Abstract
Microfluidics is a very facile and fast method of particulate production. Besides, it enables the manufacturing of size tuned particulate systems. Niosomes due to structural similarities have importance as alternative drug delivery systems to liposomes. Niosomes can be encapsulated or co-encapsulated with hydrophilic and lipophilic drugs. The research presented here includes the optimization of method parameters for niosome production as well as evaluation of the efficiency of microfluidics to encapsulate and co-encapsulate the drugs. For this purpose, metformin (MET) and garcinol (GC) were the model drugs. Two different non-ionic surfactants (NIS), namely Tween-20 and Span-60 with significant difference in hydrophilic-lipophilic balance (HLB) value, were chosen to analyze their efficiency to form niosomes and encapsulate one or more drugs.
1. Introduction
For two decades, microfluidics has been gaining fame as a new manufacturing method for nanoparticles, including liposomes [1], polymer nanoparticles [2], niosomes [3], etc. While liposomes have been extensively investigated, there is a range of alternative options that can be considered, including the use of non-ionic surfactants (NIS) for the preparation of bilayer vesicles called “niosomes”. These systems often referred to as niosomes, were launched first for cosmetic applications by L’Oreal [4]. Similar to liposomes, niosomes are broadly classified into three categories, namely small unilamellar vesicles (SUV), multilamellar vesicles (MLV) and large unilamellar vesicles (LUV) and are formed using protocols similar to liposomes [5].
The microfluidic device can be used to produce various vesicular drug delivery systems [6]. The microfluidic device used in this research included a chip with 0.3-micron channels of the staggered herringbone micromixer based on patterns of grooves. This patterned micromixer chip initiates chaotic flow in the channels by repetitive and rotation flow of the fluid running through it, which was driven by the altered grooves and their axial position in the microchannel [7].
In the formulation of niosomes, the selection of surfactants is based on hydrophilic-lipophilic balance (HLB) value. HLB values between 4 and 8 recommended for the facile formation of niosomes and surfactants with an HLB value of more than 8 are required to optimize cholesterol concentration [4,8]. However, it has been widely observed that HLB value between 4 and 8 is highly recommended for better encapsulation efficiency, of niosomes [4,5,9,10]. For example, long stearyl and short lauryl chain length increase and decrease the entrapment efficiency of niosomes, respectively [4]. Long hydrophilic chains result in increased encapsulation of hydrophilic drugs, and long hydrophobic chains result in improved encapsulation of lipophilic drugs [4].
Considering the facts above, two surfactants, namely Tween-20 (HLB = 16.7) and Span-60 (HLB = 4.7), were chosen for this comprehensive study as there is a considerable difference in the HLB value of the two surfactants. Niosomes are the structures that are efficient to accommodate the active ingredient of divergent solubility [11]. Hence the niosomes have the potential to be used in a variety of therapeutic applications [12,13]. Although the thin-film hydration method widely being used for the preparation of niosomes at a laboratory scale, new techniques like microfluidics are emerging the stream too [3]. However, there are several gaps in the development of microfluidic-based niosome production [3]. Hence, the purpose of this study was to fill the major gaps through this comprehensive screening. This report covers several factors that could contribute to the enhance the drug or co-drug encapsulation into the niosomes prepared using the benchtop microfluidic device. In other words, the studies were performed involving preparation four different formulations, namely empty niosomes, niosomes encapsulating lipophilic drug, hydrophilic drug, and co-encapsulation of divergent solubility drugs. The quality of these niosomes was determined based on their size, surface charge, percent drug encapsulation [14]. The development of niosomal formulation involves the addition of compounds like diacetyl phosphate (DCP) to impart charge on the particles to avoid aggregation. This research also describes the effect of cholesterol on the formation of niosomes. The observations made during these studies confirm that microfluidics is a facile and fastest method of niosomes production. Niosomes produced using this technique hold the potential to be used for the delivery of single and multiple drugs.
2. Materials and Methods
2.1. Materials
The non-ionic surfactants (Tween-20 and Span-60), Diacetyl phosphate (DCP), and cholesterol were obtained from Millipore-Sigma (St. Louis, MO, USA). Tablets to prepare the phosphate-buffered saline (PBS) were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Each tablet of PBS was dissolved in 200 mL of milli-Q grade water. Ethanol was also purchased from Fisher Scientific, Fair Lawn, NJ, USA. All the reagents used for the experiments were of analytical grade.
2.2. Preparation of Niosomes Using Microfluidics
To prepare niosomes, the NanoAssemblrTM benchtop (Precision Nanosystems, Vancouver, BC, Canada) was used with a 300-micron Staggered Herringbone Micromixer (Figure 1). Briefly, the non-ionic surfactants at the appropriate ratio were dissolved in ethanol. The aqueous buffer used in all studies was PBS, 10 mM, pH 7.4. The microfluidics method parameters during this process of niosome productions involved Three different flow rate ratios (FRR) 1:1, 3:1 to 5:1 (aq: organic ratio), and the total flow rate (TFR) 5, 10 and 12 mL/min and the sample volume was 2.0 mL. The encapsulation of drugs can be performed by the addition of the drug into the appropriate phase. During the preparation of niosomes, the garcinol was dissolved in the organic phase, while the metformin was dissolved in PBS before microfluidic mixing. The niosome formulations were collected from the chamber outlet and centrifuged (3500 rpm, 3 times) at room temperature for the removal of residual solvent and non-encapsulated drug.
