Effect of Gd and Dy Concentrations in Layered Double Hydroxides on Contrast in Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of LDH
2.2. Characterization of Materials
3. Results and Discussion
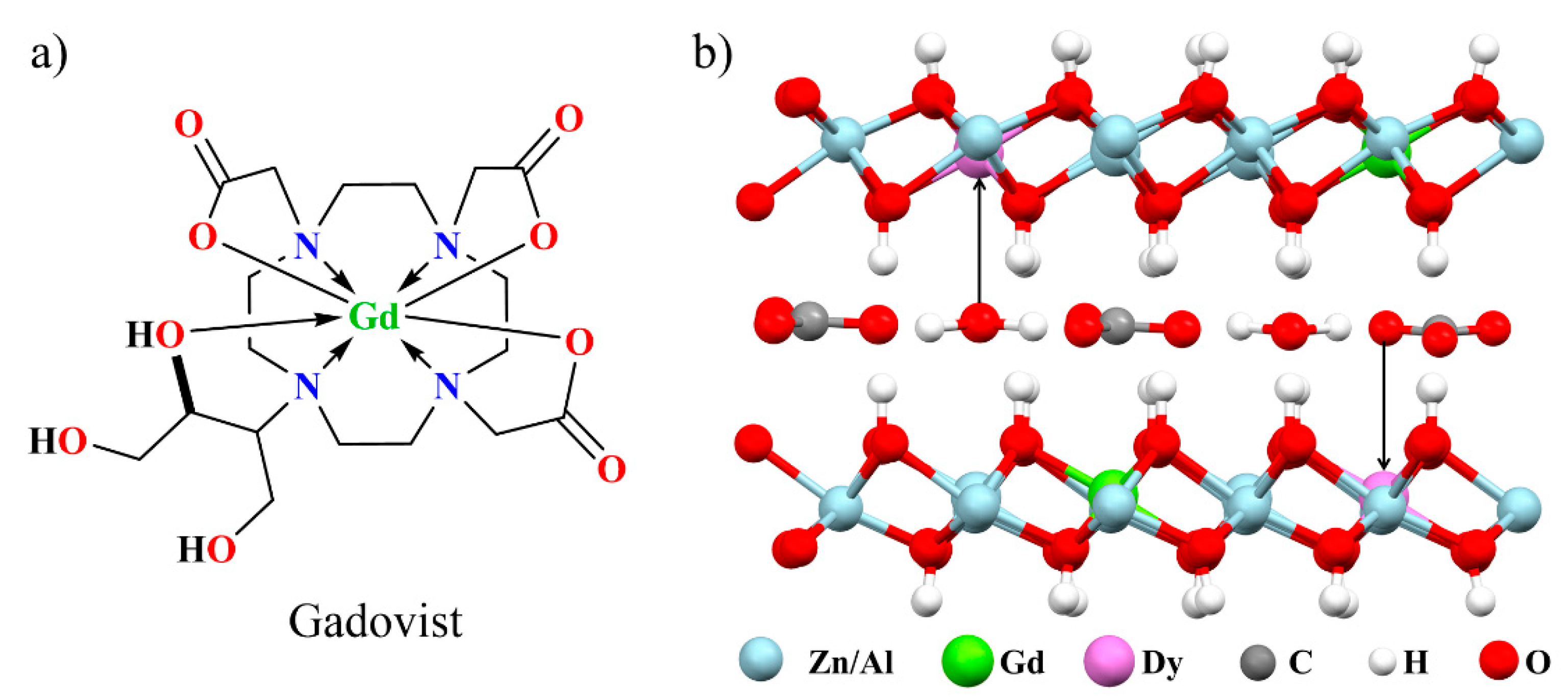
3.1. Characterization of LDH
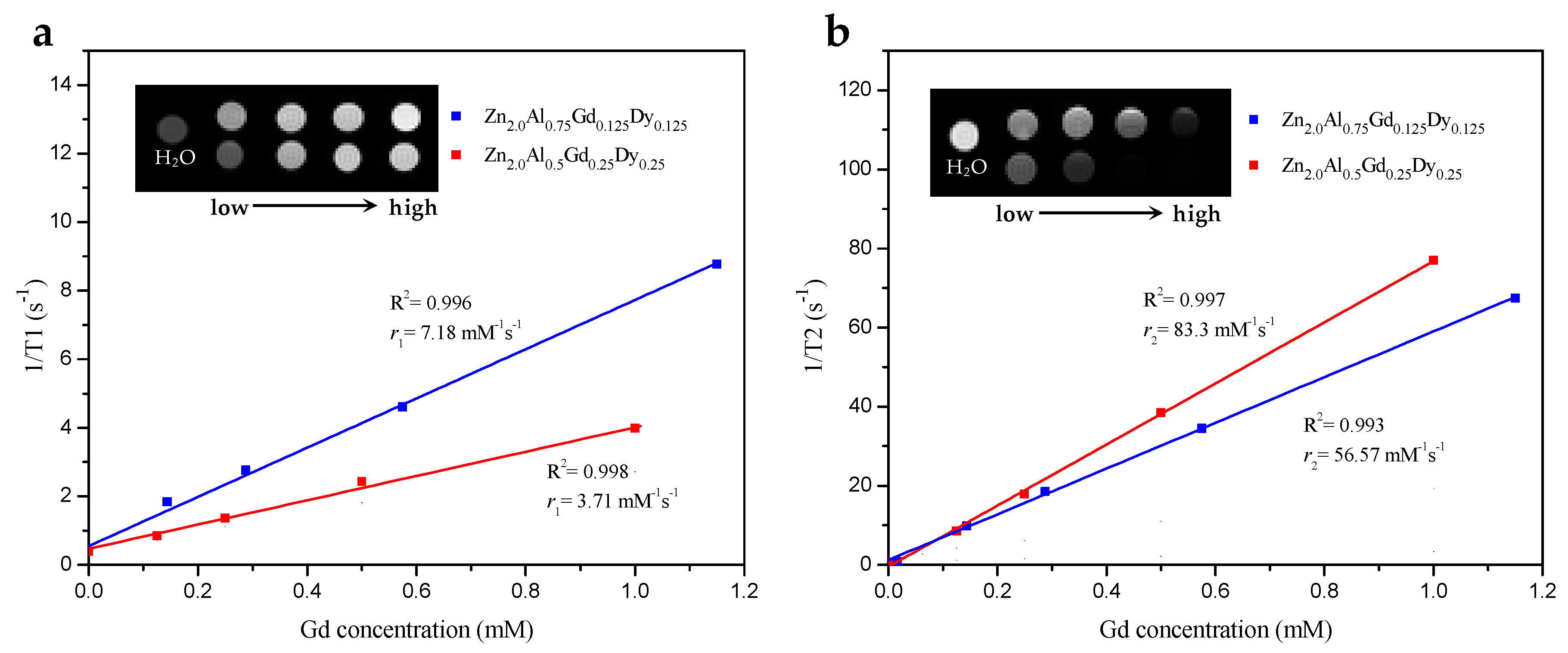
3.2. MRI Contrast Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reith, W. Magnetic Resonance Imaging. In Diagnostic and Interventional Radiology; Vogl, T.J., Reith, W., Rummeny, E.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 31–38. [Google Scholar] [CrossRef]
- Costa, S.J.; Soria, J.J.A. Resonancia Magnética Dirigida a Técnicos Superiores En Imagen Para El Diagnóstico; Elsevier: Barcelona, España, 2015. [Google Scholar]
- Zlatkin, M.B. MRI of the Shoulder: Diagnosis, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Merbach, A.; Helm, L.; Tóth, É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, Z.R. Gadolinium-Based Contrast Agents for Magnetic Resonance Cancer Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norek, M.; Peters, J.A. MRI Contrast Agents Based on Dysprosium or Holmium. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Rocklage, S.M.; Watson, A.D. Chelates of Gadolinium and Dysprosium as Contrast Agents for MR Imaging. J. Magn. Reson. Imaging 1993, 3, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bottrill, M.; Kwok, L.; Long, N.J. Lanthanides in Magnetic Resonance Imaging. Chem. Soc. Rev. 2006, 35, 557–571. [Google Scholar] [CrossRef]
- Sánchez, J.C.; Pacheco, M.F.P.; Cano, M.E.; Nava, A.K.; Briones, T.A.L.; Carbajal, A.G.G. Folate- and Glucuronate-Functionalization of Layered Double Hydroxides Containing Dysprosium and Gadolinium and the Effect on Oxidative Stress in Rat Liver Mitochondria. Heliyon 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xing, H.; Zhang, S.; Ren, Q.; Pan, L.; Zhang, K.; Bu, W.; Zheng, X.; Zhou, L.; Peng, W.; et al. A Gd-Doped Mg-Al-LDH/Au Nanocomposite for CT/MR Bimodal Imagings and Simultaneous Drug Delivery. Biomaterials 2013, 34, 3390–3401. [Google Scholar] [CrossRef]
- Guan, S.; Liang, R.; Li, C.; Wei, M. A Supramolecular Material for Dual-Modal Imaging and Targeted Cancer Therapy. Talanta 2017, 165, 297–303. [Google Scholar] [CrossRef]
