Graphene-Based Hydrogen Gas Sensors: A Review
Abstract
:1. Introduction
1.1. Surface Functionalization of Graphene and Other Carbon Materials by Creation of Surface Heterogenous Centers
1.2. Carbon Surface Functionalization by Tailoring Structural Parameters
2. Hydrogen Gas Sensors
2.1. Semiconductor Metal Oxides as Receptor Materials for Hydrogen Sensing
2.2. Other Sensing Materials Applicable to Hydrogen Sensing
2.3. Graphene-Based Materials for Hydrogen Sensors
2.3.1. Graphene-Polymer Modified
2.3.2. Graphene-Metal Modified
2.3.3. Graphene-Metal Oxide Nanocomposite
3. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Korotcenkov, G. Chemical Sensors: Comprehensive Sensor Technologies Volume 5: Electrochemical and Optical Sensors; Momentum Press: New York, NY, USA, 2011. [Google Scholar]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Scipioni, A.; Manzardo, A.; Ren, J. Hydrogen Economy: Supply Chain, Life Cycle Analysis and Energy Transition for Sustainability; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Hydrogen Isn’t the Fuel of the Future. It’s Already Here. Available online: https://www.weforum.org/agenda/2019/06/the-clean-energy-of-the-future-is-already-here/ (accessed on 17 May 2020).
- Ramachandran, R.; Menon, R.K. An overview of industrial uses of hydrogen. Int. J. Hydrogen Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Maynart, M.C.; Aveiro, L.R.; Da Paz, E.C.; Pinheiro, V.D.S. Carbon-Based Materials: Recent Advances, Challenges, and Perspectives. Ref. Modul. Mater. Sci. Mater. Eng. 2017. [Google Scholar] [CrossRef]
- 60 Uses of Graphene—The Ultimate Guide to Graphene’s (Potential) Applications 2016. Available online: https://nanografi.com/blog/60-uses-of-graphene/ (accessed on 17 May 2020).
- Lukaszewicz, J.P. Carbon Materials for Chemical Sensors: A Review. Sens. Lett. 2006, 4, 53–98. [Google Scholar] [CrossRef]
- Lukaszewicz, J.P. Carbon Films for Humidity Sensors. Sens. Lett. 2006, 4, 281–304. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.C.; Freitas, M.M.; Órfão, J. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Yadav, R.; Dixit, C. Synthesis, characterization and prospective applications of nitrogen-doped graphene: A short review. J. Sci. Adv. Mater. Devices 2017, 2, 141–149. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Dinh, N.X.; Van Quy, N.; Huy, T.Q.; Le, A.-T. Decoration of Silver Nanoparticles on Multiwalled Carbon Nanotubes: Antibacterial Mechanism and Ultrastructural Analysis. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cyganiuk, A.; Klimkiewicz, R.; Olejniczak, A.; Lukaszewicz, J.P. Biotechnological fabrication of LaMnO3-carbon catalyst for n-butanol conversion to ketones. Carbon 2010, 48, 99–106. [Google Scholar] [CrossRef]
- Cyganiuk, A.; Klimkiewicz, R.; Bumajdad, A.; Ilnicka, A.; Lukaszewicz, J.P. Nanostructured composite TiO2/carbon catalysts of high activity for dehydration of n-butanol. Mater. Sci. Eng. B 2015, 198, 35–42. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, G.-C. Stacking control in graphene-based materials: A promising method for fascinating physical properties. Front. Phys. 2018, 14, 23301. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.N.; Ghosh, A. Ultralow noise field-effect transistor from multilayer graphene. Appl. Phys. Lett. 2009, 95, 82105. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. AIP Adv. 2014, 4, 27116. [Google Scholar] [CrossRef]
