD anodes at low current densities (≤20 mA/cm2) seems promising as a sustainable method to transform urine into a liquid fertilizer rich in nitrogen, phosphorus, and other micronutrients (potassium, sodium, calcium, etc.) free of hazardous substances. This liquid fertilizer is safe to be used as it is or after dilution in gardening, landscaping, and agriculture to supply the plants with the needed nutrients.
Author Contributions
Funding
Conflicts of Interest
References
- Kirchmann, H.; Pettersson, S. Human Urine—Chemical Composition and Fertilizer Use Efficiency. Fertil. Res. 1994. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, S.K.L.; Boyer, T.H. Life Cycle Comparison of Centralized Wastewater Treatment and Urine Source Separation with Struvite Precipitation: Focus on Urine Nutrient Management. Water Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Tang, W.T.; Zheng, Y.S.; Mackey, H.R.; Chui, H.K.; van Loosdrecht, M.C.M.; Chen, G.H. An Exploratory Study on Seawater-Catalysed Urine Phosphorus Recovery (SUPR). Water Res. 2014. [Google Scholar] [CrossRef]
- Heinonen-Tanski, H.; Sjöblom, A.; Fabritius, H.; Karinen, P. Pure Human Urine Is a Good Fertiliser for Cucumbers. Bioresour. Technol. 2007. [Google Scholar] [CrossRef]
- Spångberg, J.; Tidåker, P.; Jönsson, H. Environmental Impact of Recycling Nutrients in Human Excreta to Agriculture Compared with Enhanced Wastewater Treatment. Sci. Total Environ. 2014. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, H.; Stenström, T.A.; Svensson, J.; Sundin, A. Source Separated Urine-Nutrient and Heavy Metal Content, Water Saving and Faecal Contamination. Water Sci. Technol. 1997. [Google Scholar] [CrossRef]
- Tun, L.L.; Jeong, D.; Jeong, S.; Cho, K.; Lee, S.; Bae, H. Dewatering of Source-Separated Human Urine for Nitrogen Recovery by Membrane Distillation. J. Memb. Sci. 2016. [Google Scholar] [CrossRef]
- Simha, P.; Zabaniotou, A.; Ganesapillai, M. Continuous Urea–Nitrogen Recycling from Human Urine: A Step towards Creating a Human Excreta Based Bio–Economy. J. Clean. Prod. 2018. [Google Scholar] [CrossRef]
- Bonvin, C.; Etter, B.; Udert, K.M.; Frossard, E.; Nanzer, S.; Tamburini, F.; Oberson, A. Plant Uptake of Phosphorus and Nitrogen Recycled from Synthetic Source-Separated Urine. Ambio 2015. [Google Scholar] [CrossRef] [Green Version]
- Karak, T.; Bhattacharyya, P. Human Urine as a Source of Alternative Natural Fertilizer in Agriculture: A Flight of Fancy or an Achievable Reality. Resour. Conserv. Recycl. 2011. [Google Scholar] [CrossRef]
- Winker, M.; Vinnerås, B.; Muskolus, A.; Arnold, U.; Clemens, J. Fertiliser Products from New Sanitation Systems: Their Potential Values and Risks. Bioresour. Technol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Igos, E.; Besson, M.; Navarrete Gutiérrez, T.; Bisinella de Faria, A.B.; Benetto, E.; Barna, L.; Ahmadi, A.; Spérandio, M. Assessment of Environmental Impacts and Operational Costs of the Implementation of an Innovative Source-Separated Urine Treatment. Water Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wilsenach, J.; Van Loosdrecht, M. Impact of Separate Urine Collection on Wastewater Treatment Systems. Water Sci. Technol. 2003. [Google Scholar] [CrossRef]
- Simha, P.; Ganesapillai, M. Ecological Sanitation and Nutrient Recovery from Human Urine: How Far Have We Come? A Review. Sustain. Environ. Res. 2017. [Google Scholar] [CrossRef]
- Udert, K.M.; Buckley, C.A.; Wächter, M.; McArdell, C.S.; Kohn, T.; Strande, L.; Zöllig, H.; Fumasoli, A.; Oberson, A.; Etter, B. Technologies for the Treatment of Source-Separated Urine in the EThekwini Municipality. Water SA 2015. [Google Scholar] [CrossRef]
