Activated Sludge Respiration Activity Inhibition Caused by Mobile Toilet Chemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Mobile Toilet Chemicals
2.2. Other Chemicals
2.3. Sludge Samples
2.4. Respiratory Activity Testing
2.5. Isolation of Pure Bacterial Strains
2.6. Bacterial Strains Identification
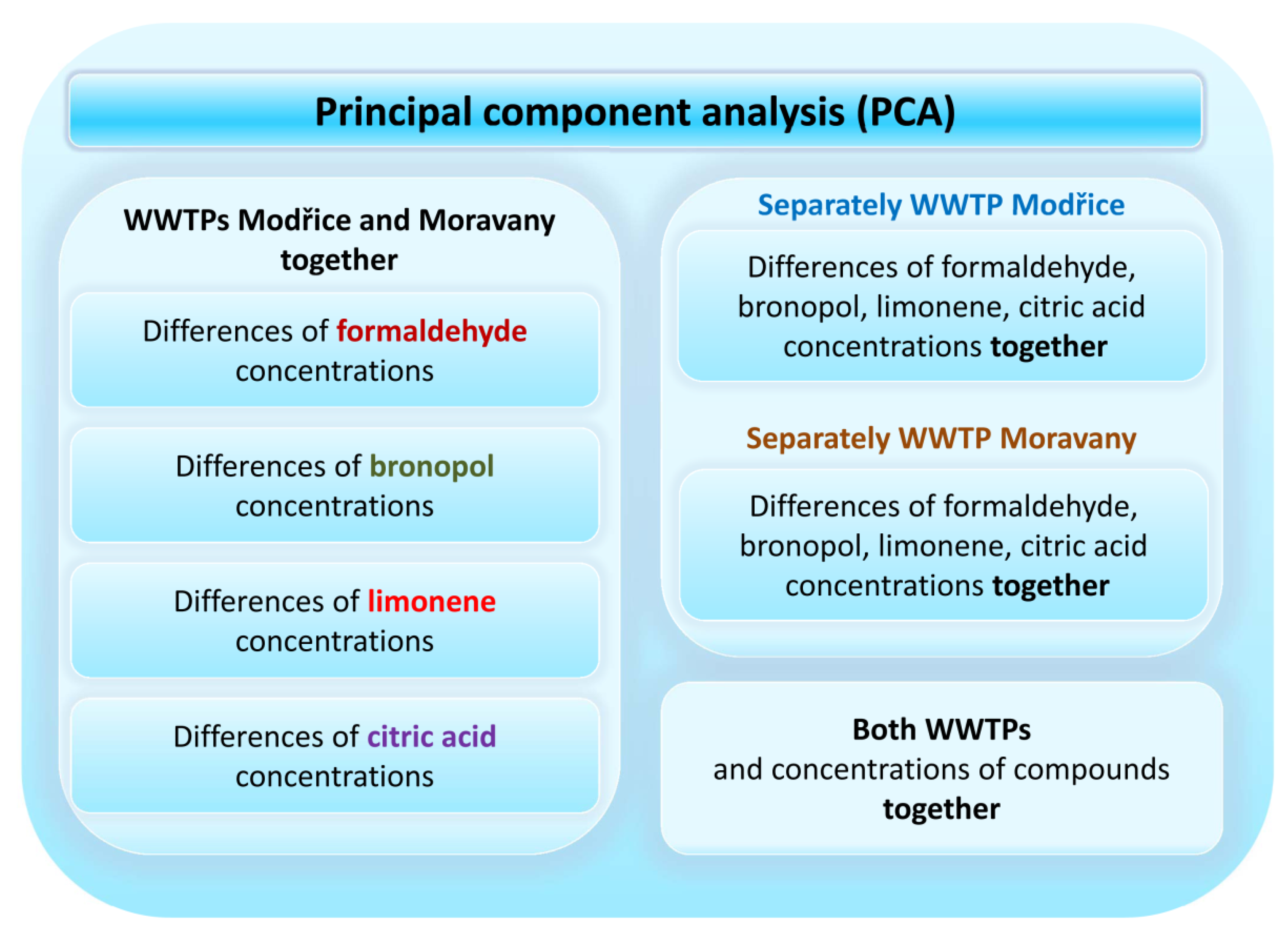
2.7. Statistical Analysis
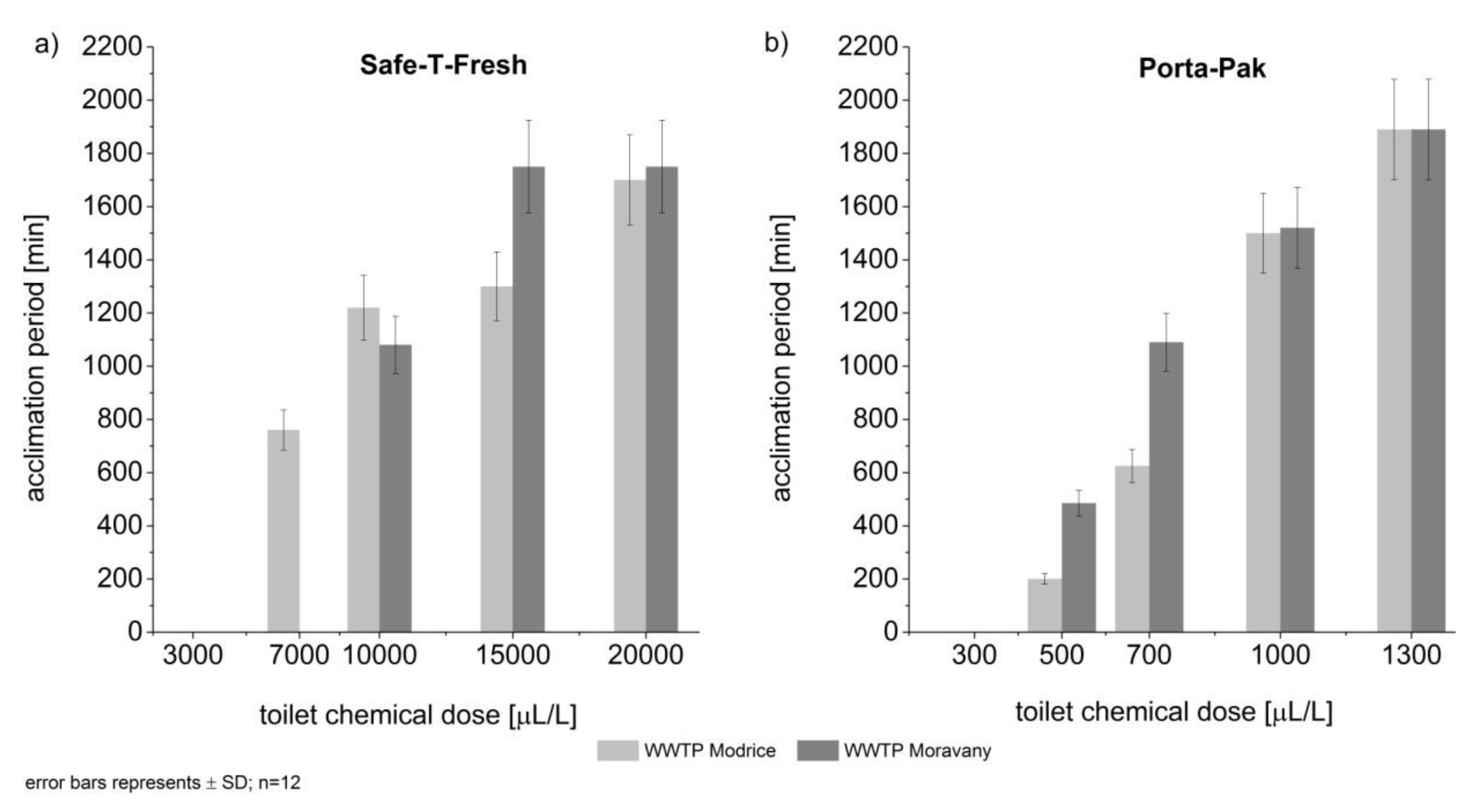
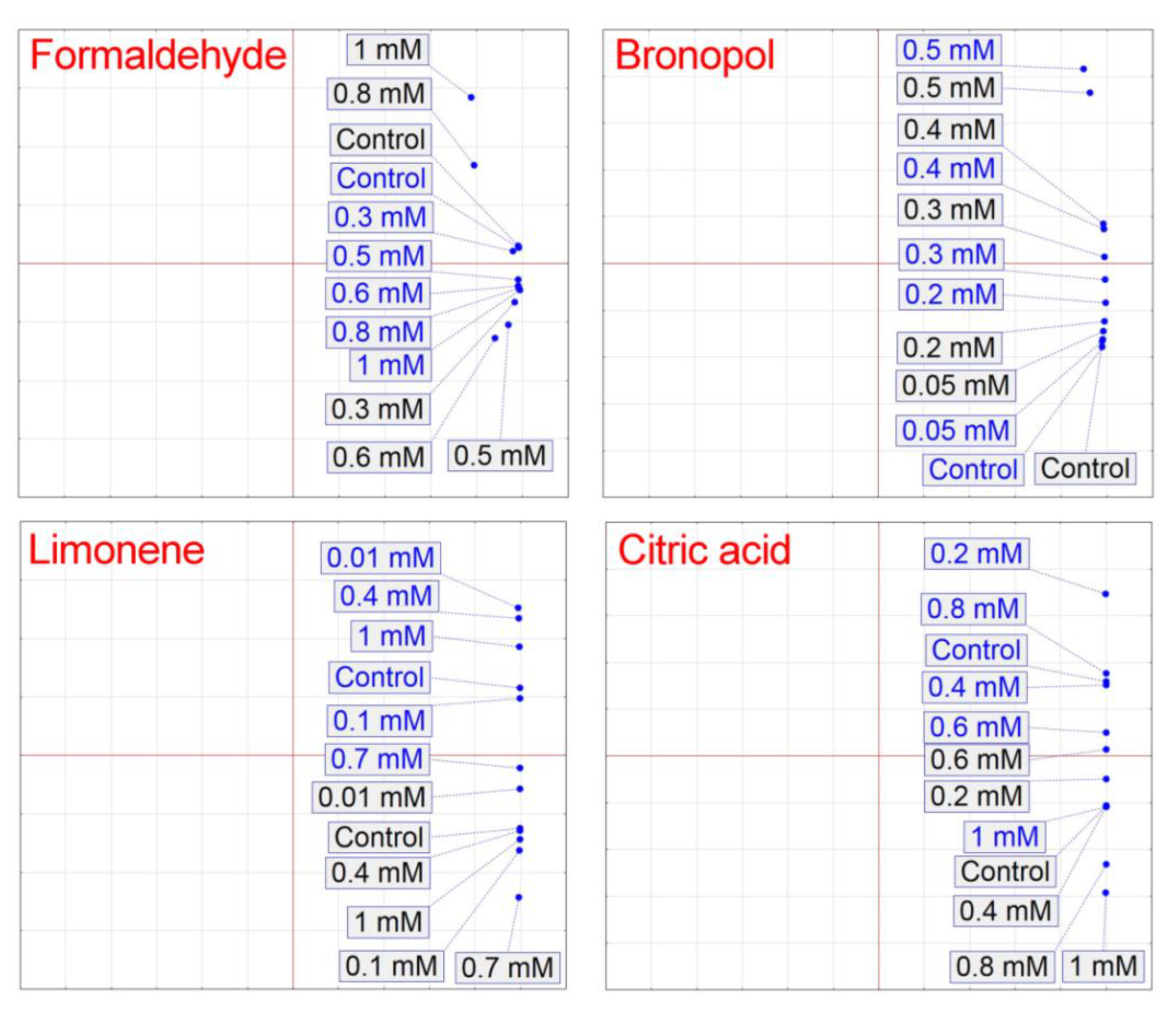
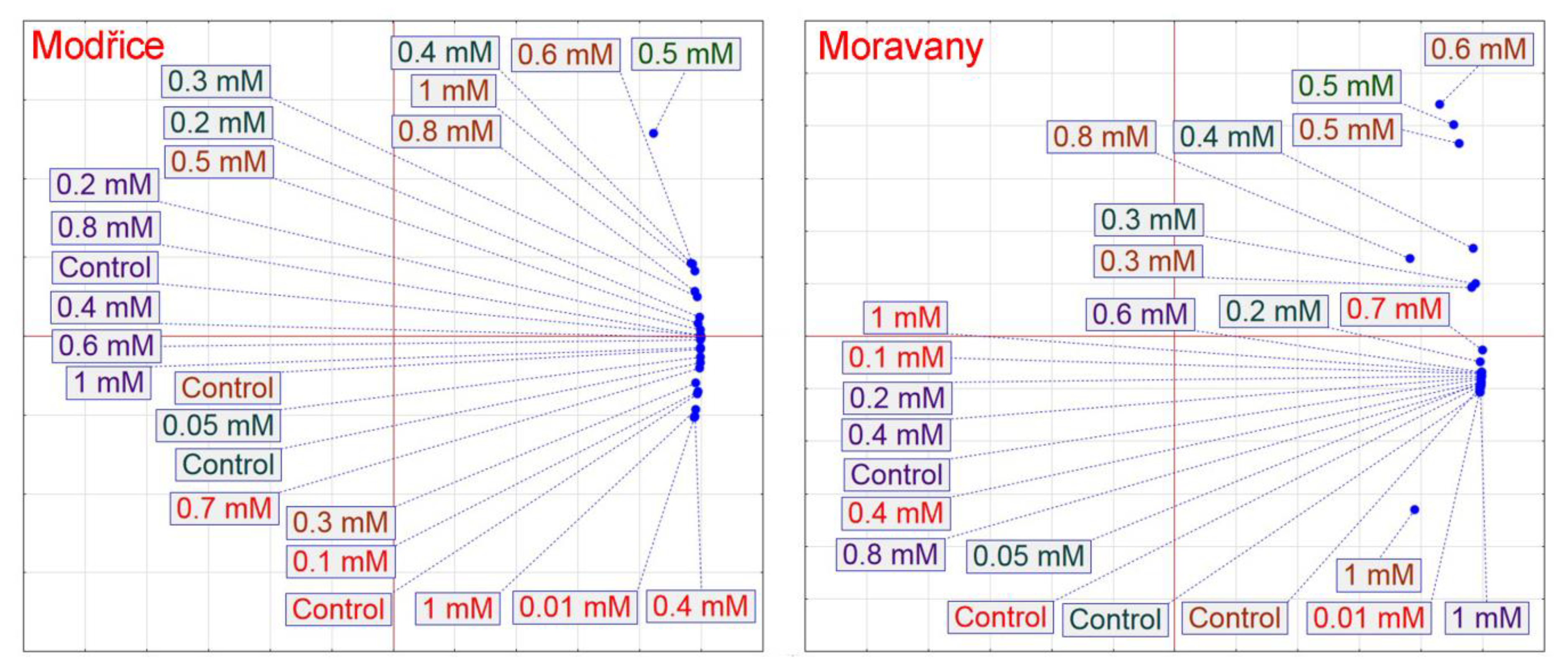
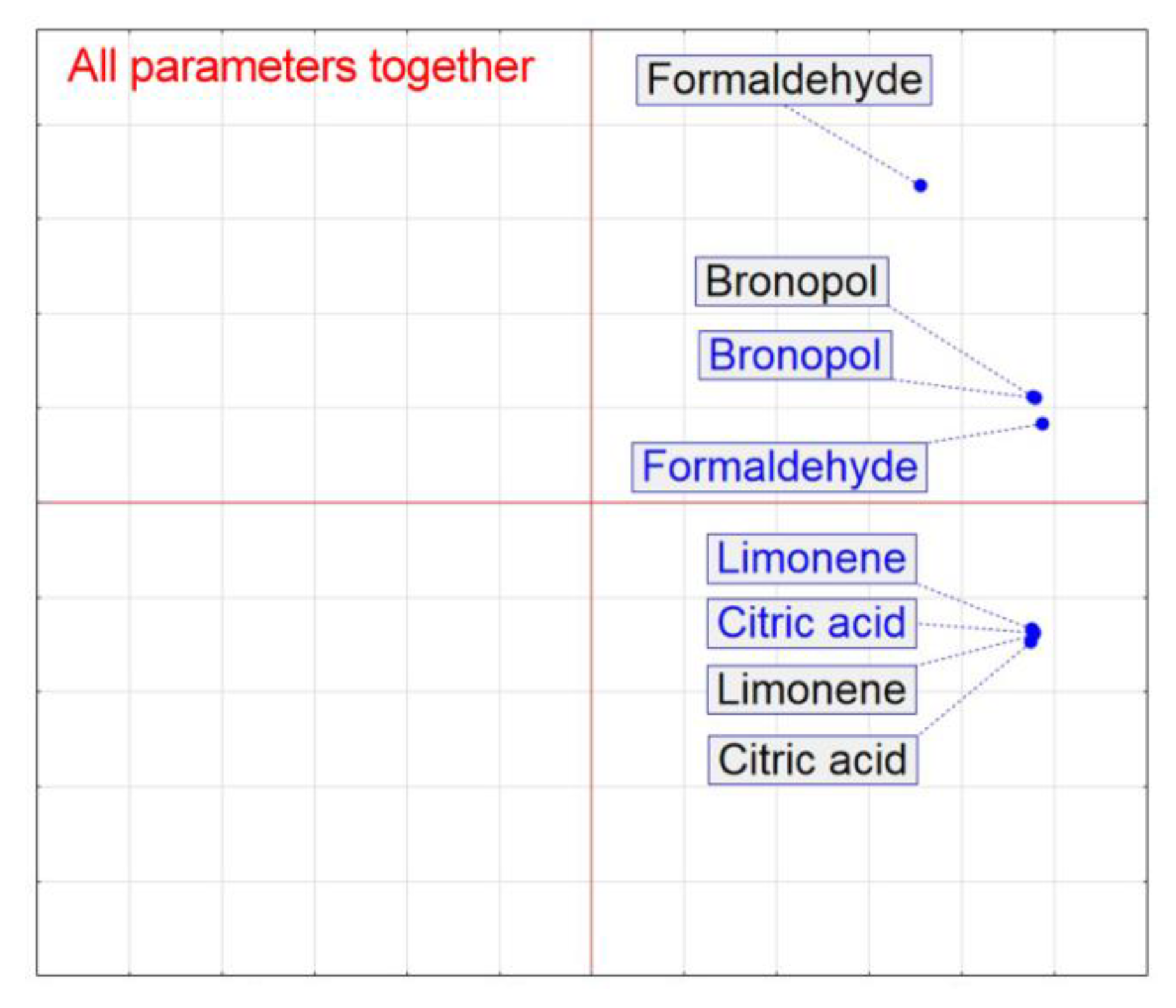
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 25 January 2020).
- Watson, J.T. Tennessee valley marina and campground wastewater characterization screening study. Proc. Water Environ. Fed. 2005, 12, 3685–3712. [Google Scholar]
- Kersh, J.; James, C.A.; Gugh, H.L. Impacts of High-Strength Boat Waste on Activated Sludge Processes. J. Environ. Eng. 2020, 146, 04020023. [Google Scholar] [CrossRef]
- Novak, J.T.; McDaniel, C.R.; Howard, S.C. The effect of boat holding tank chemicals on treatment plant performance. Res. J. Water Pollut. Control Fed. 1990, 62, 288–295. [Google Scholar]