- Kamedulski, P.; Ilnicka, A.; Lukaszewicz, J.P. Selected Aspects of Graphene Exfoliation as an Introductory Step Towards 3D Structuring of Graphene Nano-Sheets. Curr. Graphene Sci. 2019, 2, 106–117. [Google Scholar] [CrossRef]
- Taguchi, N. Gas Detecting Element and Method of Making It. U.S. Patent US3695848A, 22 February 1972. [Google Scholar]
- Ihokura, K.; Watson, J. The Stannic Oxide Gas SensorPrinciples and Applications; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Chen, W.; Zhou, Q.; Wan, F.; Gao, T. Gas Sensing Properties and Mechanism of Nano-SnO2 -Based Sensor for Hydrogen and Carbon Monoxide. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Wisitsora-At, A.; Tuantranont, A.; Comini, E.; Sberveglieri, G.; Wlodarski, W. Characterization of n-type and p-type semiconductor gas sensors based on NiOx doped TiO2 thin films. Thin Solid Films 2009, 517, 2775–2780. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwak, S.; Lee, J.-H.; Kim, I.; Yoo, Y.K.; Lee, T.H.; Shim, Y.-S.; Kim, J.; Lee, K.H. Sputtered PdO Decorated TiO2 Sensing Layer for a Hydrogen Gas Sensor. J. Nanomater. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Yu, Y.; Hahn, Y.-B. Ultra thin NiO nanosheets for high performance hydrogen gas sensor device. Appl. Surf. Sci. 2020, 506, 144971. [Google Scholar] [CrossRef]
- Raza, M.H.; Kaur, N.; Comini, E.; Pinna, N. Toward Optimized Radial Modulation of the Space-Charge Region in One-Dimensional SnO2–NiO Core–Shell Nanowires for Hydrogen Sensing. ACS Appl. Mater. Interfaces 2020, 12, 4594–4606. [Google Scholar] [CrossRef] [PubMed]
- Vomiero, A.; Ferroni, M.; Comini, E.; Faglia, G.; Sberveglieri, G. Preparation of Radial and Longitudinal Nanosized Heterostructures of In2O3 and SnO2. Nano Lett. 2007, 7, 3553–3558. [Google Scholar] [CrossRef]
- Thai, N.X.; Van Duy, N.; Van Toan, N.; Hung, C.M.; Van Hieu, N.; Hoa, N.D. Effective monitoring and classification of hydrogen and ammonia gases with a bilayer Pt/SnO2 thin film sensor. Int. J. Hydrogen Energy 2020, 45, 2418–2428. [Google Scholar] [CrossRef]
- Wu, C.-H.; Zhu, Z.; Chang, H.-M.; Jiang, Z.-X.; Hsieh, C.-Y.; Wu, R.-J. Pt@NiO core–shell nanostructure for a hydrogen gas sensor. J. Alloy. Compd. 2020, 814, 151815. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Chandra, R. Porous silicon filled with Pd/WO3 –ZnO composite thin film for enhanced H2 gas-sensing performance. RSC Adv. 2017, 7, 39666–39675. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, N.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen Gas Sensors Based on Semiconductor Oxide Nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [Green Version]
- Sitarz, M.; Kwoka, M.; Comini, E.; Zappa, D.; Szuber, J. Surface chemistry of SnO2 nanowires on Ag-catalyst-covered Si substrate studied using XPS and TDS methods. Nanoscale Res. Lett. 2014, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Trung, D.D.; Hoa, N.D.; Van Tong, P.; Van Duy, N.; Dao, T.; Chung, H.; Nagao, T.; Van Hieu, N. Effective decoration of Pd nanoparticles on the surface of SnO2 nanowires for enhancement of CO gas-sensing performance. J. Hazard. Mater. 2014, 265, 124–132. [Google Scholar] [CrossRef]
- Helwig, A.; Müller, G.; Sberveglieri, G.; Faglia, G. Catalytic enhancement of SnO2 gas sensors as seen by the moving gas outlet method. Sens. Actuators B Chem. 2008, 130, 193–199. [Google Scholar] [CrossRef]
- Available online: http://www.figarosensor.com/ (accessed on 17 May 2020).