- Reyes Jiménez, A.; Klöpsch, R.; Wagner, R.; Rodehorst, U.C.; Kolek, M.; Nölle, R.; Winter, M.; Placke, T. A Step toward High-Energy Silicon-Based Thin Film Lithium Ion Batteries. ACS Nano 2017, 11, 4731–4744. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical Advanced Oxidation Processes: Today and Tomorrow. A Review. Environ. Sci. Pollut. Res. 2014. [Google Scholar] [CrossRef]
- Cano, A.; Cañizares, P.; Barrera-Díaz, C.; Sáez, C.; Rodrigo, M.A. Use of Conductive-Diamond Electrochemical-Oxidation for the Disinfection of Several Actual Treated Wastewaters. Chem. Eng. J. 2012. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017. [Google Scholar] [CrossRef]
- Bruguera-Casamada, C.; Sirés, I.; Brillas, E.; Araujo, R.M. Effect of Electrogenerated Hydroxyl Radicals, Active Chlorine and Organic Matter on the Electrochemical Inactivation of Pseudomonas Aeruginosa Using BDD and Dimensionally Stable Anodes. Sep. Purif. Technol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Oturan, M.A.; Brillas, E. Electrochemical Advanced Oxidation Processes (EAOPs) for Environmental Applications. Port. Electrochim. Acta 2009. [Google Scholar] [CrossRef]
- Bensalah, N.; Bedoui, A. Enhancing the Performance of Electro-Peroxone by Incorporation of UV Irradiation and BDD Anodes. Environ. Technol. 2017, 38, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Brillas, E. Remediation of Water Pollution Caused by Pharmaceutical Residues Based on Electrochemical Separation and Degradation Technologies: A Review. Environ. Int. 2012. [Google Scholar] [CrossRef]
- Dbira, S.; Bensalah, N.; Zagho, M.M.; Ennahaoui, M.; Bedoui, A. Oxidative Degradation of Tannic Acid in Aqueous Solution by UV/S2O82- and UV/H2O2/Fe2+ Processes: A Comparative Study. Appl. Sci. 2019, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Chair, K.; Bedoui, A.; Bensalah, N.; Sáez, C.; Fernández-Morales, F.J.; Cotillas, S.; Cañizares, P.; Rodrigo, M.A. Treatment of Soil-Washing Effluents Polluted with Herbicide Oxyfluorfen by Combined Biosorption-Electrolysis. Ind. Eng. Chem. Res. 2017, 56. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous Photo-Fenton Processes at near Neutral PH: A Review. Appl. Catal. B Environ. 2017. [CrossRef]
- Ameta, R.; Chohadia, A.K.; Jain, A.; Punjabi, P.B. Fenton and Photo-Fenton Processes. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Academic Press, Elsevier: Amsterdam, Netherlands, 2018. [Google Scholar] [CrossRef]
- Oturan, M.A.; Oturan, N.; Edelahi, M.C.; Podvorica, F.I.; El Kacemi, K. Oxidative Degradation of Herbicide Diuron in Aqueous Medium by Fenton’s Reaction Based Advanced Oxidation Processes. Chem. Eng. J. 2011. [Google Scholar] [CrossRef]
- Marselli, B.; Garcia-Gomez, J.; Michaud, P.; Rodrigo, M.A.; Comninellis, C. Electrogeneration of Hydroxyl Radicals on Boron-Doped Diamond Electrodes. J. Electrochem. Soc. 2003. [Google Scholar] [CrossRef]
- Loos, G.; Scheers, T.; Van Eyck, K.; Van Schepdael, A.; Adams, E.; Van der Bruggen, B.; Cabooter, D.; Dewil, R. Electrochemical Oxidation of Key Pharmaceuticals Using a Boron Doped Diamond Electrode. Sep. Purif. Technol. 2018. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Cotillas, S.; Lacasa, E.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Removal of Pharmaceuticals from the Urine of Polymedicated Patients: A First Approach. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Amstutz, V.; Katsaounis, A.; Kapalka, A.; Comninellis, C.; Udert, K.M. Effects of Carbonate on the Electrolytic Removal of Ammonia and Urea from Urine with Thermally Prepared IrO 2 Electrodes. J. Appl. Electrochem. 2012. [Google Scholar] [CrossRef]
- Scialdone, O.; Randazzo, S.; Galia, A.; Silvestri, G. Electrochemical Oxidation of Organics in Water: Role of Operative Parameters in the Absence and in the Presence of NaCl. Water Res. 2009. [Google Scholar] [CrossRef]
- Karlsson, R.K.B.; Cornell, A. Selectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes. Chem. Rev. 2016. [Google Scholar] [CrossRef]