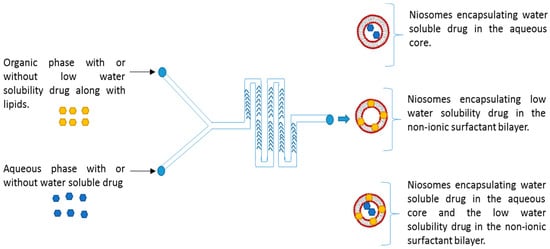
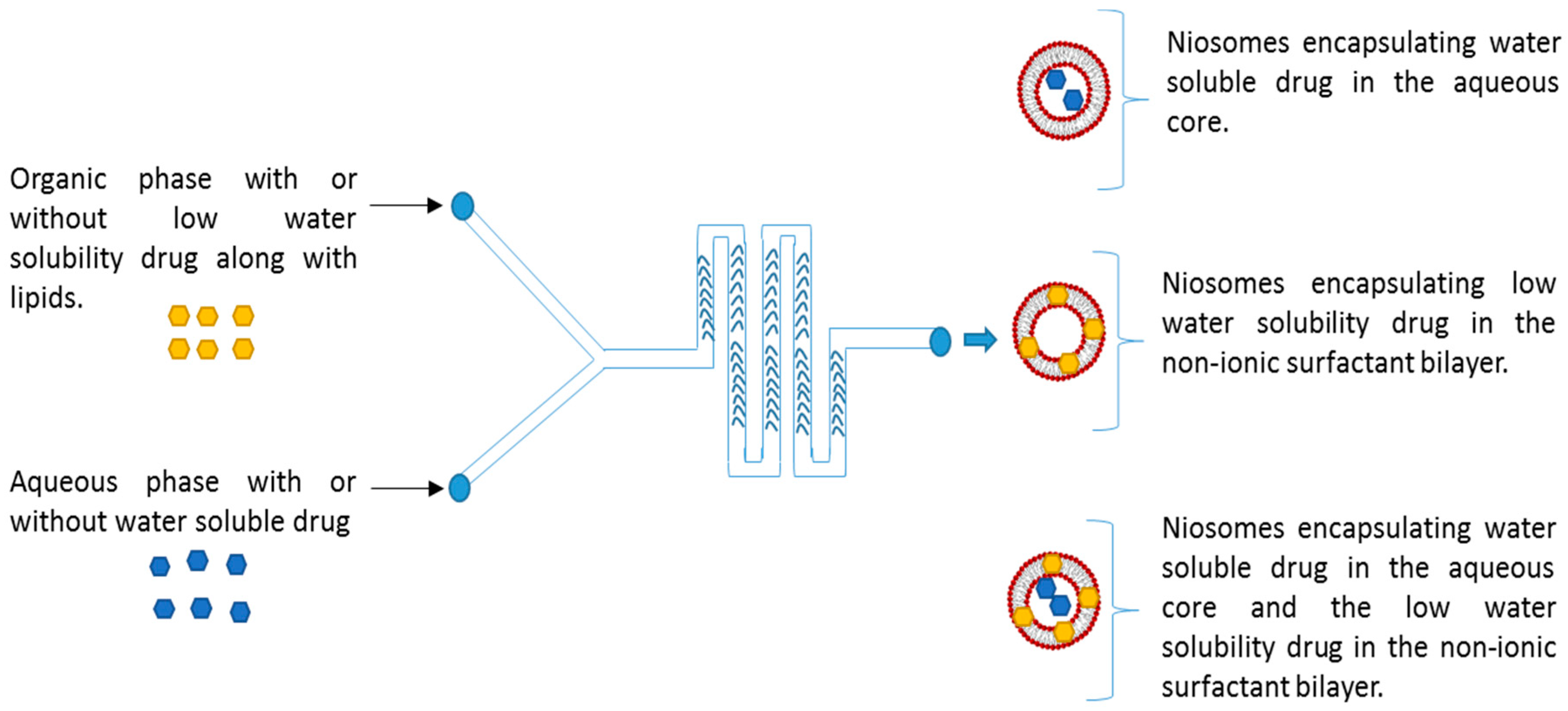
Figure 1.
Schematic representing the microfluidic chip structure is having a staggered herringbone micromixer and the whole process of niosome formation.
2.3. Particle Characteristics
Dynamic light scattering (DLS) (Zetasizer Nano-S, Malvern Instruments, Westborough, MA, USA) was used for size determination of empty and drug-loaded niosomes. Zeta potential was determined using laser Doppler velocimetry (Zetasizer Nano-S, Malvern Instruments, Westborough, MA, USA). Samples were prepared using PBS diluted 1 to 300 times (pH 7.4, 25 °C).
2.4. Determination of Percent Encapsulation
The percent drug encapsulation was determined using the UV-Visible spectrophotometry (Nanodrop, Thermo Fisher Scientific, Waltham, MA, USA). The analysis of niosomal liquid dispersion was performed at 276 nm and 232 nm for garcinol and metformin, respectively. Using the absorbance values obtained the determination of percent encapsulation based on a standard curve.
2.5. Transmission Electron Microscopy (TEM)
Niosomal suspension of both Tween-20 and Span-60 was freshly prepared, and a drop of the solution was added to the grid followed by staining with phosphotungstic acid (PTA). The grid was then imaged under the TEM (JEOL, Peabody, MA, USA) at 60 kV, 2 s exposure time, and 20 k magnification.
2.6. Statistical Analysis
Unless stated otherwise, the results were calculated as the mean ± standard deviation (SD). Data were analyzed by the student’s t-test alone or ANOVA, followed by Dunnett’s post-hoc analysis for comparison, and significance was acknowledged for p values < 0.05.
3. Results
3.1. Optimization of Microfluidics Method Parameters for the Production of Niosomes
Since the structure of niosomes resembles liposomes, many of the liposome formulation recommendations (e.g., the concentration of cholesterol) apply to niosomal formulation development [5]. Several studies have shown that cholesterol can reduce the leakage of the water-soluble drug [15]; it can affect the size of the vesicle [16,17]. Cholesterol concentration up to 50% of total lipid content is recommended for the liposomal formulation. Since niosome resembles liposomes, a NIS:Chol:DCP weight ratio of 5:2.5:1 was selected to meet the needs of both good aqueous drug retention as well as bilayer drug loading.