- Gaiardo, A.; Fabbri, B.; Guidi, V.; Bellutti, P.; Giberti, A.; Gherardi, S.; Vanzetti, L.; Malagù, C.; Zonta, G. Metal Sulfides as Sensing Materials for Chemoresistive Gas Sensors. Sensors 2016, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- Gottam, S.R.; Tsai, C.-T.; Wang, L.-W.; Wang, C.-T.; Lin, C.-C.; Chu, S.-Y. Highly sensitive hydrogen gas sensor based on a MoS2-Pt nanoparticle composite. Appl. Surf. Sci. 2020, 506, 144981. [Google Scholar] [CrossRef]
- Guidi, V.; Fabbri, B.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Malagù, C.; Zonta, G.; Bellutti, P. Metal Sulfides as a New Class of Sensing Materials. Procedia Eng. 2015, 120, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiang, H.; Zhang, J.; Huang, Y.; Tian, J.; Deng, X.; Zhao, X.; Zhang, W. Flexible nanofiber sensor for low-concentration hydrogen detection. Nanotechnology 2019, 31, 015504. [Google Scholar] [CrossRef]
- Cho, K.H.; Yu, H.; Lee, J.S.; Jang, J. Facile synthesis of palladium-decorated three-dimensional conducting polymer nanofilm for highly sensitive H2 gas sensor. J. Mater. Sci. 2020, 55, 5156–5165. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Wlodarski, W.; Kalantar-Zadeh, K.; Baker, C.; Kaner, R. Doped and dedoped polyaniline nanofiber based conductometric hydrogen gas sensors. Sens. Actuators A Phys. 2007, 139, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, M.; Weppner, W. Response Behaviour of a Hydrogen Sensor Based on Ionic Conducting Polymer-metal Interfaces Prepared by the Chemical Reduction Method. Sensors 2006, 6, 284–297. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Kim, S.-J.; Shin, H.; Koo, W.-T.; Jang, J.-S.; Kang, J.-Y.; Jeong, Y.J.; Kim, I.-D. High-Resolution, Fast, and Shape-Conformable Hydrogen Sensor Platform: Polymer Nanofiber Yarn Coupled with Nanograined Pd@Pt. ACS Nano 2019, 13, 6071–6082. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Mukhopadhyay, S.; Xu, Y. Carbon nanotubes and its gas-sensing applications: A review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Kim, M.I.; Lee, Y.-S. A Comprehensive Review of Gas Sensors Using Carbon Materials. J. Nanosci. Nanotechnology 2016, 16, 4310–4319. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Bhattacharya, S. Hydrogen gas sensing methods, materials, and approach to achieve parts per billion level detection: A review. Int. J. Hydrogen Energy 2019, 44, 26076–26099. [Google Scholar] [CrossRef]
- McConnell, C.; Kanakaraj, S.N.; Dugre, J.; Malik, R.; Zhang, G.; Haase, M.R.; Hsieh, Y.-Y.; Fang, Y.; Mast, D.; Shanov, V. Hydrogen Sensors Based on Flexible Carbon Nanotube-Palladium Composite Sheets Integrated with Ripstop Fabric. ACS Omega 2019, 5, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Jaggi, N. Hydrogen Gas Sensing Characteristics of Multiwalled Carbon Nanotubes Based Hybrid Composites. J. Electron. Mater. 2015, 45, 695–702. [Google Scholar] [CrossRef]
- Dhall, S.; Sood, K.; Jaggi, N. A hydrogen gas sensor using a Pt-sputtered MWCNTs/ZnO nanostructure. Meas. Sci. Technol. 2014, 25, 085103. [Google Scholar] [CrossRef]
- Dhall, S.; Jaggi, N. Room temperature hydrogen gas sensing properties of Pt sputtered F-MWCNTs/SnO2 network. Sens. Actuators B Chem. 2015, 210, 742–747. [Google Scholar] [CrossRef]
- Du, Y.G.; Zheng, H.X.; Ni, H. A Room-temperature Hydrogen Gas Sensor Using Palladium-decorated Single-Walled Carbon Nanotube/Si Heterojunction. Mater. Sci. 2016, 22, 209–212. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.; Han, M.; Lee, G.S. Fast-Response Room Temperature Hydrogen Gas Sensors Using Platinum-Coated Spin-Capable Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 3050–3057. [Google Scholar] [CrossRef]
