Applications of Electrolyzed Water as a Sanitizer in the Food and Animal-By Products Industry
Abstract
:1. Introduction
2. Electrolyzed Water
3. Pork
4. Fish
4.1. Salmon
4.2. Tuna Fish
4.3. Catfish
4.4. Tilapia
4.5. Other Types of Fish
4.6. Shrimp
4.7. Bivalve Mollusk
5. Chicken
6. Egg
7. Cattle Products
7.1. Beef
7.2. Milk
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Department of Economic and Social Affairs, UN. World Population Prospects 2019. Volume II: Demographic Profiles; United Nations: New York, NY, USA, 2019; Volume 2, ISBN 9789211483284. [Google Scholar]
- Mylius, M.; Dreesman, J.; Pulz, M.; Pallasch, G.; Beyrer, K.; Claußen, K.; Allerberger, F.; Fruth, A.; Lang, C.; Prager, R.; et al. Shiga toxin-producing Escherichia coli O103:H2 outbreak in Germany after school trip to Austria due to raw cow milk, 2017—The important role of international collaboration for outbreak investigations. Int. J. Med. Microbiol. 2018, 308, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Morton, V.K.; Kearney, A.; Coleman, S.; Viswanathan, M.; Chau, K.; Orr, A.; Hexemer, A. Outbreaks of Salmonella illness associated with frozen raw breaded chicken products in Canada, 2015–2019. Epidemiol. Infect. 2019, 147, 2019–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesema, I.; de Jong, A.; Hofhuis, A.; Heck, M.; van den Kerkhof, H.; de Jonge, R.; Hameryck, D.; Nagel, K.; van Vilsteren, G.; van Beek, P.; et al. Large outbreak of Salmonella Tompson related to smoked salmon in the Netherlands, August to December 2012. Eurosurveillance 2014, 19, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchatchouang, C.K.; Fri, J.; De Santi, M.; Ateba, C.N. Listeriosis Outbreak in South Africa: A Comparative Analysis with Previously Reported Cases Worldwide. Microorganisms 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabal, A.; Allerberger, F.; Huhulescu, S.; Kornschober, C.; Springer, B.; Schlagenhaufen, C.; Wassermann-Neuhold, M.; Fötschl, H.; Pless, P.; Krause, R.; et al. Listeriosis outbreak likely due to contaminated liver pâté consumed in a tavern, Austria, December 2018. Euro Surveill. 2019, 24, 1–6. [Google Scholar] [CrossRef]
- Sinulingga, T.S.; Aziz, S.A.; Bitrus, A.A.; Zunita, Z.; Abu, J. Occurrence of Campylobacter species from broiler chickens and chicken meat in Malaysia. Trop. Anim. Health Prod. 2019, 52, 151–157. [Google Scholar] [CrossRef]
- Lung, H.M.; Cheng, Y.C.; Chang, Y.H.; Huang, H.W.; Yang, B.B.; Wang, C.Y. Microbial decontamination of food by electron beam irradiation. Trends Food Sci. Technol. 2015, 44, 66–78. [Google Scholar] [CrossRef]
- Deng, L.Z.; Mujumdar, A.S.; Pan, Z.; Vidyarthi, S.K.; Xu, J.; Zielinska, M.; Xiao, H.W. Emerging chemical and physical disinfection technologies of fruits and vegetables: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 1–28. [Google Scholar] [CrossRef]
- Len, S.V.; Hung, Y.C.; Erickson, M.; Kim, C. Ultraviolet spectrophotometric characterization and bactericidal properties of electrolyzed oxidizing water as influenced by amperage and pH. J. Food Prot. 2000, 63, 1534–1537. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirakata, Y.; Kaku, M.; Yoshida, R.; Takemura, H.; Mizukane, R.; Ishida, K.; Tomono, K.; Koga, H.; Kohno, S.; et al. Antimicrobial activity of superoxidized water. J. Hosp. Infect. 1996, 34, 43–49. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.H. Hurdle technology: A novel approach for enhanced food quality and safety—A review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Morita, C.; Sano, K.; Morimatsu, S.; Kiura, H.; Goto, T.; Kohno, T.; Hong, W.; Miyoshi, H.; Iwasawa, A.; Nakamura, Y.; et al. Disinfection potential of electrolyzed solutions containing sodium chloride at low concentrations. J. Virol. Methods 2000, 85, 163–174. [Google Scholar] [CrossRef]