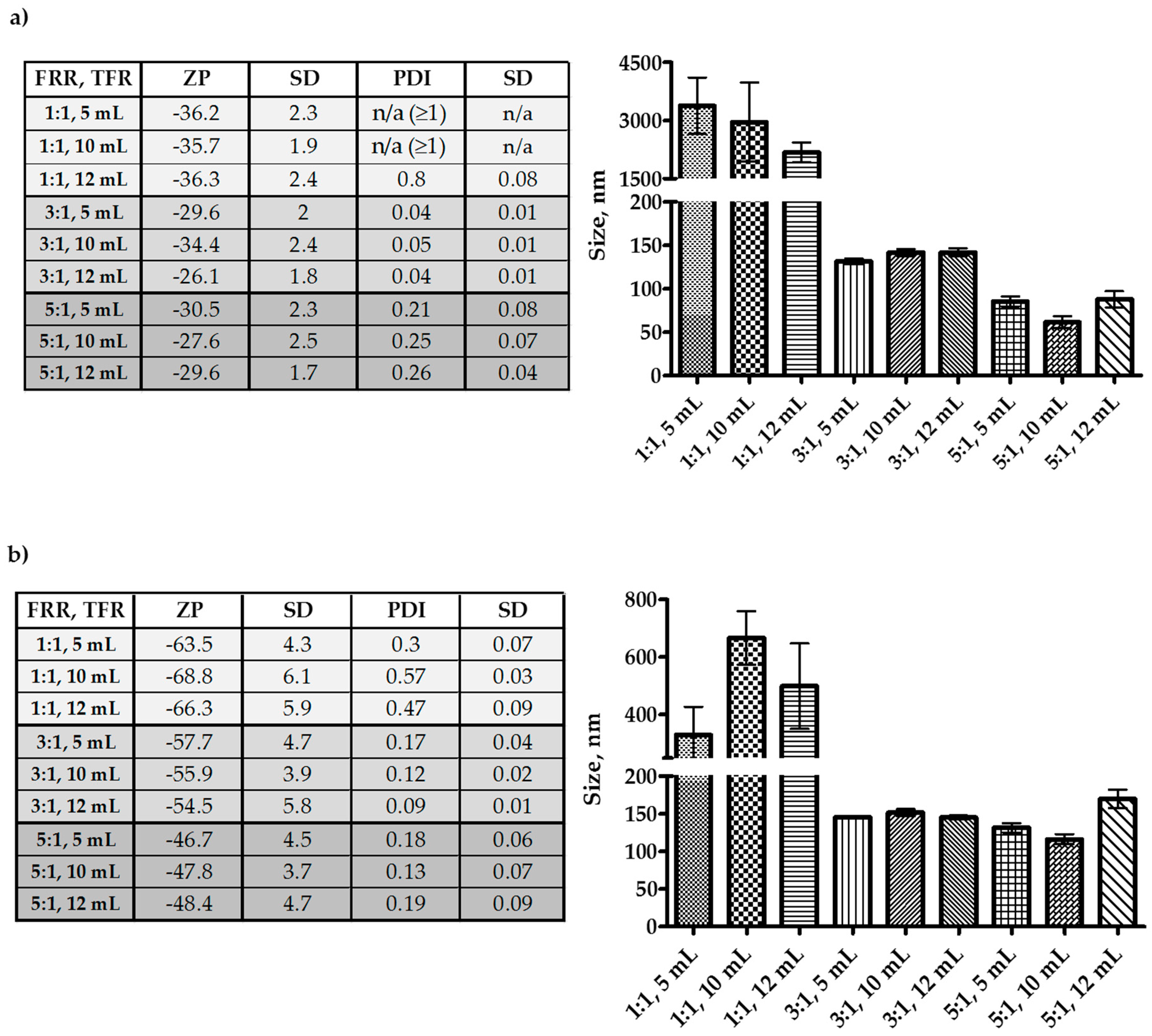
Results in Figure 2 demonstrate irrespective that at flow rate ratio (FRR) 1:1 (aqueous:solvent), both Tween-20 and Span-60 produced larger niosomes. However, Span-60 produced particles below 1 µm, and Tween-20 niosomes were over a µm in size. For both Tween-20 and Span-60, the FRR 3:1 (aqueous:solvent) produced niosomes in the range of 100–150 nm. However, at FRR 5:1 (aqueous:solvent), the Tween-20 niosomes were below 100 nm, and the Span-60 were nearly the same in size as the FRR 3:1 except for the total flow rate (TFR) 10 mL/min; where the size was close to 100 nm. Through all the experiments, only the FRR had a significant impact on the size of the niosomes. The TFR did not affect the size, but size observed with FRR 5:1 and TFR 10 mL/min can be considered as the better among all. The reason for this is, the Tween-20 niosome size at FRR 5:1 and TFR 10 mL/min was below 100 nm, but the polydispersity index (PDI) was more than Span-60 niosomes. Additionally, the zeta-potential of Span-60 niosomes was higher, which will contribute to the stability of the niosomes. The diacetyl phosphate (DCP) was added to the formulation to gain a negative charge on the niosomes and restrict the aggregation. Hence, all the niosome formulations were negative in zeta potential. From the optimization study, it can be seen that FRR 5:1 and TFR 10 mL/min was the best suit; hence, the FRR 5:1 and TFR 10 mL/min was adopted for further studies involving drug encapsulation.
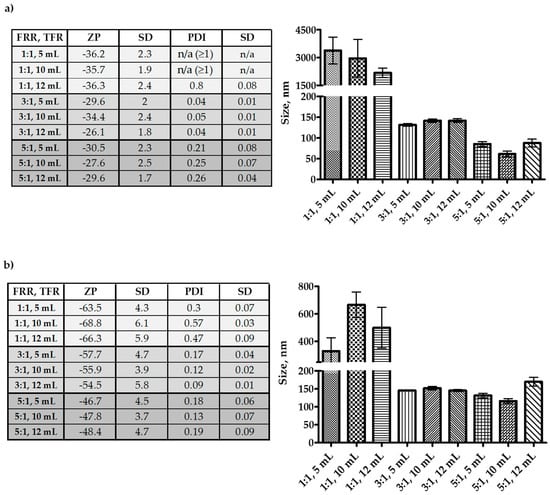
Figure 2.
Optimization of microfluidics parameters for the production of nano-sized niosomes. All the niosomes were formulated with NIS:Cholesterol: DCP (5:2.5:1 w/w/w) (a) niosomes formulated with Tween-20 and (b) niosomes formulated with Span-60. The results presented here are representing n = 3, ± SD. ZP = zeta potential, PDI = polydispersity index, FRR (1:1, 3:1, 5:1), TFR (5, 10, 12 mL/min), SD = Standard Deviation.
3.2. Effect of Cholesterol Concentration on Niosome Size
Optimization of cholesterol has a crucial role in the formation of niosomes. Microfluidics is an emerging technique in the production of niosomes. However, it is essential to optimize the cholesterol concentration to study the effect on particle characteristics. Although the results show that a cholesterol concentration, 50% of NIS can produce niosomes, the size can be controlled with microfluidic parameters. However, keeping optimized microfluidics parameters if the concentration of cholesterol reduced, then it could alter the characteristics of the niosomes.
