Deacidification of Palm Oil Using Betaine Monohydrate-Carboxylic Acid Deep Eutectic Solvents: Combined Extraction and Simple Solvent Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
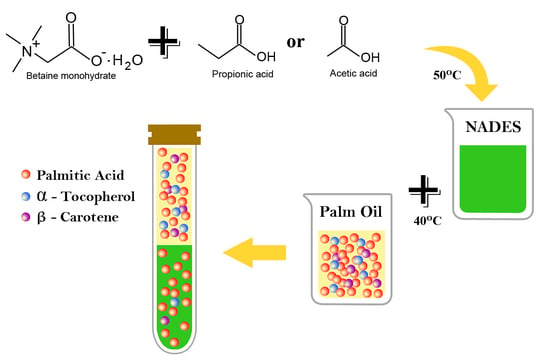
2.2. Preparation of DESs and Palm Oil
2.3. Liquid–Liquid Extraction
2.4. FT-IR and NMR Investigation of Oil-Rich and DES-Rich Phases
2.5. Solvent Recovery
3. Results and Discussion
3.1. Betaine Monohydrate-Carboxylic Acid DES as a Solvent for Deacidification of Palm Oil
3.2. Effect of Extraction Conditions on the Distribution Coefficients of Palmitic Acid
3.3. FT-IR and NMR Analysis of Oil-Rich and DES-Rich Phases
3.4. Solvent Recovery by Cooling the DES-Rich Phase to Solidify Palmitic Acid
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gee, P.T. Analytical characteristics of crude and refined palm oil and fractions. Eur. J. Lipid Sci. Technol. 2007, 109, 373–379. [Google Scholar] [CrossRef]
- Shahidi, F. Bailey’s Industrial Oil and Fat Products; Wiley Interscience: Hoboken, NJ, USA, 2005; Volume 2, pp. 333–429. [Google Scholar]
- Goh, S.; Choo, Y.; Ong, S. Minor constituents of palm oil. J. Am. Oil Chem. Soc. 1985, 62, 237–240. [Google Scholar] [CrossRef]
- Craft, B.D.; Nagy, K.; Seefelder, W.; Dubois, M.; Destaillats, F. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions. Part II: Practical recommendations for effective mitigation. Food Chem. 2012, 132, 73–79. [Google Scholar] [CrossRef]
- Ermacora, A.; Hrncirik, K. Influence of oil composition on the formation of fatty acid esters of 2-chloropropane-1,3-diol (2-MCPD) and 3-chloropropane-1,2-diol (3-MCPD) under conditions simulating oil refining. Food Chem. 2014, 161, 383–389. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Ceriani, R.; Silva, S.M.; Taham, T.; Meirelles, A.J. Steam deacidification of palm oil. Food Bioprod. Process. 2011, 89, 383–390. [Google Scholar] [CrossRef]
- Pirola, C.; Galli, F.; Comazzi, A.; Manenti, F.; Bianchi, C. Preservation of carotenes in the deacidification of crude palm oil. RSC Adv. 2014, 4, 46922–46925. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.P.; Krishna, A.G. Effect of different deacidification methods on phytonutrients retention in deacidified fractionated palm oil. J. Am. Oil Chem. Soc. 2015, 92, 645–658. [Google Scholar] [CrossRef]
- Gonçalves, C.B.; Meirelles, A.J.A. Liquid–liquid equilibrium data for the system palm oil + fatty acids + ethanol + water at 318.2K. Fluid Phase Equilib. 2004, 221, 139–150. [Google Scholar]
- da Silva, A.E.; Lanza, M.; Batista, E.A.; Rodrigues, A.M.; Meirelles, A.J.; da Silva, L.H.M. Liquid–Liquid Equilibrium Data for Systems Containing Palm Oil Fractions+Fatty Acids + Ethanol + Water. J. Chem. Eng. Data 2011, 56, 1892–1898. [Google Scholar] [CrossRef]
- Gonçalves, C.B.; Filho, P.A.P.; Meirelles, A.J. Partition of nutraceutical compounds in deacidification of palm oil by solvent extraction. J. Food Eng. 2007, 81, 21–26. [Google Scholar] [CrossRef]
- Wazeer, I.; Hayyan, M.; Hadj-Kali, M.K. Deep eutectic solvents: Designer fluids for chemical processes. J. Chem. Technol. Biotechnol. 2018, 93, 945–958. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Coelho, M.A.Z.; Marrucho, I.M. Extraction of saponins from sisal (Agave sisalana) and juá (Ziziphus joazeiro) with cholinium-based ionic liquids and deep eutectic solvents. Eur. Food Res. Technol. 2013, 237, 965–975. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Radosevic, K.; Curko, N.; Srcek, V.G.; Bubalo, M.C.; Tomasevic, M.; Ganic, K.K.; Redovnikovic, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Zahrina, I.; Nasikin, M.; Krisanti, E.; Mulia, K. Deacidification of palm oil using betaine monohydrate-based natural deep eutectic solvents. Food Chem. 2018, 240, 490–495. [Google Scholar] [CrossRef]
- Zahrina, I.; Nasikin, M.; Mulia, K.; Prajanto, M.; Yanuar, A. Molecular interactions between betaine monohydrate-glycerol deep eutectic solvents and palmitic acid: Computational and experimental studies. J. Mol. Liq. 2018, 251, 28–34. [Google Scholar] [CrossRef]