- Mansha, M.; Qurashi, A.; Ullah, N.; Bakare, F.O.; Khan, I.; Yamani, Z.H. Synthesis of In2O3/graphene heterostructure and their hydrogen gas sensing properties. Ceram. Int. 2016, 42, 11490–11495. [Google Scholar] [CrossRef]
- Wang, J.; Rathi, S.; Singh, B.; Lee, I.; Joh, H.-I.; Kim, G.-H. Alternating Current Dielectrophoresis Optimization of Pt-Decorated Graphene Oxide Nanostructures for Proficient Hydrogen Gas Sensor. ACS Appl. Mater. Interfaces 2015, 7, 13768–13775. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, Q.; Du, Y.; Ling, C.; Xing, W. Highly enhanced sensitivity of hydrogen sensors using novel palladium-decorated graphene nanoribbon film/SiO2/Si structures. J. Mater. Chem. A 2014, 2, 15931–15937. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Wang, J.; Kwak, Y.; Lee, I.-Y.; Maeng, S.; Kim, G.-H. Highly responsive hydrogen gas sensing by partially reduced graphite oxide thin films at room temperature. Carbon 2012, 50, 4061–4067. [Google Scholar] [CrossRef]
- Pavithra, A.; Rakkesh, R.A.; Durgalakshmi, D.; Balakumar, S. Room Temperature Detection of Hydrogen Gas Using Graphene Based Conductometric Gas Sensor. J. Nanosci. Nanotechnol. 2017, 17, 3449–3453. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and application of nanoporous graphene oxide film for CO2, H2, and C2H2 gases sensing. J. Mater. Res. Technol. 2019, 8, 4510–4520. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Wang, W.D.; Liang, X.-Q.; Chu, W.-S.; Song, W.; Wu, Z.-Y. Characterization of partially reduced graphene oxide as room temperature sensor for H2. Nanoscale 2011, 3, 2458–2460. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Hazra, S.K. Graphene—Noble Metal Nano-Composites and Applications for Hydrogen Sensors. C J. Carbon Res. 2017, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Kim, J.-S. MEMS based highly sensitive dual FET gas sensor using graphene decorated Pd-Ag alloy nanoparticles for H2 detection. Sci. Rep. 2018, 8, 5902. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Meng, F.; Guo, Z.; Huang, X.-J. Graphene-based hybrids for chemiresistive gas sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In Situ Polymerization Deposition of Porous Conducting Polymer on Reduced Graphene Oxide for Gas Sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, D.; Koo, H.Y.; Maeng, S. Chemically modified graphene/PEDOT: PSS nanocomposite films for hydrogen gas sensing. Carbon 2015, 81, 54–62. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Shin, K.; Kalantar-Zadeh, K.; Plessis, J.D.; Han, S.H.; Kojima, R.W.; Kaner, R.B.; Li, D.; Gou, X.; Ippolito, S.J.; et al. Graphene/Polyaniline Nanocomposite for Hydrogen Sensing. J. Phys. Chem. C 2010, 114, 16168–16173. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Q.; Xiang, C.; Tang, C.; Chu, H.; Qiu, S.; Yan, E.; Xu, F.; Sun, L. Doping composite of polyaniline and reduced graphene oxide with palladium nanoparticles for room-temperature hydrogen-gas sensing. Int. J. Hydrogen Energy 2016, 41, 5396–5404. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Lee, S.; Seo, J.; Pyo, S.; Kim, J.; Lee, T. A Highly Sensitive Hydrogen Sensor with Gas Selectivity Using a PMMA Membrane-Coated Pd Nanoparticle/Single-Layer Graphene Hybrid. ACS Appl. Mater. Interfaces 2015, 7, 3554–3561. [Google Scholar] [CrossRef]
- Alfano, B.; Polichetti, T.; Miglietta, M.L.; Massera, E.; Schiattarella, C.; Ricciardella, F.; Di Francia, G. Fully eco-friendly H2 sensing device based on Pd-decorated graphene. Sens. Actuators B Chem. 2017, 239, 1144–1152. [Google Scholar] [CrossRef]