- Tagawa, M.; Yamaguchi, T.; Osamu, Y.; Matsutani, S.; Maeda, T.; Saisho, H. Inactivation of a hepadnavirus by electrolysed acid water. J. Antimicrob. Chemother. 2000, 46, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Espinosa, D.; Cervantes-Aguilar, F.J.; Río-García, J.C.; Villarreal-Barajas, T.; Vázquez-Durán, A.; Méndez-Albores, A. Ameliorative effects of neutral electrolyzed water on growth performance, biochemical constituents, and histopathological changes in Turkey poults during aflatoxicosis. Toxins 2017, 9, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, S.; Zhang, F.; Liu, S.; Yu, C.; Guan, D.; Pan, C. Removal of aflatoxin B1 in edible plant oils by oscillating treatment with alkaline electrolysed water. Food Chem. 2013, 141, 3118–3123. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Itakura, J.; Watanabe, M.; Ohta, M.; Sato, Y.; Yamaya, Y. Inactivation of staphylococcal enterotoxin-A with an electrolyzed anodic solution. J. Agric. Food Chem. 2002, 50, 230–234. [Google Scholar] [CrossRef]
- Al-Haq, M.I.; Sugiyama, J.; Isobe, S. Applications of Electrolyzed Water in Agriculture & Food Industries. Food Sci. Technol. Res. 2005, 1, 135–150. [Google Scholar]
- Ngnitcho, P.F.K.; Khan, I.; Tango, C.N.; Hussain, M.S.; Oh, D.H. Inactivation of bacterial pathogens on lettuce, sprouts, and spinach using hurdle technology. Innov. Food Sci. Emerg. Technol. 2017, 43, 68–76. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, I.; Oh, D.H. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef] [Green Version]
- Hati, S.; Mandal, S.; Minz, P.S.; Vij, S.; Khetra, Y.; Singh, B.P.; Yadav, D. Electrolyzed Oxidized Water (EOW): Non-Thermal Approach for Decontamination of Food Borne Microorganisms in Food Industry. Food Nutr. Sci. 2012, 3, 760–768. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Feirtag, J.; Diez-Gonzalez, F. Sanitizing effectiveness of commercial “active water” technologies on Escherichia coli O157:H7, Salmonella enterica and Listeria monocytogenes. Food Control 2013, 33, 232–238. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Yang, H. Efficacy of low concentration neutralised electrolysed water and ultrasound combination for inactivating Escherichia coli ATCC 25922, Pichia pastoris GS115 and Aureobasidium pullulans 2012 on stainless steel coupons. Food Control 2017, 73, 889–899. [Google Scholar] [CrossRef]
- Cui, X.; Shang, Y.; Shi, Z.; Xin, H.; Cao, W. Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions. J. Food Eng. 2009, 91, 582–586. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Chen, K.K.; Tajima, K.; Kakigawa, H.; Kozono, Y. Durability of Bactericidal Activity in Electrolyzed Neutral Water by Storage. Dent. Mater. J. 2002, 21, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gudiño, J.; Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Paez-Esquiliano, D.; Martinez-Vidal, S.; Andrade-Esquivel, E.; Cano-Buendia, J.A. Analysis of Neutral Electrolyzed Water anti-bacterial activity on contaminated eggshells with Salmonella enterica or Escherichia coli. Int. J. Food Microbiol. 2020, 320, 108538. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Paez-Esquiliano, D.; Andrade-Esquivel, E.; Cano-Buendia, J.A. The effect of neutral electrolyzed water as a disinfectant of eggshells artificially contaminated with Listeria monocytogenes. Food Sci. Nutr. 2019, 7, 2252–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrich, J.M.; Gilbaugh, J.H.; Callahan, K.B.; Hurst, J.K. Effects of the putative neutrophil-generated toxin, hypochlorous acid, on membrane permeability and transport systems of Escherichia coli. J. Clin. Invest. 1986, 78, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Hurst, J.K.; Barrette, W.C.; Michel, B.R.; Rosen, H. Hypochlorous acid and myeloperoxidase-catalyzed oxidation of iron-sulfur clusters in bacterial respiratory dehydrogenases. Eur. J. Biochem. 1991, 202, 1275–1282. [Google Scholar] [CrossRef]