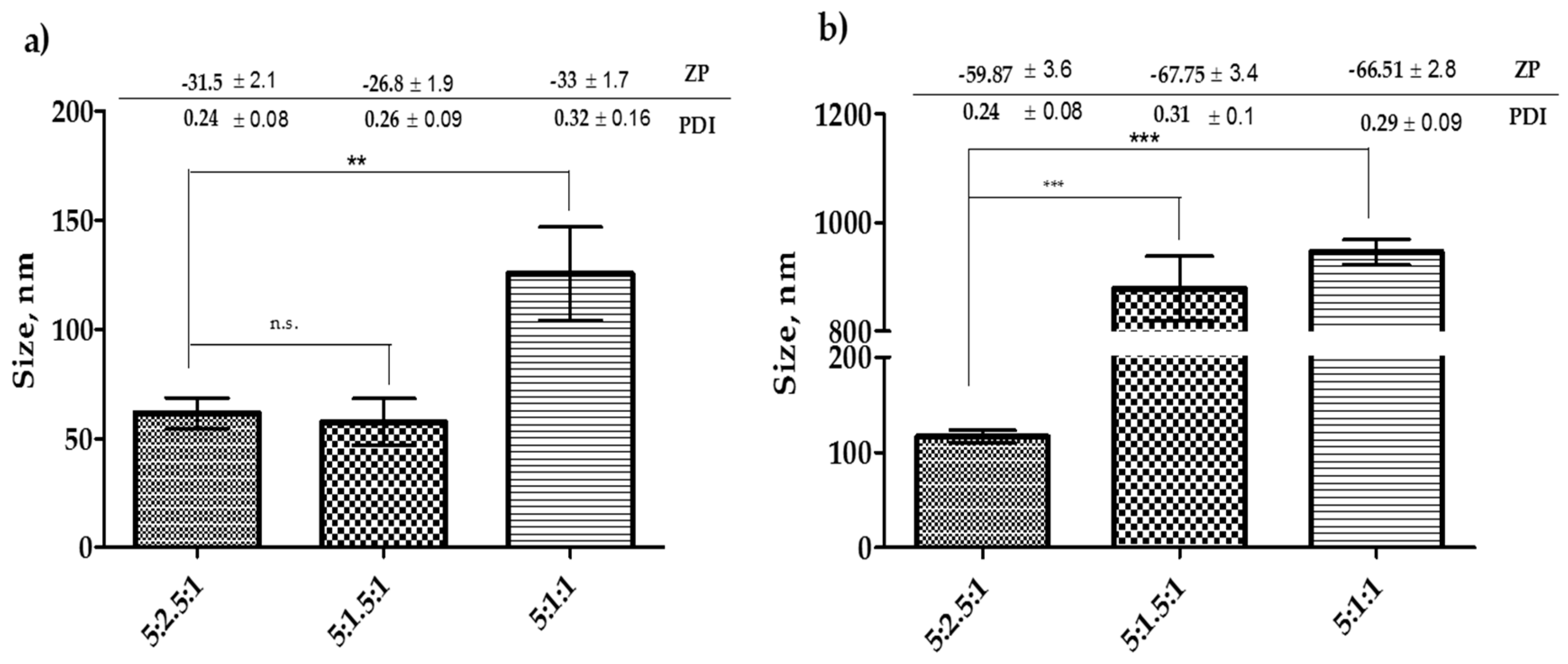
Results in Figure 3 show that lowering the amount of cholesterol to 1.5 mg did not affect the size of Tween-20 niosomes. However, for the further reduction of cholesterol amount significantly increased (p < 0.05) the size of the niosomes. On the other hand, lowering the amount of cholesterol to 1.5 mg or 1.0 mg in Span-60 formulation significantly increased (p < 0.05) the size of the niosomes. It was also noticed that the reduction in cholesterol amount to 1 mg resulted in an approximately 2.5-fold increase in the size of Tween-20 niosomes. Whereas, the same reduction in Span-60 formulation resulted in approximately 8 folds increase in size (Figure 3a,b). Considering these results and the fact mentioned in the previous section that cholesterol can prevent leakage of the drug, keeping the higher concentration of cholesterol would be a better choice to proceed for the drug encapsulation.
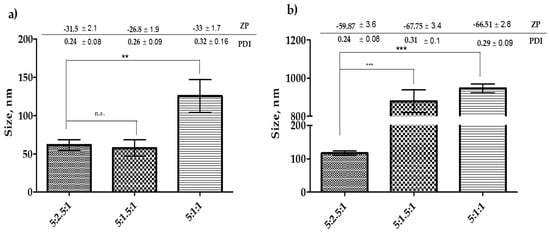
Figure 3.
Optimization of cholesterol concentration before encapsulation of drugs into the niosomes. (a) Niosomes formulated with Tween-20 and (b) niosomes formulated with Span-60. The results presented here are representing n = 3, ± SD. ZP = zeta potential, PDI = polydispersity index, FRR (1:1, 3:1, 5:1), TFR (5, 10, 12 mL/min), SD = Standard Deviation. n.s. = not significant, ** p < 0.01, *** p < 0.001.
3.3. Effect of Drug Encapsulation on the Size of the Niosomes
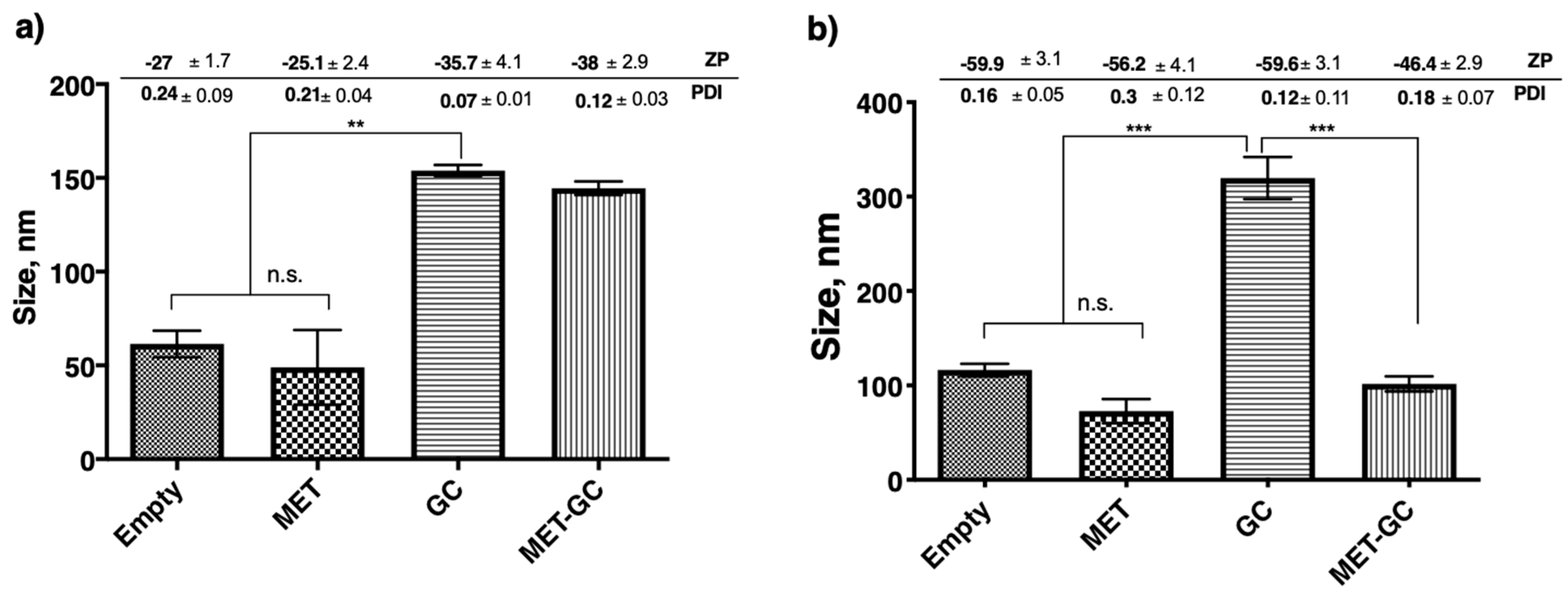
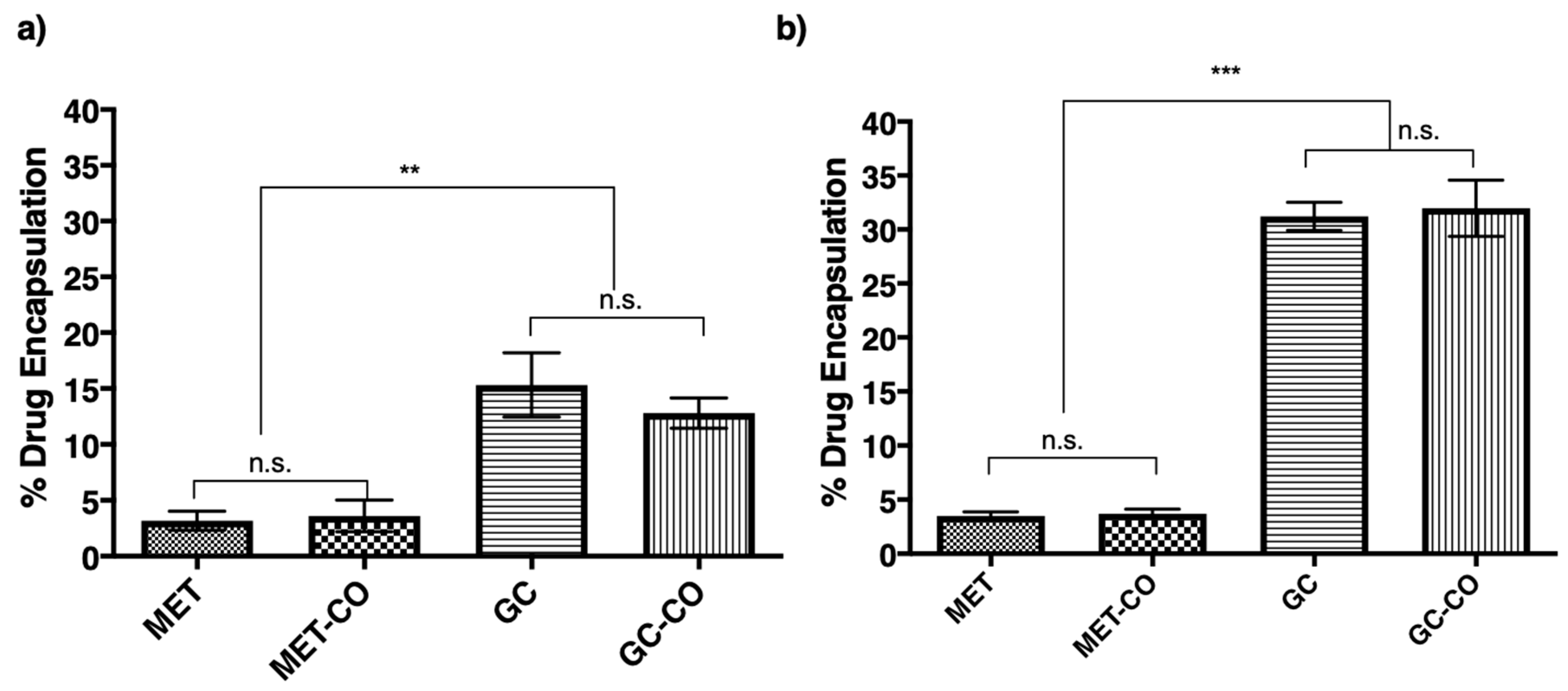