- Guan, S.; Weng, Y.; Li, M.; Liang, R.; Sun, C.; Qu, X.; Zhou, S. An NIR-Sensitive Layered Supramolecular Nanovehicle for Combined Dual-Modal Imaging and Synergistic Therapy. Nanoscale 2017, 9, 10367–10374. [Google Scholar] [CrossRef] [Green Version]
- Vargas, D.R.M.; Oviedo, M.J.; Da Silva Lisboa, F.; Wypych, F.; Hirata, G.A.; Arizaga, G.G.C. Phosphor Dysprosium-Doped Layered Double Hydroxides Exchanged with Different Organic Functional Groups. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Viruete, A.; Carbajal, G.G.A.; Hernández Gutiérrez, R.; Oaxaca Camacho, A.R.; Arratia-Quijada, J. Passive Targeting Effect of Dy-Doped LDH Nanoparticles Hybridized with Folic Acid and Gallic Acid on HEK293 Human Kidney Cells and HT29 Human Cells. J. Nanopart. Res. 2018, 20, 1–10. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Nava, K.; Puebla, A.M.; Carbajal, G.G. Passive and active targeting strategies in hybrid layered double hydroxides nanoparticles for tumor bioimaging and therapy. Appl. Clay Sci. 2019, 181, 105214. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Hou, X.; Bish, D.L.; Wang, S.L.; Johnston, C.T.; Kirkpatrick, R.J. Hydration, expansion, structure, and dynamics of layered double hydroxides. Am. Mineral. 2003, 88, 167–179. [Google Scholar] [CrossRef]
- Posati, T.; Costantino, F.; Latterini, L.; Nocchetti, M.; Tarpani, L. New Insights on the Incorporation of Lanthanide Ions Into. Inorg. Chem. 2012, 51, 13229–13236. [Google Scholar] [CrossRef]
- Vicente, P.; Pérez-Bernal, M.E.; Ruano-Casero, R.J.; Ananias, D.; Almeida Paz, F.A.; Rocha, J.; Rives, V. Luminescence Properties of Lanthanide-Containing Layered Double Hydroxides. Microporous Mesoporous Mater. 2016, 226, 209–220. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. FT-Raman and FT-IR spectroscopic study of synthetic Mg/Zn/Al-hydrotalcites. J. Raman Spectrosc. 2004, 35, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-like Compounds and Their Structures and Physico-Chemical Properties-i: The Systems Mg2+-Al3+-NO3 -, Mg2+-Al3+-Cl-, Mg2+-Al3+-ClO4 -, Ni2+-Al3+-Cl- and Zn2+-Al3+-Cl-. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared Spectroscopic Identification of Species Arising from Reactive Adsorption of Carbon Oxides on Metal Oxide Surfaces. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. The Principles of Distribution of Chemical Elements in Minerals and Rocks. J. Chem. Soc. 1937, 655–673. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii in Halides and Chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Thermogravimetric Analysis of Selected Layered Double Hydroxides. J. Therm. Anal. Calorim. 2013, 112, 649–657. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Xiao, H.; Zhang, L.; Qin, Y.; Liu, D.; Hou, W.; Du, N. Synthesis and Thermal Properties of ZnAl Layered Double Hydroxide by Urea Hydrolysis. Powder Technol. 2014, 253, 41–45. [Google Scholar] [CrossRef]
- Perez, J.R.; Mul, G.; Kapteijn, F.; Moulijn, J.A. A spectroscopic study of the effect of the trivalent cation on the thermal decomposition behaviour of Co-based hydrotalcites. J. Mater. Chem. 2001, 11, 2529–2536. [Google Scholar] [CrossRef]
- Fowler, R.A.; Fossheim, S.L.; Mestas, J.L.; Ngo, J.; Canet-Soulas, E.; Lafon, C. Non-Invasive Magnetic Resonance Imaging Follow-up of Sono-Sensitive Liposome Tumor Delivery and Controlled Release After High-Intensity Focused Ultrasound. Ultrasound Med. Biol. 2013, 39, 2342–2350. [Google Scholar] [CrossRef]
- Haraldseth, O.; Jones, R.A.; Müller, T.B.; Fahlvik, A.K.; Øksendal, A.N. Comparison of Dysprosium DTPA BMA and Superparamagnetic Iron Oxide Particles as Susceptibility Contrast Agents for Perfusion Imaging of Regional Cerebral Ischemia in the Rat. J. Magn. Reson. Imaging 1996, 6, 714–717. [Google Scholar] [CrossRef]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on Gadolinium Chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Caravan, P.; Ellison, J.J.; Mcmurry, T.J.; Lauffer, R.B. Gadolinium (III) Chelates as MRI Contrast Agents: Structure, Dynamics and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]



| Sample | Metal Salts, g (mmol) | |||
|---|---|---|---|---|
| Zn(NO3)2·6H2O | Al(NO3)3·9H2O | Gd(NO3)3·xH2O | Dy(NO3)3·xH2O | |
| Zn2.0Al1.0 | 1.190 (4) | 0.720 (1.92) | - | - |
| Zn2.0Al0.75Gd0.125Dy0.125 | 1.190 (4) | 0.562 (1.50) | 0.116 (0.25) | 0.087 (0.25) |