- Len, S.V.; Hung, Y.C.; Chung, D.; Anderson, J.L.; Erickson, M.C.; Morita, K. Effects of storage conditions and pH on chlorine loss in electrolyzed oxidizing (EO) water. J. Agric. Food Chem. 2002, 50, 209–212. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hung, Y.C.; Hsu, S.Y.; Huang, Y.W.; Hwang, D.F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The generation and inactivation mechanism of oxidation-reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007, 78, 1326–1332. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Jin, Y.G.; Oh, D.H. Combined Effects of Alkaline Electrolyzed Water and Citric Acid with Mild Heat to Control Microorganisms on Cabbage. J. Food Sci. 2010, 75, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, J.; Lim, Z.Y.; Aggarwal, A.; Yang, H.; Wang, S. Evaluation of the metabolic response of Escherichia coli to electrolysed water by 1H NMR spectroscopy. LWT Food Sci. Technol. 2017, 79, 428–436. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, J.; Lim, Z.Y.; Lai, S.; Lee, N.; Yang, H. Metabolite profiling of Listeria innocua for unravelling the inactivation mechanism of electrolysed water by nuclear magnetic resonance spectroscopy. Int. J. Food Microbiol. 2018, 271, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Chan, J.Z.Y. Development of Portable Flow-Through Electrochemical Sanitizing Unit to Generate Near Neutral Electrolyzed Water. J. Food Sci. 2018, 83, 780–790. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Oliveira, M.; Alegre, I.; Viñas, I. Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally-processed vegetables. Int. J. Food Microbiol. 2008, 123, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.E.; Wang, J.; Oh, D.H. Synergistic effect of low concentration electrolyzed water and calcium lactate to ensure microbial safety, shelf life and sensory quality of fresh pork. Food Control 2013, 30, 176–183. [Google Scholar] [CrossRef]
- Tyszkiewicz, I.; Kłossowska, B.M.; Wieczorek, U.; Jakubiec-Puka, A. Mechanical Tenderisation of Pork Meat: Protein and Water Release due to Tissue Damage. J. Sci. Food Agric. 1997, 73, 179–185. [Google Scholar] [CrossRef]
- Fabrizio, K.A.; Cutter, C.N. Comparison of electrolyzed oxidizing water with other antimicrobial interventions to reduce pathogens on fresh pork. Meat Sci. 2004, 68, 463–468. [Google Scholar] [CrossRef]
- Fabrizio, K.A.; Cutter, C.N. Application of electrolyzed oxidizing water to reduce Listeria monocytogenes on ready-to-eat meats. Meat Sci. 2005, 71, 327–333. [Google Scholar] [CrossRef]
- Mansur, A.R.; Oh, D.H. Combined effects of thermosonication and slightly acidic electrolyzed water on the microbial quality and shelf life extension of fresh-cut kale during refrigeration storage. Food Microbiol. 2015, 51, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Athayde, D.R.; Flores, D.R.M.; da Silva, J.S.; Genro, A.L.G.; Silva, M.S.; Klein, B.; Mello, R.; Campagnol, P.C.B.; Wagner, R.; de Menezes, C.R.; et al. Application of electrolyzed water for improving pork meat quality. Food Res. Int. 2017, 100, 757–763. [Google Scholar] [CrossRef]
- Rigdon, M.; Hung, Y.C.; Stelzleni, A.M. Evaluation of alkaline electrolyzed water to replace traditional phosphate enhancement solutions: Effects on water holding capacity, tenderness, and sensory characteristics. Meat Sci. 2017, 123, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brychcy, E.; Malik, M.; Drozdzewski, P.; Ulbin-Figlewicz, N.; Jarmoluk, A. Low-concentrated acidic electrolysed water treatment of pork: Inactivation of surface microbiota and changes in product quality. Int. J. Food Sci. Technol. 2015, 50, 2340–2350. [Google Scholar] [CrossRef]
- Belton, B.; Bush, S.R.; Little, D.C. Not just for the wealthy: Rethinking farmed fi sh consumption in the Global South. Glob. Food Sec. 2018, 16, 85–92. [Google Scholar] [CrossRef]