Results in Figure 4a,b demonstrate a significant difference (p < 0.01 and p < 0.001) in size between niosomes encapsulated with the hydrophilic drug (MET) and niosomes encapsulated with the lipophilic drug (GC). However, there were two interesting findings in these results; the first is in both cases (encapsulation and co-encapsulation) of metformin encapsulation, a slight reduction in size was observed. The second is co-encapsulation of MET and GC in the Span-60 niosomes significantly decreased (p < 0.001) the size, and this was not seen in Tween-20 niosomes.
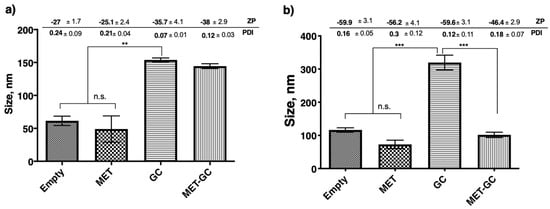
Figure 4.
Changes in niosomal size due to encapsulation or co-encapsulation of the drug(s). All the niosomes were formulated with NIS:Cholesterol: DCP (5:2.5:1 w/w/w) (a) niosomes formulated with Tween-20 and (b) niosomes formulated with Span-60. The results presented here are representing n = 3, ± SD. ZP = zeta potential, PDI = polydispersity index, FRR (1:1, 3:1, 5:1), TFR (5, 10, 12 mL/min), SD = Standard Deviation, n.s. = not significant, ** p < 0.01, *** p < 0.001.
3.4. Encapsulation of Drug into the Niosomes
Figure 5a,b demonstrates the percent drug encapsulation into Tween-20 and Span-60 niosomes, respectively. Here, the Span-60 niosomes found significantly efficient (p < 0.05) efficient in encapsulation and co-encapsulation of the lipophilic drug (GC). The encapsulation of MET was not significantly different in both Span-60 and Tween-20 niosomes. Another noticeable thing in these results is that co-encapsulation of GC in Tween-20 lowered (not significant) the percent encapsulation of the GC. However, in the case of Span-60, the percent encapsulation of GC was not in the presence of another drug in the niosomes. Overall, in both the cases (Figure 5a,b) the encapsulation of lipophilic drug was significantly better (p < 0.01 and p < 0.005) than the hydrophilic drug (MET).