- Vítězová, M.; Nitayapat, N.; Vítěz, T. Temperature and De-icing Salt, Effect on the Activated Sludge Respiration. Clean Soil Air Water 2018, 46, 1800050. [Google Scholar] [CrossRef]
- Walker, W.; Haley, C.; Bridgeman, P.; Goldstein, S. Effects of deodorants on treatment of boat holding-tank waste. Environ. Manag. 1991, 15, 441–449. [Google Scholar] [CrossRef]
- Garrido, J.M.; Mendez, R.; Lema, J.M. Treatment of wastewaters from a formaldehyde–urea adhesives. Water Sci. Technol. 2000, 42, 293–301. [Google Scholar] [CrossRef]
- Karsa, D.R. Biocides. In Handbook for Cleaning/Decontamination of Surfaces; Johansson, I., Somasundaran, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 593–623. [Google Scholar]
- Zoutberg, G.R.; de Been, P. The Biobed® EGSB (expanded granular sludge bed) system covers shortcomings of the upflow anaerobic sludge blanket reactor in the chemical industry. Water Sci. Technol. 1997, 35, 183–188. [Google Scholar] [CrossRef]
- Kumar, A. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–163. [Google Scholar] [CrossRef]
- Cvetkovic, Z.; Suhadolnik, Z.; Grilc, V. Biological treatment of wastewater from a phenol–formaldehyde resins production. Chem. Eng. J. 1998, 2, 262–273. [Google Scholar]