- Ozer, N.P.; Demirci, A. Electrolyzed oxidizing water treatment for decontamination of raw salmon inoculated with Escherichia coli O157:H7 and Listeria monocytogenes Scott A and response surface modeling. J. Food Eng. 2006, 72, 234–241. [Google Scholar] [CrossRef]
- McCarthy, S.; Burkhardt, W. Efficacy of electrolyzed oxidizing water against Listeria monocytogenes and Morganella morganii on conveyor belt and raw fish surfaces. Food Control 2012, 24, 214–219. [Google Scholar] [CrossRef]
- Miks-Krajnik, M.; Feng, L.X.J.; Bang, W.S.; Yuk, H.-G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolyzed water, ultraviolet light or/and ultrasounds. Food Control 2017, 74, 54–60. [Google Scholar] [CrossRef]
- Ovissipour, M.; Shiroodi, S.G.; Rasco, B.; Tang, J.; Sablani, S.S. Electrolyzed water and mild-thermal processing of Atlantic salmon (Salmo salar): Reduction of Listeria monocytogenes and changes in protein structure. Int. J. Food Microbiol. 2018, 276, 10–19. [Google Scholar] [CrossRef]
- Ghorban Shiroodi, S.; Ovissipour, M.; Ross, C.F.; Rasco, B.A. Efficacy of electrolyzed oxidizing water as a pretreatment method for reducing Listeria monocytogenes contamination in cold-smoked Atlantic salmon (Salmo salar). Food Control 2016, 60, 401–407. [Google Scholar] [CrossRef]
- Phuvasate, S.; Su, Y. Effects of electrolyzed oxidizing water and ice treatments on reducing histamine-producing bacteria on fish skin and food contact surface. Food Control 2010, 21, 286–291. [Google Scholar] [CrossRef]
- Huang, Y.R.; Shiau, C.Y.; Hung, Y.C.; Hwang, D.F. Change of hygienic quality and freshness in tuna treated with electrolyzed water and carbon monoxide gas during refrigerated and frozen storage. J. Food Sci. 2006, 71, M127–M133. [Google Scholar] [CrossRef]
- Abou-Taleb, M.; Kawai, Y. Shelf life of semifried tuna slices coated with essential oil compounds after treatment with anodic electrolyzed NaCl solution. J. Food Prot. 2008, 71, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Rajkowski, K.T.; Sommers, C.H. Effect of anolyte on background microflora, salmonella, and listeria monocytogenes on catfish fillets. J. Food Prot. 2012, 75, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-M.; Hung, Y.-C.; Deng, S.-G. Effect of partial replacement of polyphosphate with alkaline electrolyzed water (AEW) on the quality of catfish fillets. Food Control 2020, 112, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hsieh, H.S.; Lin, S.Y.; Lin, S.J.; Hung, Y.C.; Hwang, D.F. Application of electrolyzed oxidizing water on the reduction of bacterial contamination for seafood. Food Control 2006, 17, 987–993. [Google Scholar] [CrossRef]
- Feliciano, L.; Lee, J.; Lopes, J.A.; Pascall, M.A. Efficacy of Sanitized Ice in Reducing Bacterial Load on Fish Fillet and in the Water Collected from the Melted Ice. J. Food Sci. 2010, 75, 231–238. [Google Scholar] [CrossRef]
- Mahmoud, B.S.M.; Yamazaki, K.; Miyashita, K.; Il-Shik, S.; Dong-Suk, C.; Suzuki, T. Decontamination effect of electrolysed NaCl solutions on carp. Lett. Appl. Microbiol. 2004, 39, 169–173. [Google Scholar] [CrossRef]
- Kim, W.T.; Lim, Y.S.; Shin, I.S.; Park, H.; Chung, D.; Suzuki, T. Use of electrolyzed water ice for preserving freshness of pacific saury (Cololabis saira). J. Food Prot. 2006, 69, 2199–2204. [Google Scholar] [CrossRef]
- Al-holy, M.A.; Rasco, B.A. The bactericidal activity of acidic electrolyzed oxidizing water against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on raw fish, chicken and beef surfaces. Food Control 2015, 54, 317–321. [Google Scholar] [CrossRef]
- Xu, G.; Tang, X.; Tang, S.; You, H.; Shi, H.; Gu, R. Combined effect of electrolyzed oxidizing water and chitosan on the microbiological, physicochemical, and sensory attributes of American shad (Alosa sapidissima) during refrigerated storage. Food Control 2014, 46, 397–402. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Deng, S.; Huang, Y. Effect of Combined Pretreatment with Slightly Acidic Electrolyzed Water and Botanic Biopreservative on Quality and Shelf Life of Bombay Duck (Harpadon nehereus). J. Food Qual. 2016, 39, 116–125. [Google Scholar] [CrossRef]