- Valero, D.; García-García, V.; Expósito, E.; Aldaz, A.; Montiel, V. Electrochemical Treatment of Wastewater from Almond Industry Using DSA-Type Anodes: Direct Connection to a PV Generator. Sep. Purif. Technol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Dbira, S.; Bensalah, N.; Ahmad, M.I.; Bedoui, A. Electrochemical Oxidation/Disinfection of Urine Wastewaters with Different Anode Materials. Materials 2019, 12, 1254. [Google Scholar] [CrossRef] [Green Version]
- Dbira, S.; Bensalah, N.; Cañizares, P.; Rodrigo, M.A.; Bedoui, A. The Electrolytic Treatment of Synthetic Urine Using DSA Electrodes. J. Electroanal. Chem. 2015. [Google Scholar] [CrossRef]
- Dbira, S.; Bensalah, N.; Bedoui, A.; Cañizares, P.; Rodrigo, M.A. Treatment of Synthetic Urine by Electrochemical Oxidation Using Conductive-Diamond Anodes. Environ. Sci. Pollut. Res. 2015. [CrossRef]
- HACH. Chemical Oxygen Demand, Dichromate Method; Hach Company: Lange, Germany, 2014. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. 4500-Cl Chlorine (Residual). Stand. Methods Exam. Water Wastewater 2017. [Google Scholar] [CrossRef]
- Bensalah, N.; Gadri, A.; Cañizares, P.; Sáez, C.; Lobato, J.; Rodrigo, M.A. Electrochemical Oxidation of Hydroquinone, Resorcinol, and Catechol on Boron-Doped Diamond Anodes. Environ. Sci. Technol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Polcaro, A.M.; Vacca, A.; Mascia, M.; Palmas, S.; Rodiguez Ruiz, J. Electrochemical Treatment of Waters with BDD Anodes: Kinetics of the Reactions Involving Chlorides. J. Appl. Electrochem. 2009. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical Oxidation of Organic Pollutants for Wastewater Treatment. Curr. Opin. Electrochem. 2018. [Google Scholar] [CrossRef]
- Trellu, C.; Coetsier, C.; Rouch, J.C.; Esmilaire, R.; Rivallin, M.; Cretin, M.; Causserand, C. Mineralization of Organic Pollutants by Anodic Oxidation Using Reactive Electrochemical Membrane Synthesized from Carbothermal Reduction of TiO2. Water Res. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elahmadi, M.F.; Bensalah, N.; Gadri, A. Treatment of Aqueous Wastes Contaminated with Congo Red Dye by Electrochemical Oxidation and Ozonation Processes. J. Hazard. Mater. 2009. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-Dimensional Electrochemical Process for Wastewater Treatment: A General Review. Chem. Eng. J. 2013. [Google Scholar] [CrossRef]
- Lacasa, E.; Llanos, J.; Cañizares, P.; Rodrigo, M.A. Electrochemical Denitrificacion with Chlorides Using DSA and BDD Anodes. Chem. Eng. J. 2012. [Google Scholar] [CrossRef]
- Gutser, R.; Ebertseder, T.; Weber, A.; Schraml, M.; Schmidhalter, U. Short-Term and Residual Availability of Nitrogen after Long-Term Application of Organic Fertilizers on Arable Land. J. Plant Nutr. Soil Sci. 2005. [Google Scholar] [CrossRef]
- Ranasinghe, E.S.S.; Karunarathne, C.L.S.M.; Beneragama, C.K.; Wijesooriya, B.G.G. Human Urine as a Low Cost and Effective Nitrogen Fertilizer for Bean Production. Procedia Food Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Randall, D.G.; Naidoo, V. Urine: The Liquid Gold of Wastewater. J. Environ. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Haaken, D.; Dittmar, T.; Schmalz, V.; Worch, E. Influence of Operating Conditions and Wastewater-Specific Parameters on the Electrochemical Bulk Disinfection of Biologically Treated Sewage at Boron-Doped Diamond (BDD) Electrodes. Desalin. Water Treat. 2012. [Google Scholar] [CrossRef]
- Bensalah, N.; Dbira, S.; Bedoui, A. The Contribution of Mediated Oxidation Mechanisms in the Electrolytic Degradation of Cyanuric Acid Using Diamond Anodes. J. Environ. Sci. 2015, 45. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Coetsier, C.; Causserand, C.; Groenen Serrano, K. On the Role of Salts for the Treatment of Wastewaters Containing Pharmaceuticals by Electrochemical Oxidation Using a Boron Doped Diamond Anode. Electrochim. Acta 2017. [Google Scholar] [CrossRef] [Green Version]