- Alvarez-Rivera, G.; Llompart, M.; Lores, M.; Garcia–Jares, C. Preservatives in cosmetics: Regulatory aspects and analytical methods. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–224. [Google Scholar]
- Bryce, D.M.; Croshaw, B.; Hall, J.E.; Holland, V.R.; Lessel, B. The activity and safety of the antimicrobial agent Bronopol (2-bromo-2-nitropropan-1,3-diol). J. Soc. Cosmet. Chem. 1978, 29, 3–24. [Google Scholar]
- Challis, B.C.; Yousaf, T.I. The reaction of geminal bromonitroalkanes with nucleophiles. Part 1. The decomposition of 2-bromo-2-nitropropane-1,3-diol (Bronopol) in aqueous base. J. Chem. Soc. Perkin Trans. 1991, 2, 283–286. [Google Scholar] [CrossRef]
- Kajimura, K.; Tagami, T.; Yamamoto, T.; Iwagami, S. The release of formaldehyde upon decomposition of 2-bromo-2-nitropropan-1,3-diol (bronopol). J. Health Sci. 2008, 54, 488–492. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Chapter 11—Terpenoids. In Pharmacognosy Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Academic Press: London, UK, 2017; pp. 233–266. [Google Scholar]
- Chikhoune, A.; Hazzit, M.; Kerbouche, L.; Baaliouamer, A.; Aissat, K. Tetraclinis articulata (Vahl) Masters essential oils: Chemical composition and biological activities. J. Essent. Oil Res. 2013, 25, 300–307. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1, 8-cineole alone and in combination. Flavour Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Zukerman, I. Effect of oxidized d-limonene on micro-organisms. Nature 1951, 168, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Søltoft-Jensen, J.; Hansen, F. New chemical and biochemical hurdles. In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Academic Press: San Diego, CA, USA, 2005; pp. 387–416. [Google Scholar]
- OECD. Test No. 301F: Ready Biodegradability, Manometric Respirometry, Test OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 1992. [Google Scholar]
- Bailey, N.T.J. Statistical Methods in Biology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Vítězová, M.; Vítěz, T.; Nitayapat, N. Effects of De-icing Salts on the Respiration of the Microorganisms of Activated Sludge. Water Air Soil Pollut. 2016, 227, 416. [Google Scholar] [CrossRef]