- Wang Jing, J.; Sun, W.S.; Jin, M.T.; Liu, H.Q.; Zhang, W.; Sun, X.H.; Pan, Y.J.; Zhao, Y. Fate of Vibrio parahaemolyticus on shrimp after acidic electrolyzed water treatment. Int. J. Food Microbiol. 2014, 179, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J.J.; Sun, X.H.; Pan, Y.J.; Zhao, Y. Preliminary mechanism of acidic electrolyzed water ice on improving the quality and safety of shrimp. Food Chem. 2015, 176, 333–341. [Google Scholar] [CrossRef]
- Ren, T.; Su, Y.C. Effects of electrolyzed oxidizing water treatment on reducing Vibrio parahaemolyticus and Vibrio vulnificus in raw oysters. J. Food Prot. 2006, 69, 1829–1834. [Google Scholar] [CrossRef]
- Al-Qadiri, H.M.; Al-Holy, M.A.; Shiroodi, S.G.; Ovissipour, M.; Govindan, B.N.; Al-Alami, N.; Sablani, S.S.; Rasco, B. Effect of acidic electrolyzed water-induced bacterial inhibition and injury in live clam (Venerupis philippinarum) and mussel (Mytilus edulis). Int. J. Food Microbiol. 2016, 231, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.M.E.; Park, J.; Song, K.B.; Al-Harbi, N.A.; Oh, D.H. Effects of slightly acidic low concentration electrolyzed water on microbiological, physicochemical, and sensory quality of fresh chicken breast meat. J. Food Sci. 2012, 77, M35–M41. [Google Scholar] [CrossRef]
- Shimamura, Y.; Shinke, M.; Hiraishi, M.; Tsuchiya, Y.; Masuda, S. The application of alkaline and acidic electrolyzed water in the sterilization of chicken breasts and beef liver. Food Sci. Nutr. 2016, 4, 431–440. [Google Scholar] [CrossRef]
- Cichoski, A.J.; Flores, D.R.M.; De Menezes, C.R.; Jacob-Lopes, E.; Zepka, L.Q.; Wagner, R.; Barin, J.S.; de Moraes Flores, É.M.; da Cruz Fernandes, M.; Campagnol, P.C.B. Ultrasound and slightly acid electrolyzed water application: An efficient combination to reduce the bacterial counts of chicken breast during pre-chilling. Int. J. Food Microbiol. 2019, 301, 27–33. [Google Scholar] [CrossRef]
- Fabrizio, K.A.; Sharma, R.R.; Demirci, A.; Cutter, C.N. Comparison of electrolyzed oxidizing water with various antimicrobial interventions to reduce Salmonella species on poultry. Poult. Sci. 2002, 81, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Wang, H.; Xue, S.; Li, M.; Xu, X. Application of disinfectant sprays after chilling to reduce the initial microbial load and extend the shelf-life of chilled chicken carcasses. Food Control 2017, 75, 70–77. [Google Scholar] [CrossRef]
- Wang, H.; Qi, J.; Duan, D.; Dong, Y.; Xu, X.; Zhou, G. Combination of a novel designed spray cabinet and electrolyzed water to reduce microorganisms on chicken carcasses. Food Control 2018, 86, 200–206. [Google Scholar] [CrossRef]
- Rasschaert, G.; Piessens, V.; Scheldeman, P.; Leleu, S.; Stals, A.; Herman, L.; Heyndrickx, M.; Messens, W. Efficacy of electrolyzed oxidizing water and lactic acid on the reduction of Campylobacter on naturally contaminated broiler carcasses during processing. Poult. Sci. 2013, 92, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hung, Y.-C.; Brackett, R.E. Antimicrobial effect of electrolyzed water for inactivating Campylobacter jejuni during poultry washing. Int. J. Food Microbiol. 2002, 72, 77–83. [Google Scholar] [CrossRef]
- Russell, S.M. The effect of electrolyzed oxidative water applied using electrostatic spraying on pathogenic and indicator bacteria on the surface of eggs. Poult. Sci. 2003, 82, 158–162. [Google Scholar] [CrossRef]
- Zang, Y.T.; Bing, S.; Li, Y.J.; Shu, D.Q.; Huang, A.M.; Wu, H.X.; Lan, L.T.; Wu, H.D. Efficacy of slightly acidic electrolyzed water on the microbial safety and shelf life of shelled eggs. Poult. Sci. 2019, 98, 5932–5939. [Google Scholar] [CrossRef]