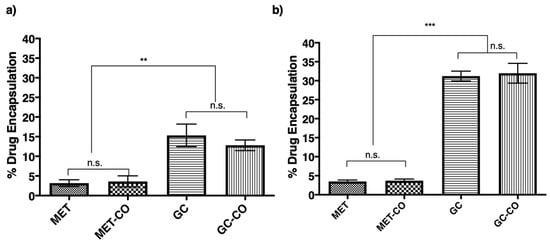
Figure 5.
Percent encapsulation or co-encapsulation of the drug(s) into the niosomes. All the niosomes were formulated with NIS:Cholesterol: DCP (5:2.5:1 w/w/w) (a) niosomes formulated with Tween-20 and (b) niosomes formulated with Span-60. Results presented here are representing % encapsulation n = 3, ± SD, n.s. = not significant, ** p < 0.01, *** p < 0.001.
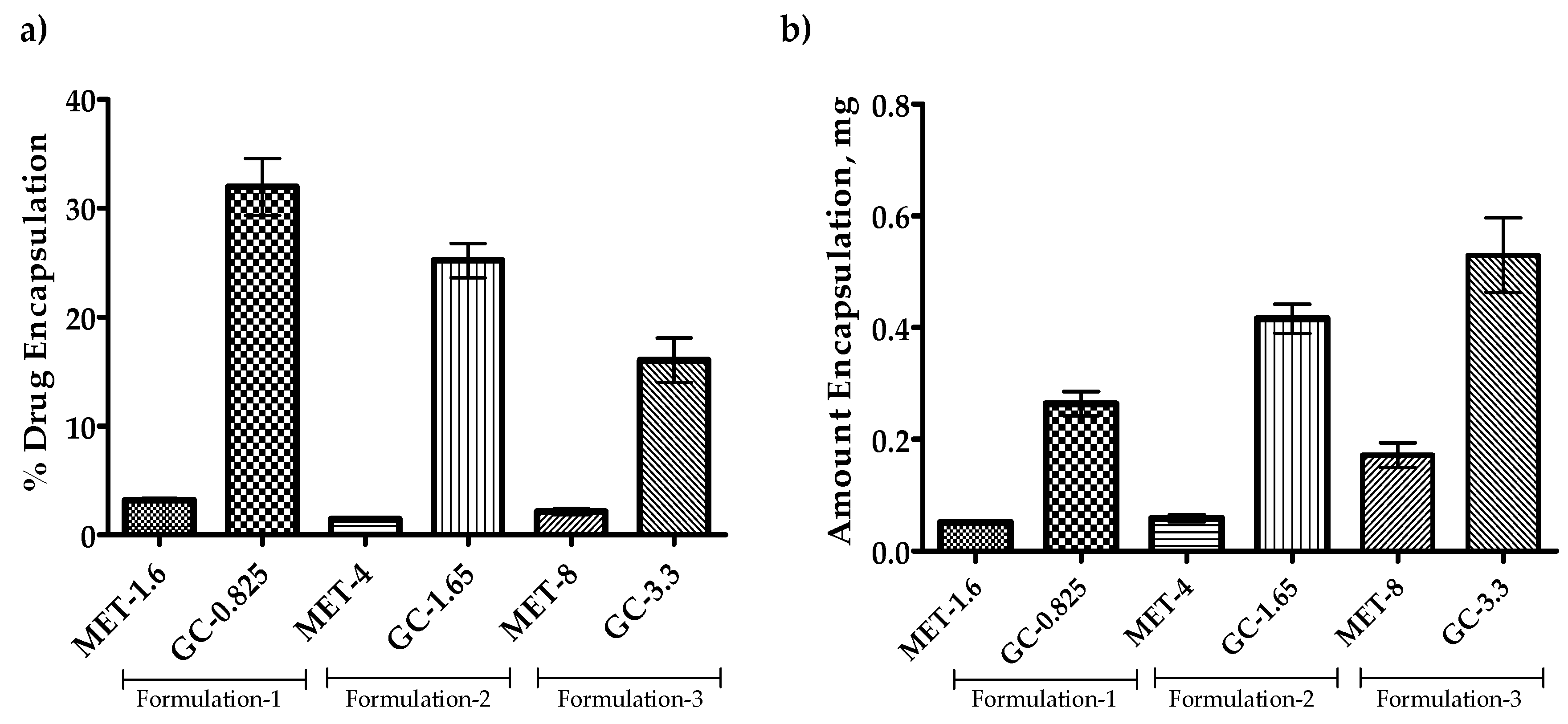
3.5. Effect of Drug Amount Escalation on Percent Encapsulation
Since the findings from the previous section represent that the Span-60 niosomes are more efficient in encapsulation or co-encapsulation of drugs. The drug amount escalation study was performed to evaluate the efficiency of Span-60 niosomes for higher drug accommodation with the same amount of NIS and cholesterol. The results in Figure 6 show that the increase in the amount of drug available for encapsulation decreases the percent encapsulation. However, when converted this percent encapsulation into the amount encapsulated, it appears that the amount encapsulated increases with an increase in the drug amount available for the encapsulation (Figure 6b). This information promotes the Span-60 niosomes as a potential candidate for the encapsulation of both hydrophilic and lipophilic drugs.
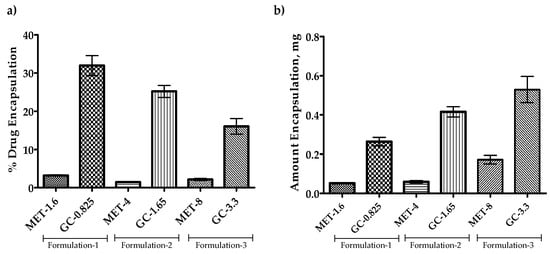
Figure 6.
Co-encapsulation of the drug(s) into the Span-60 niosomes. All the niosomes were formulated with NIS:Cholesterol:DCP (5:2.5:1 w/w/w) (a) Percent co-drug encapsulation and (b) Amount of drugs co-encapsulated. Results presented here are representing % encapsulation n = 3, ± SD.