- Gilligan, P.H.; Lum, G.; Vandamme, P.A.R.; Whittier, S. Burkholderia, stenotrophomonas, ralstonia, brevundimonas, comamonas, delftia, pandoraea and acidovorax. In Manual of Clinical Microbiology, 8th ed.; Murray, P.R., Baron, E.J., Jorgensen, J.H., Pfaller, M.A., Yolken, R.H., Eds.; ASM: Washington, DC, USA, 2003; pp. 729–748. [Google Scholar]
- Ryan, M.; Pembroke, J.; Adley, C. Ralstonia pickettii in environmental biotechnology: Potential and applications. J. Appl. Microbiol. 2007, 103, 754–764. [Google Scholar] [CrossRef] [PubMed]


| Product | Manufacturer | Prevailed Chemical Substance |
|---|---|---|
| Qualicar | STACHEMA CZ, CZ | formaldehyde (<16% w/w) |
| Instagreen Spezial | Campingaz, FR | citric acid (>5% w/w) |
| Elsan Double Blue | Elsan Limited, UK | formaldehyde (20–30% w/w) |
| Aqua Kem Blue | Thetford, US | formaldehyde (20–30% w/w) |
| Aqua Rinse Spray | Thetford, US | bronopol (2-bromo-2-nitropropan-1,3-diol) (0.1–1% w/w) |
| Aqua Kem Sachets | Thetford, US | bronopol (2-bromo-2-nitropropan-1,3-diol) (20–30% w/w) |
| Porta-Pak * | Walex, US | bronopol (2-bromo-2-nitropropan-1,3-diol) (3–8% w/w) |