- Bialka, K.; Demirci, A.; Knabel, S.; Patterson, P.; Puri, V. Efficacy of electrolyzed oxidizing water for the microbial safety and quality of eggs. Poult. Sci. 2004, 83, 2071–2078. [Google Scholar] [CrossRef]
- Fasenko, G.M.; O’Dea Christopher, E.E.; McMullen, L.M. Spraying hatching eggs with electrolyzed oxidizing water reduces eggshell microbial load without compromising broiler production parameters. Poult. Sci. 2009, 88, 1121–1127. [Google Scholar] [CrossRef]
- Killinger, K.M.; Kannan, A.; Bary, A.I.; Cogger, C.G. Validation of a 2 percent lactic acid antimicrobial rinse for mobile poultry slaughter operations. J. Food Prot. 2010, 73, 2079–2083. [Google Scholar] [CrossRef]
- Veasey, S.; Muriana, P. Evaluation of Electrolytically-Generated Hypochlorous Acid (‘Electrolyzed Water’) for Sanitation of Meat and Meat-Contact Surfaces. Foods 2016, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Duan, D.; Wu, Z.; Xue, S.; Xu, X.; Zhou, G. Primary concerns regarding the application of electrolyzed water in the meat industry. Food Control 2019, 95, 50–56. [Google Scholar] [CrossRef]
- Ding, T.; Rahman, S.M.E.; Purev, U.; Oh, D.H. Modelling of Escherichia coli O157:H7 growth at various storage temperatures on beef treated with electrolyzed oxidizing water. J. Food Eng. 2010, 97, 497–503. [Google Scholar] [CrossRef]
- Liao, X.; Xiang, Q.; Cullen, P.J.; Su, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Plasma-activated water (PAW) and slightly acidic electrolyzed water (SAEW) as beef thawing media for enhancing microbiological safety. LWT 2020, 117, 108649. [Google Scholar] [CrossRef]
- Sheng, X.; Shu, D.; Tang, X.; Zang, Y. Effects of slightly acidic electrolyzed water on the microbial quality and shelf life extension of beef during refrigeration. Food Sci. Nutr. 2018, 6, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Ferrocino, I.; Cavallero, M.C.; Riva, S.; Giordano, M.; Cocolin, L. Potentially active spoilage bacteria community during the storage of vacuum packaged beefsteaks treated with aqueous ozone and electrolyzed water. Int. J. Food Microbiol. 2018, 266, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Kalchayanand, N.; Arthur, T.M.; Bosilevac, J.M.; Brichta-Harhay, D.M.; Guerini, M.N.; Wheeler, T.L.; Koohmaraie, M. Evaluation of Various Antimicrobial Interventions for the Reduction of Escherichia coli O157:H7 on Bovine Heads during Processing. J. Food Prot. 2008, 71, 621–624. [Google Scholar] [CrossRef]
- Signorini, M.; Costa, M.; Teitelbaum, D.; Restovich, V.; Brasesco, H.; García, D.; Superno, V.; Petroli, S.; Bruzzone, M.; Arduini, V.; et al. Evaluation of decontamination efficacy of commonly used antimicrobial interventions for beef carcasses against Shiga toxin-producing Escherichia coli. Meat Sci. 2018, 142, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kalit, S.; Kos, T.; Kalit, M.T.; Kos, I. The Efficacy of Electrolysed Oxidizing Water as a Disinfectant in the Dairy Industry. J. Hyg. Eng. Des. 2015, 12, 28–32. [Google Scholar]
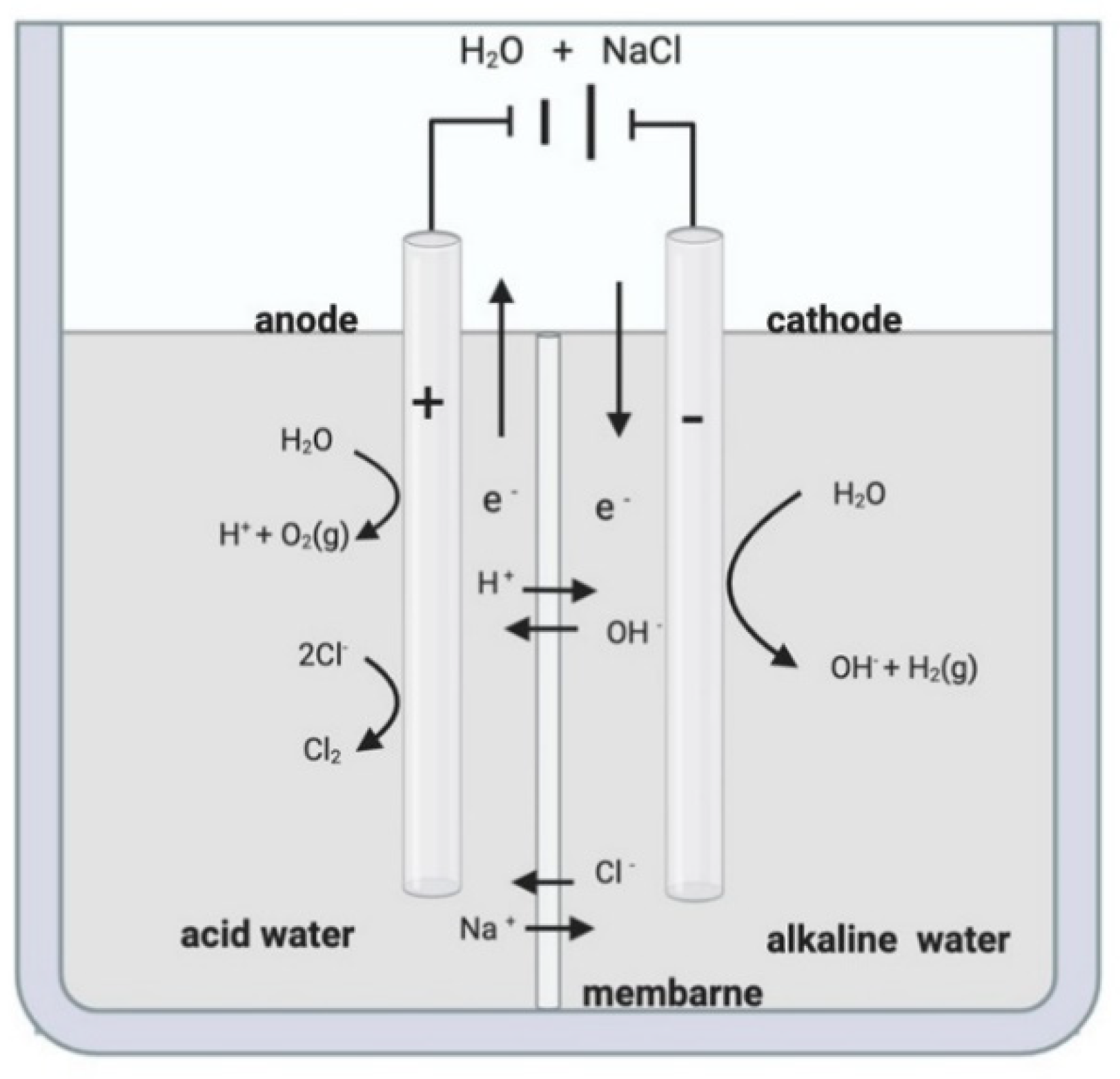
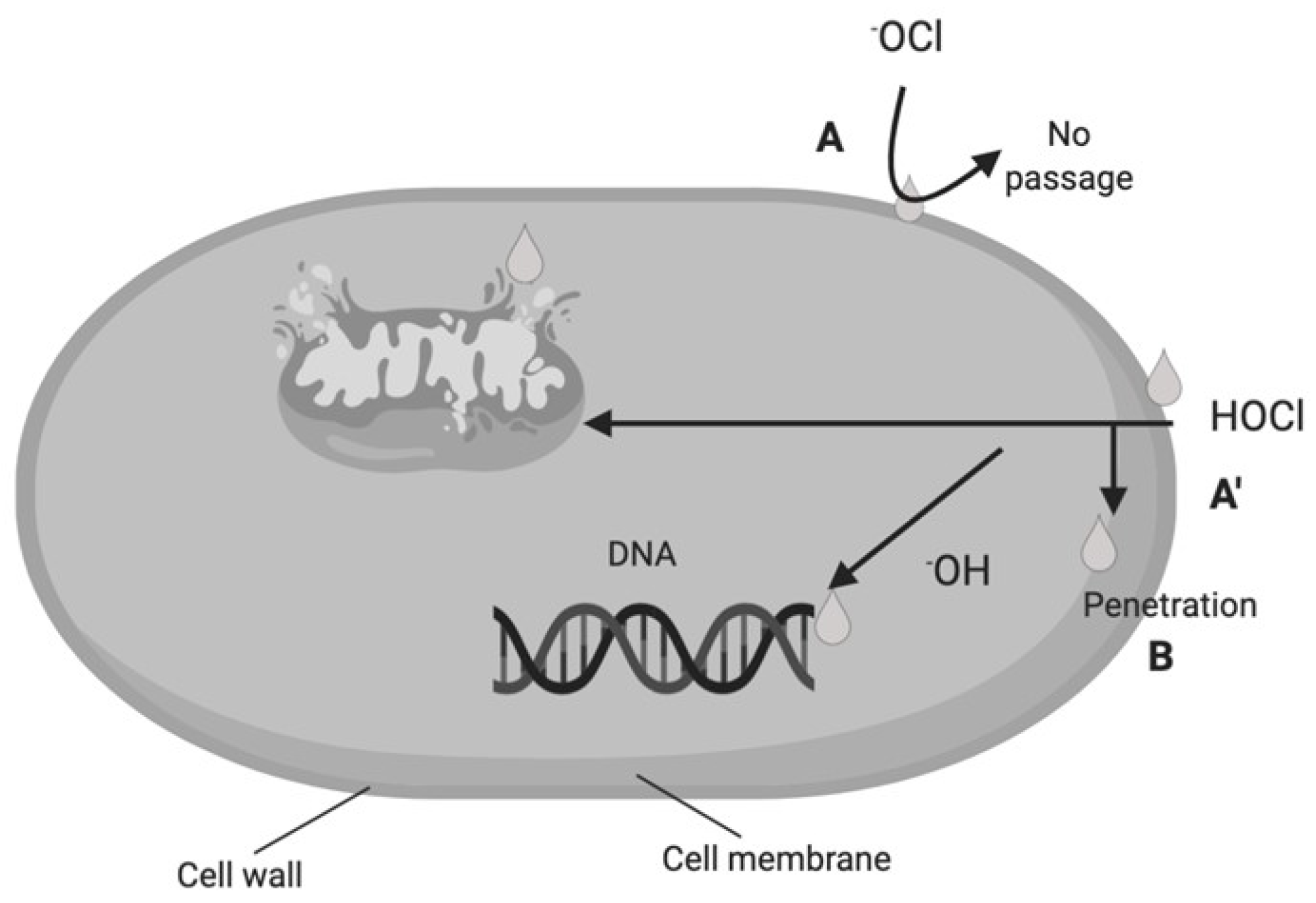
| Type of Electrolyzed Water | pH | ORP a (mV) |
|---|---|---|
| Acidic electrolyzed water | 2–3 | >1100 |
| Basic electrolyzed water | 10–13 | −800 to −900 |
| Neutral electrolyzed water | 6.5–7.5 | 700 to 800 |
| Material | Type of Electrolyzed Water | Concentration of EW (ppm) | Microorganisms | Inoculum Concentration b | Type of Treatment | Duration of Treatment | Reference |
|---|---|---|---|---|---|---|---|
| Pork belly | AEW, AEW + lactic acid | 50 | L. monocytogenes Salmonella Typhimurium Campylobacter coli | 7 log CFU/mL | Spray | 15 s | [40] |
| Frankfurters Ham | AEW and BEW | 50 | L. monocytogenes | 5 log CFU/mL | Spray, dip | 15 s | [41] |
| Fresh pork loin | AEW + fumaric acid | 30 | E. coli L. monocytogenes Staph. Aureus Salmonella sp | 8 log CFU/mL | Dip | 5 min | [42] |