- Zahrina, I.; Nasikin, M.; Mulia, K. Evaluation of the interaction between molecules during betaine monohydrate-organic acid deep eutectic mixture formation. J. Mol. Liq. 2017, 225, 446–450. [Google Scholar] [CrossRef]
- Zahrina, I.; Mulia, K.; Yanuar, A.; Nasikin, M. Molecular interactions in the betaine monohydrate-polyol deep eutectic solvents: Experimental and computational studies. J. Mol. Struct. 2018, 1158, 133–138. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.M.; Garavazo, B.R.; Rodrigues, C.E. Liquid–liquid equilibria for systems composed of rice bran oil and alcohol-rich solvents: Application to extraction and deacidification of oil. J. Food Eng. 2012, 110, 418–427. [Google Scholar] [CrossRef]
- Plante, M.; Bailey, B.; Acworth, I.; Clark, D. Analysis of Lipids by HPLC-CAD; ESA-Dionex Inc.: Sunnyvale, CA, USA, 2011. [Google Scholar]
- Bruscatto, M.; Zambiazi, R.; Sganzerla, M.; Pestana, V.; Otero, D.; Lima, R.; Paiva, F. Degradation of tocopherols in rice bran oil submitted to heating at different temperatures. J. Chromatogr. Sci. 2009, 47, 762–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knockaert, G.; Pulissery, S.K.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Carrot β-Carotene Degradation and Isomerization Kinetics during Thermal Processing in the Presence of Oil. J. Agric. Food Chem. 2012, 60, 10312–10319. [Google Scholar] [CrossRef]
- Peng, X.; Duan, M.H.; Yao, X.H.; Zhang, Y.H.; Zhao, C.J.; Zu, Y.G.; Fu, Y.J. Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Mulia, K.; Adam, D.; Zahrina, I.; Krisanti, E. Green extraction of palmitic acid from palm oil using betaine-based Natural Deep Eutectic Solvents. Int. J. Technol. 2018, 9, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.E.; Onoyama, M.M.; Meirelles, A.J. Optimization of the rice bran oil deacidification process by liquid–liquid extraction. J. Food Eng. 2006, 73, 370–378. [Google Scholar] [CrossRef]
- Gonçalves, C.B.; Rodrigues, C.E.; Marcon, E.C.; Meirelles, A.J. Deacidification of palm oil by solvent extraction. Sep. Purif. Technol. 2016, 160, 106–111. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Israyandi; Zahrina, I.; Mulia, K. Optimization process condition for deacidification of palm oil by liquid-liquid extraction using NADES (Natural Deep Eutectic Solvent). AIP Conf. Proc. 2017, 1823, 020107. [Google Scholar]
- Koca, N.; Rodriguez-Saona, L.; Harper, W.; Alvarez, V. Application of Fourier transform infrared spectroscopy for monitoring short-chain free fatty acids in Swiss cheese. J. Dairy Sci. 2007, 90, 3596–3603. [Google Scholar] [CrossRef]
- SDBSWeb. National Institute of Advanced Industrial Science and Technology. Available online: http://riodb01.ibase.aist.go.jp/sdbs/ (accessed on 30 April 2020).






| Fatty Acid | % Mass |
|---|---|
| Palmitic (c16:0) | 44.6 |
| Oleic (c18:1) | 38.1 |
| Linoleic (c18:2) | 9.5 |
| Stearic (c18:0) | 4.3 |
| Myristic (c14:0) | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulia, K.; Nasikin, M.; Krisanti, E.A.; Zahrina, I. Deacidification of Palm Oil Using Betaine Monohydrate-Carboxylic Acid Deep Eutectic Solvents: Combined Extraction and Simple Solvent Recovery. Processes 2020, 8, 543. https://doi.org/10.3390/pr8050543
Mulia K, Nasikin M, Krisanti EA, Zahrina I. Deacidification of Palm Oil Using Betaine Monohydrate-Carboxylic Acid Deep Eutectic Solvents: Combined Extraction and Simple Solvent Recovery. Processes. 2020; 8(5):543. https://doi.org/10.3390/pr8050543
Chicago/Turabian StyleMulia, Kamarza, Mohammad Nasikin, Elsa Anisa Krisanti, and Ida Zahrina. 2020. "Deacidification of Palm Oil Using Betaine Monohydrate-Carboxylic Acid Deep Eutectic Solvents: Combined Extraction and Simple Solvent Recovery" Processes 8, no. 5: 543. https://doi.org/10.3390/pr8050543
APA StyleMulia, K., Nasikin, M., Krisanti, E. A., & Zahrina, I. (2020). Deacidification of Palm Oil Using Betaine Monohydrate-Carboxylic Acid Deep Eutectic Solvents: Combined Extraction and Simple Solvent Recovery. Processes, 8(5), 543. https://doi.org/10.3390/pr8050543