- Trasatti, S. Electrocatalysis: Understanding the Success of DSA®. Electrochim. Acta 2000. [Google Scholar] [CrossRef]
- Cotillas, S.; Llanos, J.; Cañizares, P.; Mateo, S.; Rodrigo, M.A. Optimization of an Integrated Electrodisinfection/Electrocoagulation Process with Al Bipolar Electrodes for Urban Wastewater Reclamation. Water Res. 2013. [Google Scholar] [CrossRef]
- US EPA, O. Drinking Water Contaminants—Standards and Regulations. Drink. Water Contam. 2013. [Google Scholar]
- Groenen Serrano, K. Indirect Electrochemical Oxidation Using Hydroxyl Radical, Active Chlorine, and Peroxodisulfate. In Electrochemical Water and Wastewater Treatment; Elsevier: Amsterdam, Netherlands, 2018. [Google Scholar] [CrossRef]
- Dionisio, D.; Motheo, A.J.; Sáez, C.; Rodrigo, M.A. Effect of the Electrolyte on the Electrolysis and Photoelectrolysis of Synthetic Methyl Paraben Polluted Wastewater. Sep. Purif. Technol. 2019. [Google Scholar] [CrossRef]
- Sánchez-Carretero, A.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Electrochemical Production of Perchlorates Using Conductive Diamond Electrolyses. Chem. Eng. J. 2011. [Google Scholar] [CrossRef]
- Lan, Y.; Coetsier, C.; Causserand, C.; Serrano, G.K. An experimental and modelling study of the electrochemical oxidation of pharmaceuticals using a boron-doped diamond anode. Chem. Eng. J. 2018. [Google Scholar] [CrossRef] [Green Version]
- Chuang, Y.H.; Lin, A.Y.C.; Wang, X.; Tung, H. The Contribution of Dissolved Organic Nitrogen and Chloramines to Nitrogenous Disinfection Byproduct Formation from Natural Organic Matter. Water Res. 2013. [Google Scholar] [CrossRef]
- Wang, F.; Gao, B.; Ma, D.; Li, R.; Sun, S.; Yue, Q.; Wang, Y.; Li, Q. Effects of Operating Conditions on Trihalomethanes Formation and Speciation during Chloramination in Reclaimed Water. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef]
- Cañizares, P.; Sáez, C.; Sánchez-Carretero, A.; Rodrigo, M.A. Influence of the Characteristics of P-Si BDD Anodes on the Efficiency of Peroxodiphosphate Electrosynthesis Process. Electrochem. Commun. 2008. [Google Scholar] [CrossRef]
- Cañizares, P.; Larrondo, F.; Lobato, J.; Rodrigo, M.A.; Sáez, C. Electrochemical Synthesis of Peroxodiphosphate Using Boron-Doped Diamond Anodes. J. Electrochem. Soc. 2005. [Google Scholar] [CrossRef]





| Component | Parameter |
|---|---|
| Urea (CH4N2O) | 3333.3 mg/L |
| Uric acid (C5H4N4O3) | 50.0 mg/L |
| Creatinine (C4H7N3O) | 166.7 mg/L |
| Potassium (K+) | 1000.0 |
| Sodium (Na+) | 166.7 mg/L |
| Ammonium (NH4+) | 25.0 mg/L |
| Magnesium (Mg2+) | 16.7 mg/L |
| Calcium (Ca2+) | 25.0 mg/L |
| Chloride (Cl−) | 1000.0 mg/L |
| Phosphate (PO43−) | 25.0 mg/L |
| Sulfate (SO42−) | 300.0 mg/L |
| Carbonates (CO3−) | 166.7 mg/L |
| TOC | 750 mg C/L |
| COD | 825 mg O2/L |
| pH | 5.5 |
| Conductivity | 6.5 mS/cm |
| Flow Rate (mL/min) | Chlorates (mg Cl/L) | Perchlorates (mg Cl/L) | ||
|---|---|---|---|---|
| BDD | DSA | BDD | DSA | |
| 800 | 325.4 | 41.1 | 20.5 | 0.1 |
| 1250 | 198.9 | 15.9 | 8.3 | ND |
| 1780 | 84.2 | 5.2 | 1.2 | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensalah, N.; Dbira, S.; Bedoui, A.; Ahmad, M.I. Electrolytic Oxidation as a Sustainable Method to Transform Urine into Nutrients. Processes 2020, 8, 460. https://doi.org/10.3390/pr8040460
Bensalah N, Dbira S, Bedoui A, Ahmad MI. Electrolytic Oxidation as a Sustainable Method to Transform Urine into Nutrients. Processes. 2020; 8(4):460. https://doi.org/10.3390/pr8040460
Chicago/Turabian StyleBensalah, Nasr, Sondos Dbira, Ahmed Bedoui, and Mohammad I. Ahmad. 2020. "Electrolytic Oxidation as a Sustainable Method to Transform Urine into Nutrients" Processes 8, no. 4: 460. https://doi.org/10.3390/pr8040460