| Carcass | low concentration EW | 10 | E. coli O157:H7 | 9 log CFU/mL | Dip | 5 min | [38] |
| (NEW) | L. monocytogenes | ||||||
| Pork loin | AEW and | 74 | Mesophilic and | 4.12 log10 CFU g−1 | Spray | 20–40 s | [43] |
| slight AEW | 51 | psycrotrophs | |||||
| Pork loin | BEW | ND a | ND | ND | Injection | 15 min | [44] |
| Pork loin | NEW | 16.6 | Aerobic bacteria Psychrotrophs Yeast and moulds | 0.55 to 0.57 log CFU/cm−2 | Spray | 120 s | [45] |
| 0.49 to 0.54 log CFU/cm−2 | |||||||
| 0.52 to 0.60 log CFU/cm−2 |
| Material | Type of Electrolyzed Water | Concentration of EW (ppm) | Microorganisms | Inoculum Concentrationb | Type of Treatment | Duration of Treatment | Reference |
|---|---|---|---|---|---|---|---|
| Salmon | AEW and | 76.9 | E. coli O157:H7 | 8.7 log CFU/mL | Immersion | 64 min | [47] |
| BEW | ND | L. monocytogenes | |||||
| Salmon, mahi mahi (Coryphaena hippurus) | AEW | 50 | L. monocytogenes | 4.47 log CFU/g | Immersion | 5 min | [48] |
| Morganella morganii | 4.02 log CFU/g | ||||||
| Salmon | AEW | 65 | L. monocytogenes | 6 log CFU/mL | Immersion | 5 min | [49] |
| Aerobic bacteria | 4.2 to 5.9 log CFU/g | ||||||
| Coliforms | 2.8 to 4 log CFU/g | ||||||
| Yeast and moulds | 1.3 to 2.9 log CFU/g | ||||||
| Salmon | AEW, NEW | 60 | L. monocytogenes | 7.70 log CFU/g | Immersion | 10 min | [50] |
| Smoked salmon | AEW | 60 | L. monocytogenes | 8.48 log CFU/mL | Immersion | 10 min | [51] |
| Salmon, tuna fish skin | AEW | 100 | Enterobacter aerogenes | 8 to 9 log CFU/mL | Soaking in ice | 120 min to 24 h | [52] |
| Enterobacter cloacae | |||||||
| Klebsiella pneumoniae | |||||||
| Morganella morganii | |||||||
| Proteus hauser | |||||||
| Tuna | AEW + CO gas | 10, 50 and 100 | Aerobic bacteria | 3.14 log CFU/g | Immersion | 5 min | [53] |
| Tuna | AEW | 41 | Aerobic bacteria | < 3 log CFU/mL | Immersion | 15 min | [54] |
| Catfish | AEW | 300 | L. monocytogenes | 5 log CFU/g | Wash | 3 min | [55] |
| Salmonella spp | |||||||
| Catfish | BEW + polyphosphate | NDa | ND | ND | Immersion | 2 h | [56] |
| Tilapia | AEW | 120 | E. coli | 8 log CFU/mL | Immersion | 10 min | [57] |
| Vibrio parahaemolyticus | |||||||
| Tilapia | NEW + PROSAN | 150 | Listeria innocua | 6 to 7 log CFU/g | Soaking in ice | 72 h | [58] |
| E. coli K12 | |||||||
| Pseudomona putida | |||||||
| Carp | BAE | 0.87 | Aerobic bacteria | 6 log CFU/mL | Immersion | 15 min | [59] |
| AEW | 40.8 | ||||||
| Pacific saury (Cololabis saira) | weak AEW | 34.2 to 47.2 | Aerobic bacteria | 3 log CFU/g | Soaking in ice | 30 days | [60] |
| Psychrotrophic bacteria | |||||||
| Trout | AEW | 38 | Aerobic bacteria | 9 log CFU/mL | Immersion | 5 to 10 min | [61] |
| American shad (Alosa sapidissima) | AEW + chitosan | 70 to 80 | Aerobic bacteria | 3.71 to 3.94 log CFU/g | Immersion | 15 min | [62] |
| Bombay duck | slightly AEW +ebony-bamboo leaves complex extracts | 27.37 | Aerobic bacteria | 1.5 log CFU/g | Immersion | 5 min | [63] |
| (Harpadon nehereus) | |||||||
| Shrimp | AEW | 66 | V. parahaemolyticus | 9 log CFU/mL | Immersion | 2.5 min | [64] |
| Shrimp | AEW | 44 | Aerobic bacteria | 6.04 log CFU/g | Soaking in ice | 7 days | [65] |
| Oyster | AEW | 30 | V. parahaemolyticus | 8.94 log CFU/mL | Immersion | 4 to 6 h | [66] |
| Vibrio vulnificus | |||||||
| Clams and mussels | AEW | 20 | E. coli O104:H4 | 9 log CFU/mL | Immersion | 1 to 2 h | [67] |
| BEW | 10 | L. monocytogenes | |||||
| V. parahaemolyticus | |||||||
| Aeromonas hydrophila |
| Material | Type of Electrolyzed Water | Concentration of EW (ppm) | Microorganisms | Inoculum Concentration b | Type of Treatment | Duration of Treatment | Reference |
|---|---|---|---|---|---|---|---|
| Chicken breast | Slightly AEW | 10 | L monocytogenes | 9 log CFU/mL | Immersion | 10 min | [68] |
| strong AEW | 50 | S. Typhimurium | |||||
| Chicken breast | AEW 4 °C | 30 | Salmonella Enteritidis | 9 log CFU/mL | Immersion | 3 min | [69] |