- Chu, B.H.; Lo, C.; Nicolosi, J.; Chang, C.; Chen, V.; Strupiński, W.; Pearton, S.J.; Ren, F. Hydrogen detection using platinum coated graphene grown on SiC. Sens. Actuators B Chem. 2011, 157, 500–503. [Google Scholar] [CrossRef]
- Vedala, H.; Sorescu, D.C.; Kotchey, G.P.; Star, A. Chemical Sensitivity of Graphene Edges Decorated with Metal Nanoparticles. Nano Lett. 2011, 11, 2342–2347. [Google Scholar] [CrossRef]
- Shafiei, M.; Spizzirri, P.G.; Arsat, R.; Yu, J.; Du Plessis, J.; Dubin, S.; Kaner, R.B.; Kalantar-Zadeh, K.; Wlodarski, W. Platinum/Graphene Nanosheet/SiC Contacts and Their Application for Hydrogen Gas Sensing. J. Phys. Chem. C 2010, 114, 13796–13801. [Google Scholar] [CrossRef]
- Shafiei, M.; Arsat, R.; Yu, J.; Kalantar-Zadeh, K.; Wlodarski, W.; Dubin, S.; Kaner, R. Pt/graphene nano-sheet based hydrogen gas sensor. In Proceedings of the SENSORS, 2009 IEEE, Christchurch, New Zealand, 25–28 October 2009; pp. 295–298. [Google Scholar]
- Chu, B.H.; Nicolosi, J.; Lo, C.F.; Strupiński, W.; Pearton, S.J.; Ren, F. Effect of Coated Platinum Thickness on Hydrogen Detection Sensitivity of Graphene-Based Sensors. Electrochem. Solid-State Lett. 2011, 14, K43–K45. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Jafri, R.I.; Arockiadoss, T.; Ramaprabhu, S. Nanostructured Pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor. Nanoscale 2009, 1, 382. [Google Scholar] [CrossRef]
- Harley-Trochimczyk, A.; Chang, J.; Zhou, Q.; Dong, J.; Pham, T.; Worsley, M.A.; Maboudian, R.; Zettl, A.; Mickelson, W. Catalytic hydrogen sensing using microheated platinum nanoparticle-loaded graphene aerogel. Sens. Actuators B Chem. 2015, 206, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Song, X.; Gu, C.; Ren, H.; Sun, Y.; Huang, J. Freeze drying-assisted synthesis of Pt@reduced graphene oxide nanocomposites as excellent hydrogen sensor. J. Phys. Chem. Solids 2018, 116, 324–330. [Google Scholar] [CrossRef]
- Zhu, L.; Jia, Y.; Gai, G.; Ji, X.; Luo, J.; Yao, Y. Ambipolarity of large-area Pt-functionalized graphene observed in H2 sensing. Sens. Actuators B Chem. 2014, 190, 134–140. [Google Scholar] [CrossRef]
- Yu, J.; Shafiei, M.; Ou, J.Z.; Shin, K.; Wlodarski, W. A study of hydrogen gas sensing performance of Pt/Graphene/GaN devices. In Proceedings of the 2011 IEEE SENSORS, Limerick, Ireland, 28–31 October 2011; pp. 1017–1020. [Google Scholar]
- Pak, Y.; Kim, S.-M.; Jeong, H.; Kang, C.G.; Park, J.S.; Song, H.; Lee, R.; Myoung, N.; Lee, B.H.; Seo, S.; et al. Palladium-Decorated Hydrogen-Gas Sensors Using Periodically Aligned Graphene Nanoribbons. ACS Appl. Mater. Interfaces 2014, 6, 13293–13298. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.; Hirsch, T.; Mirsky, V.M.; Wolfbeis, O.S. Hydrogen sensor based on a grapheme—Palladium nanocomposite. Electrochim. Acta 2011, 56, 3707–3712. [Google Scholar] [CrossRef]
- Johnson, J.L.; Behnam, A.; Pearton, S.J.; Ural, A. Hydrogen Sensing Using Pd-Functionalized Multi-Layer Graphene Nanoribbon Networks. Adv. Mater. 2010, 22, 4877–4880. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.-H.; Seo, D.K.; Kim, T.; Im, H.U.; Lee, H.M.; Yoo, J.B.; Hong, S.-H.; Kang, T.J.; Kim, Y.H. Flexible hydrogen sensors using graphene with palladium nanoparticle decoration. Sens. Actuators B Chem. 2012, 169, 387–392. [Google Scholar] [CrossRef]
- Lee, J.S.; Oh, J.; Jun, J.; Jang, J. Wireless Hydrogen Smart Sensor Based on Pt/Graphene-Immobilized Radio-Frequency Identification Tag. ACS Nano 2015, 9, 7783–7790. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Z.; Jauregui, L.A.; Yu, Q.; Pillai, R.; Cao, H.; Bao, J.; Chen, Y.P.; Pei, S.-S. Wafer-scale synthesis of graphene by chemical vapor deposition and its application in hydrogen sensing. Sens. Actuators B Chem. 2010, 150, 296–300. [Google Scholar] [CrossRef]