| Power Care Tabs | Dometic, SE | bronopol (2-bromo-2-nitropropan-1,3-diol) (1–10% w/w) |
| Safe-T-Fresh 5000 | Satellite, US | bronopol (2-bromo-2-nitropropan-1,3-diol) (1–10% w/w) |
| Safe-T-Fresh 4000 * | Satellite, US | bronopol (2-bromo-2-nitropropan-1,3-diol) (1–10% w/w) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vítěz, T.; Vítězová, M.; Nováčková, M.; Kushkevych, I. Activated Sludge Respiration Activity Inhibition Caused by Mobile Toilet Chemicals. Processes 2020, 8, 598. https://doi.org/10.3390/pr8050598
Vítěz T, Vítězová M, Nováčková M, Kushkevych I. Activated Sludge Respiration Activity Inhibition Caused by Mobile Toilet Chemicals. Processes. 2020; 8(5):598. https://doi.org/10.3390/pr8050598
Chicago/Turabian StyleVítěz, Tomáš, Monika Vítězová, Markéta Nováčková, and Ivan Kushkevych. 2020. "Activated Sludge Respiration Activity Inhibition Caused by Mobile Toilet Chemicals" Processes 8, no. 5: 598. https://doi.org/10.3390/pr8050598
APA StyleVítěz, T., Vítězová, M., Nováčková, M., & Kushkevych, I. (2020). Activated Sludge Respiration Activity Inhibition Caused by Mobile Toilet Chemicals. Processes, 8(5), 598. https://doi.org/10.3390/pr8050598