| AEW 25 °C | 14 | E. coli | |||||
| Staph. aureus | |||||||
| Chicken breast | Slightly AEW + ultrasound | 5 | Mesophilic bacteria | 3.8 log CFU/g | Immersion | 30 min | [70] |
| Psychrotrophic bacteria | 3.47 log CFU/g | ||||||
| Lactic acid bacteria | 3.22 log CFU/g | ||||||
| Enterobacteria | 2.1 log CFU/g | ||||||
| Staph. aureus | 2.25 log CFU/g | ||||||
| Chicken carcass | AEW, BEW | 50 | S. Typhimurium | 5 log CFU/mL | Immersion | 45 min | [71] |
| E. coli | Spray wash | 15 s | |||||
| Total coliforms | |||||||
| Chicken carcass | AEW, slightly AEW | 58 | Aerobic bacteria | 4 log CFU/cm2 | Spray wash | 15 s | [72,73] |
| 30 | Total coliforms | ||||||
| Chicken carcass | NEW + lactic acid | 50 | C. jejuni | 9 log CFU/g | Immersion | 3 min | [74] |
| Spray wash | |||||||
| Chicken wings | AEW | 50 | C. jejuni | 7 to 8 log CFU/mL | Immersion | 10 or 30 min | [75] |
| Egg | AEW | 8 | S. Typhimurium | Different values | Spray | 4 × 15 s | [76] |
| Staph. aureus | |||||||
| L monocytogenes | |||||||
| E. coli | |||||||
| Egg | slightly AEW | 26 | Salmonella Enteritidis | 8 log CFU/mL | Immersion | 3 min | [77] |
| E. coli | |||||||
| Egg | AEW, BAW | 70 to 80 | Salmonella Enteritidis | 6 log CFU/mL | Immersion | 1 to 5 min | [78] |
| E. coli K12 | |||||||
| Egg | AEW | ND | Enterobacteriaceae | Spray | ND a | [79] | |
| Aerobic bacteria | 3.5 log CFU/cm2 | ||||||
| Egg | NEW | 46 | L monocytogenes | 6 log CFU/mL | Spray | 30 s | [27] |
| Egg | NEW | 60 | Salmonella enterica | 6 log CFU/mL | Spray | 30 s | [26] |
| E. coli |
| Material | Type of Electrolyzed Water | Concentration of EW (ppm) | Microorganisms | Inoculum Concentration b | Type of Treatment | Duration of Treatment | Reference |
|---|---|---|---|---|---|---|---|
| Beef meat | Slightly AEW | 38 | E. coli 0157:H7 | 9 log CFU/mL | Immersion | 10 min | [61] |
| S. Typhimurium | |||||||
| L. monocytogenes | |||||||
| Fresh meat | NEW | 27 to 39, 50 | L. monocytogenes | 8 log CFU/mL | Spray | 30 s | [81] |
| E. coli O157:H7 | |||||||
| Salmonella sp | |||||||
| Rib meat | AEW, slightly AEW | 50 5 | E. coli O157:H7 | 9 log CFU/mL | Immersion | 3 min | [83] |
| Meat | AEW, slightly AEW | ND a | Aerobic bacteria | 4.78 log CFU/g | Immersion | ND | [84] |
| Fungi and yeast | 3.71 log CFU/g | ||||||
| Beef meat | Slightly AEW + tea polyphenols | 40 | Aerobic bacteria | 3.06 log CFU/g | Immersion | 5 min | [85] |
| Beef fillets | BEW | 100 | Aerobic bacteria | 3.82 log CFU/cm2 | Spray | 90 s | [86] |
| Total coliforms | 1.94 log CFU/cm2 | ||||||
| Yeast | 2.21 log CFU/cm2 | ||||||
| Lactic acid bacteria | 2.64 log CFU/cm2 | ||||||
| Beef head | AEW | 60 | E. coli O157:H7 | 6 log CFU/cm2 | Spray | 12 s | [87] |
| Bovine carcass | BEW, AEW | 400 | Aerobic bacteria | 5 log CFU/400 cm2 | Spray | NDa | [88] |
| E. coli O157:H7 | 0.60 log CFU/400 cm2 | ||||||
| Coliforms | 0.83 log CFU/400 cm2 | ||||||
| Milk | AEW | ND | Aerobic bacteria | 2.48 log CFU/mL | Mix | 15 min | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez Orejel, J.C.; Cano-Buendía, J.A. Applications of Electrolyzed Water as a Sanitizer in the Food and Animal-By Products Industry. Processes 2020, 8, 534. https://doi.org/10.3390/pr8050534
Ramírez Orejel JC, Cano-Buendía JA. Applications of Electrolyzed Water as a Sanitizer in the Food and Animal-By Products Industry. Processes. 2020; 8(5):534. https://doi.org/10.3390/pr8050534
Chicago/Turabian StyleRamírez Orejel, Juan C., and José A. Cano-Buendía. 2020. "Applications of Electrolyzed Water as a Sanitizer in the Food and Animal-By Products Industry" Processes 8, no. 5: 534. https://doi.org/10.3390/pr8050534
APA StyleRamírez Orejel, J. C., & Cano-Buendía, J. A. (2020). Applications of Electrolyzed Water as a Sanitizer in the Food and Animal-By Products Industry. Processes, 8(5), 534. https://doi.org/10.3390/pr8050534