| Zn2.0Al0.5Gd0.25Dy0.25 | 0.595 (2) | 0.188 (0.50) | 0.113 (0.25) | 0.102 (0.29) |
| Zn2.0Al0.25Gd0.37Dy0.37 | 0.595 (2) | 0.094 (0.25) | 0.170 (0.37) | 0.141 (0.40) |
| Zn2.0Gd0.5Dy0.5 | 0.595 (2) | - | 0.225 (0.50) | 0.174 (0.50) |
| Sample | a (Å) | c (Å) | d003 |
|---|---|---|---|
| Zn2.0Al1.0 | 3.095 | 23.1 | 7.70 |
| Zn2.0Al0.75Gd0.125Dy0.125 | 3.087 | 22.53 | 7.51 |
| Zn2.0Al0.5Gd0.25Dy0.25 | 3.096 | 23.01 | 7.67 |
| Sample | ν3(CO32-) cm−1, (intensity, %) | Δν3 |
|---|---|---|
| Zn2.0Al1.0 | 1356 | - |
| Zn2.0Al0.75Gd0.125Dy0.125 | 1359 (86), 1505 (40) | 146 |
| Zn2.0Al0.5Gd0.25Dy0.25 | 1363 (99), 1502 (63) | 139 |
| Sample | Molar Fraction 1 (Nominal) | Molar Fraction 1 (Experimental) | ||||||
|---|---|---|---|---|---|---|---|---|
| Zn | Al | Gd | Dy | Zn | Al | Gd | Dy | |
| Zn2.0Al1.0 | 0.67 | 0.33 | - | - | 0.57 | 0.43 | - | - |
| Zn2.0Al0.75Gd0.125Dy0.125 | 0.67 | 0.25 | 0.04 | 0.04 | 0.68 | 0.25 | 0.04 | 0.03 |
| Zn2.0Al0.5Gd0.25Dy0.25 | 0.67 | 0.17 | 0.08 | 0.08 | 0.41 | 0.28 | 0.17 | 0.15 |
| Sample | Temperature Range (°C) and Mass Loss (%) | |||
|---|---|---|---|---|
| 1st Loss | 2nd Loss | 3rd Loss | 4th Loss | |
| Zn2.0Al1.0 | 25–100 (4.4) | 100–200 (12.8) | 200–450 (13.7) | 450–850 (2.6) |
| Zn2.0Al0.75Gd0.125Dy0.125 | 25–80 (2.6) | 80–210 (16.1) | 210–560 (19.4) | 560–850 (4.7) |
| Zn2.0Al0.5Gd0.25Dy0.25 | 25–100 (4.3) | 100–180 (10.2) | 180–440 (11.7) | 440–850 (4.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava Andrade, K.; Carbajal Arízaga, G.G.; Rivera Mayorga, J.A. Effect of Gd and Dy Concentrations in Layered Double Hydroxides on Contrast in Magnetic Resonance Imaging. Processes 2020, 8, 462. https://doi.org/10.3390/pr8040462
Nava Andrade K, Carbajal Arízaga GG, Rivera Mayorga JA. Effect of Gd and Dy Concentrations in Layered Double Hydroxides on Contrast in Magnetic Resonance Imaging. Processes. 2020; 8(4):462. https://doi.org/10.3390/pr8040462
Chicago/Turabian StyleNava Andrade, Karina, Gregorio Guadalupe Carbajal Arízaga, and José Antonio Rivera Mayorga. 2020. "Effect of Gd and Dy Concentrations in Layered Double Hydroxides on Contrast in Magnetic Resonance Imaging" Processes 8, no. 4: 462. https://doi.org/10.3390/pr8040462