3.6. Microscopic Elucidation Niosomal Structure
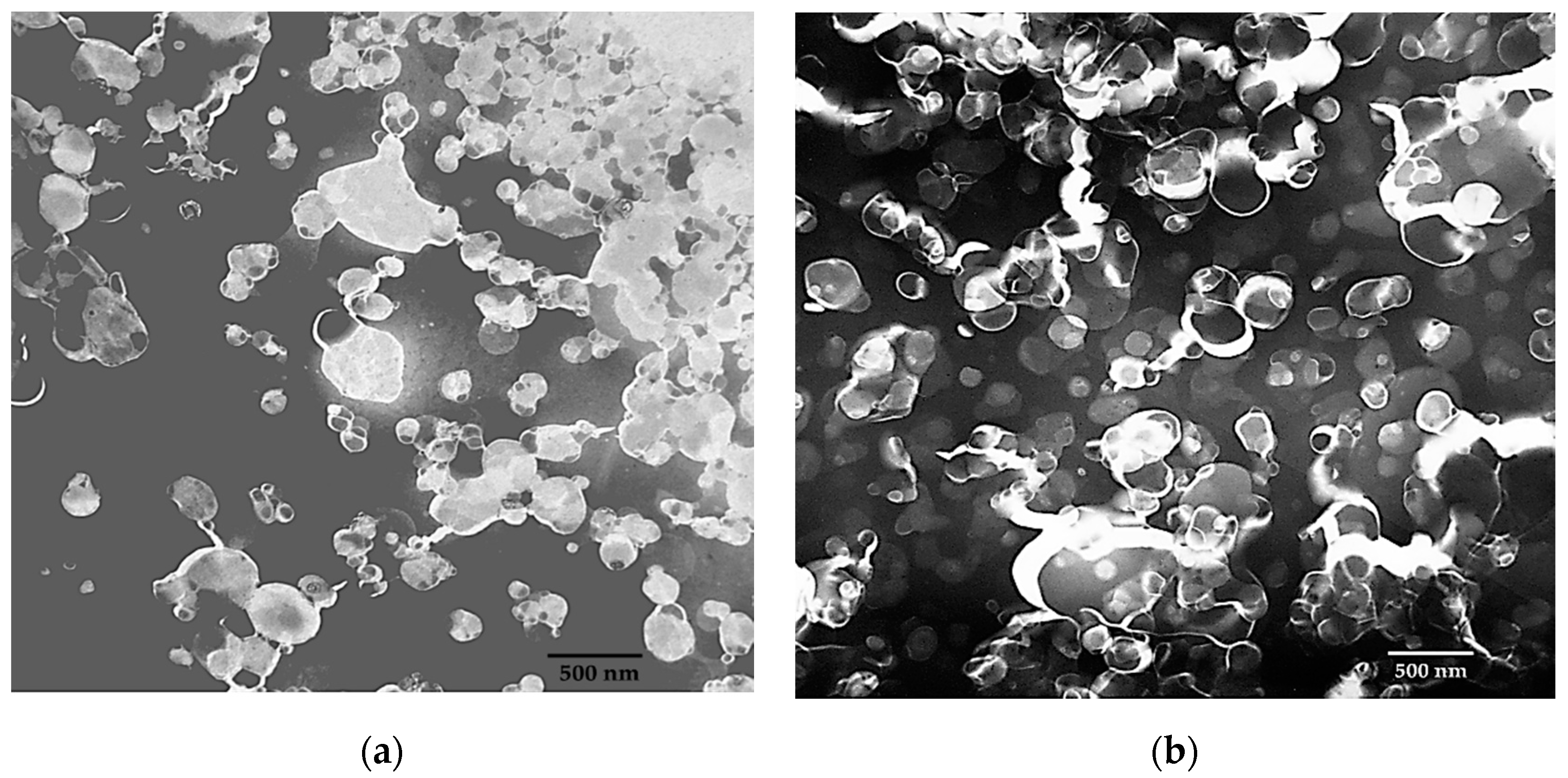
Microscopic elucidation supports the DLS data. Here, many particles in the Tween-20 suspension are below 100 nm and slightly above 100 nm in Span-60 suspension. However, the presence of larger particles represents polydispersity. Hence the PDI for the Tween-20 and Span-60 niosomes was 0.26 and 0.19 respectively. During the microscopy, it was also observed that the Tween-20 niosomes appearing in aggregates (Figure 7a), and no aggregation, during the imaging of Span-60 niosomes (Figure 7b).
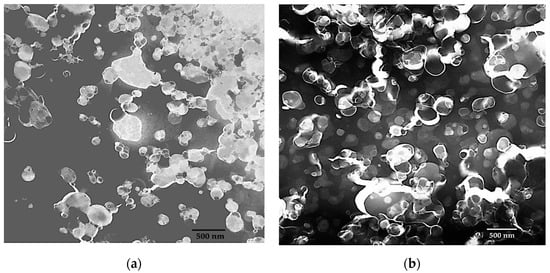
Figure 7.
TEM-based imaging of empty niosomes ((a): Tween 20 and (b): Span 60) prepared using microfluidic device parameters FRR 5:1 and TFR 10 mL/min. (HT: 60 kV, Mag. 20 k, Scale 500 nm, and Exposure time 2 s and Scale bar: 500 nm).
4. Discussion
Among the novel drug delivery systems, liposomes are the most successful carriers available in the market [18]. However, niosomes are the closest match to the structure of the liposomes, and the production of niosomes is cost-effective compared to liposomes [9,11,19]. Co-encapsulation of different solubility drugs using microfluidics has already been reported [20]. For the first time, this research is describing the screening of microfluidic techniques for the niosomal encapsulation and co-encapsulation. Thus, during this study, the potential of a microfluidic device to produce size tuned niosomes and co-encapsulate the drug of different solubility into the niosomes has been evaluated.
Like liposomes can be made with different lipids, the niosomes also can be prepared using different non-ionic surfactants. The lipids are the main component of the liposomal structure, and the non-ionic surfactants are the main component of the niosome structure. HLB value is the primary factor in the formulation development of niosomes [4]; hence the Tween-20 with the higher HLB value and Span-60 with least HLB value were used for the optimization of niosomal production using a microfluidic device. As mentioned earlier in this paper, the conventional methods of niosomes production reported that the HLB value of 4 to 8 is recommended for better niosome production [4,8]. Although microfluidics is emerging in the production of niosomes, a similar effect was observed, where during the optimization the Span-60 niosomes were better in size (Figure 2a,b) as well as percent encapsulation (Figure 5a,b). The zeta potential of the vesicles is responsible for the stability and higher zeta potential results in less particle aggregation, therefore better stability and monodispersed [21]. Although the reason for aggregation of Tween-20 niosomes was not confirmed, one of the reasons for this aggregation is possibly the effect of surface charges [5,10] (Figure 7). Moreover, with the Tween-20 niosomes the pattern of aggregation was observed, where the smaller particles are noticeably aggregated compared to the larger particles. Although the Span-60 niosomes were found larger in size than the Tween-20 niosomes but the aggregation of particles was not observed with Span-60. Here the finding suggests that along with the zeta-potential the size of niosome may also have a role in particle aggregation.
Granting microfluidics is one of the modern techniques in the preparation of niosomes, it did not affect the fact that cholesterol can reduce the leakage of the water-soluble drug [15]; it can affect the size of the vesicle [16,17]. It was witnessed that lowering the amount of cholesterol possibly had changed the assembly of the bilayer, and hence larger niosomes have been produced (Figure 3a,b). It was also found that the encapsulation of drugs could alter the size of vesicles irrespective of the type of non-ionic surfactant used (Figure 4a,b). Moreover, the reduction in the size of niosomes after encapsulation of MET was observed in both Span-60 and Tween-20 niosomes and has been reported with liposomes [20]. The reason for this is still unclear but could be the interaction of MET with the bilayer assembly.
Past research with conventional methods of the niosome production suggest the HLB value of 4 and 8 is highly recommended better encapsulation efficiency of niosomes [4,5,9,10]. Here the trend appears continued although microfluidics is a modern technique. The encapsulation of drugs into the Span-60 (4.7) niosomes was significantly better compared to Tween-20 (HLB 16.7) niosomes. In addition, it was reported that passive encapsulation of drugs depends on the solubility of the drug to be encapsulated; where lipophilic drugs encapsulation is always greater compared to the lipophilic drugs [20]. This supports the data from Figure 5a,b and Figure 6a,b; where both Tween-20 and Span-60 niosomes have accommodated the more lipophilic drug (GC) compared to the hydrophilic drug (MET). In case of bilayered vesicles, the passive encapsulation of drugs of low water-solubility is expected to remain higher than the highly water-soluble drugs [16,17]. This happens because during the encapsulation/vesicle formation process the amount of water getting encapsulated into the core of bilayered vesicle is less than the amount of water outside the bilayered vesicle. Hence the percent encapsulation of hydrophilic drug is always less than the low water-solubility drug [15,18].
Overall, compared to the Tween-20 niosomes, the Span-60 niosomes were not only competent in size but also better in encapsulation and co-encapsulation of drugs of different solubility. The two important factors contributing to this is largely the HLB value of surfactant and solubility of drug to be encapsulated. Although size varies increase in the amount of drug encapsulated, Span-60 has the perspective of accommodating more drugs (Figure 6a,b). However, further developments in formulation may be able to achieve the requirements.
5. Conclusions
Niosomes are often referred to as the alternative carrier to liposomes. There are advantages and disadvantages to both carrier systems. Thin-film hydration has been the conventional method for niosome production for the past few decades; however, in this research, niosomes produced on a microfluidics platform were screened for their particle characteristics and encapsulation efficiency. The results suggest that the particle characteristics of niosomes and encapsulation are not only depending on microfluidic process parameters but also on the properties of the surfactant used to produce the niosome carriers. Moreover, the niosomal encapsulation efficiency of low-water solubility drugs can compete for liposome, but niosomes are less efficient in encapsulating water-soluble drugs. The results obtained for these model drugs (MET and GC) will help the selection of drugs for microfluidic-based niosomal encapsulation or co-encapsulation. Overall, the aim of this work was to optimize the process parameters required for better encapsulation and co-encapsulation of the drugs in the niosomes. The continuation of this work would include the stability in terms of the drug release from the niosomes that may be based applications as well as on factors such as route of administration, site of drug delivery, etc.
Author Contributions
Conceptualization, methodology, and writing of the research were done by S.J., R.W. and R.S. did experimentation and data analysis. V.A.D. and S.R.S. did supervision, editing and review. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented in this manuscript was from the grant NSF-CREST (HRD-1241701).
Acknowledgments
We want to acknowledge Eva Dennis for her help with the artistic niosome structures in Figure 1. Additionally, we would like to acknowledge Miller (Auburn University, Auburn, AL, USA) for his assistance in TEM sample preparation and imaging.
Conflicts of Interest
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Abbreviations
| MET | Metformin |
| GC | Garcinol |
| HLB | Hydrophilic-lipophilic balance |
| SUV | Small unilamellar vesicles |
| MLV | Multilamellar vesicles |
| LUV | Large unilamellar vesicles |
| PDI | The polydispersity index |
| DCP | Diacetyl phosphate |
| PBS | Phosphate-buffered saline |
| TFR | Total flow rate |
| FRR | Flow rate ratio |
| TEM | Transmission electron microscopy |
References
- Kastner, E.; Kaur, R.; Lowry, D.; Moghaddam, B.; Wilkinson, A.; Perrie, Y. High-throughput manufacturing of size-tuned liposomes by a new microfluidics method using enhanced statistical tools for characterization. Int. J. Pharm. 2014, 477, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Dorati, R.; Modena, T.; Conti, B.; Genta, I. Multivariate analysis for the optimization of microfluidics-assisted nanoprecipitation method intended for the loading of small hydrophilic drugs into PLGA nanoparticles. Int. J. Pharm. 2018, 536, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Ag Seleci, D.; Maurer, V.; Stahl, F.; Scheper, T.; Garnweitner, G. Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery. Int. J. Mol. Sci. 2019, 20, 4696. [Google Scholar] [CrossRef] [PubMed]
- Mahale, N.B.; Thakkar, P.D.; Mali, R.G.; Walunj, D.R.; Chaudhari, S.R. Niosomes: Novel sustained release nonionic stable vesicular systems—An overview. Adv. Colloid Interface Sci. 2012, 183–184, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [PubMed]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Florence, A.T. Non-ionic surfactant vesicles (niosomes): Physical and pharmaceutical chemistry. Adv. Colloid Interface Sci. 1995, 58, 1–55. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Jadon, P.S.; Gajbhiye, V.; Jadon, R.S.; Gajbhiye, K.R.; Ganesh, N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech 2009, 10, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Attia, I.A.; El-Gizawy, S.A.; Fouda, M.A.; Donia, A.M. Influence of a niosomal formulation on the oral bioavailability of acyclovir in rabbits. AAPS PharmSciTech 2007, 8, E106. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, Z.S.; Yuksel, N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J. Pharm. Sci. 2010, 99, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Kirby, C.; Clarke, J.; Gregoriadis, G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem. J. 1980, 186, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Ali, M.H.; Kirby, D.J.; Mohammed, A.R.; Perrie, Y. Solubilisation of drugs within liposomal bilayers: Alternatives to cholesterol as a membrane stabilising agent. J. Pharm. Pharmacol. 2010, 62, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Bawage, S.; Tiwari, P.; Kirby, D.; Perrie, Y.; Dennis, V.; Singh, S.R. Liposomes: A promising carrier for respiratory syncytial virus therapeutics. Expert Opin. Drug Deliv. 2019, 16, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).