- Pandey, P.; Wilson, N.R.; Covington, J.A. Pd-doped reduced graphene oxide sensing films for H2 detection. Sens. Actuators B Chem. 2013, 183, 478–487. [Google Scholar] [CrossRef]
- Tang, X.; Haddad, P.-A.; Mager, N.; Geng, X.; Reckinger, N.; Hermans, S.; Debliquy, M.; Raskin, J.-P. Chemically deposited palladium nanoparticles on graphene for hydrogen sensor applications. Sci. Rep. 2019, 9, 3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, D.-T.; Chung, G.-S. Characteristics of resistivity-type hydrogen sensing based on palladium-graphene nanocomposites. Int. J. Hydrogen Energy 2014, 39, 620–629. [Google Scholar] [CrossRef]
- Phan, D.-T.; Chung, G.-S. A novel Pd nanocube–graphene hybrid for hydrogen detection. Sens. Actuators B Chem. 2014, 199, 354–360. [Google Scholar] [CrossRef]
- Phan, D.-T.; Chung, G.-S. Reliability of hydrogen sensing based on bimetallic Ni–Pd/graphene composites. Int. J. Hydrogen Energy 2014, 39, 20294–20304. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.-S. Graphene decorated Pd-Ag nanoparticles for H2 sensing. Int. J. Hydrogen Energy 2018, 43, 11397–11402. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Li, Z.; Chen, B.; Ma, X.; Dong, L.; Peng, L.-M. Multifunctional Graphene Sensors for Magnetic and Hydrogen Detection. ACS Appl. Mater. Interfaces 2015, 7, 9581–9588. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, D.; Chang, H.; Zhang, Y. Fabrication of palladium–zinc oxide–reduced graphene oxide hybrid for hydrogen gas detection at low working temperature. J. Mater. Sci. Mater. Electron. 2016, 28, 1667–1673. [Google Scholar] [CrossRef]
- Kaur, J.; Anand, K.; Kohli, N.; Kaur, A.; Singh, R.C. Temperature dependent selective detection of hydrogen and acetone using Pd doped WO3/reduced graphene oxide nanocomposite. Chem. Phys. Lett. 2018, 701, 115–125. [Google Scholar] [CrossRef]
- Du, Y.; Xue, Q.; Zhang, Z.; Xia, F. Great enhancement in H2 response using graphene-based Schottky junction. Mater. Lett. 2014, 135, 151–153. [Google Scholar] [CrossRef]
- Shiraz, H.G. Efficient room temperature hydrogen gas sensing based on graphene oxide and decorated porous silicon. Int. J. Hydrogen Energy 2017, 42, 15966–15972. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, Y.; Bai, X.; Chen, K.; Guan, B.-O. High-sensitivity and fast-response fiber-tip Fabry-Pérot hydrogen sensor with suspended palladium-decorated graphene. Nanoscale 2019, 11, 15821–15827. [Google Scholar] [CrossRef]
- Shin, N.H.; Lee, J.S.; Jun, J.; An, J.H.; Kim, S.G.; Cho, K.H.; Jang, J. Flower-like Palladium Nanoclusters Decorated Graphene Electrodes for Ultrasensitive and Flexible Hydrogen Gas Sensing. Sci. Rep. 2015, 5, 12294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Varandani, D.; Mehta, B.R.; Singh, V.; Wen, Z.; Feng, X.; Müllen, K. Fast response and recovery of hydrogen sensing in Pd–Pt nanoparticle–graphene composite layers. Nanotechnology 2011, 22, 275719. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Basumatari, B.; Das, J.; Roychaudhury, C.; Saha, H.; Mukherjee, N. ZnO–SnO2 based composite type gas sensor for selective hydrogen sensing. Sens. Actuators B Chem. 2014, 194, 389–396. [Google Scholar] [CrossRef]
- Ha, Y.; Jung, H.; Lee, Y.; Kim, M.H.; Lee, Y. Alteration of the morphology and electrocatalytic activity of IrO2 nanowires upon reduction by hydrogen gas. Sens. Actuators B Chem. 2015, 216, 159–164. [Google Scholar] [CrossRef]
- Huang, H.; Gong, H.; Chow, C.L.; Guo, J.; White, T.J.; Tse, M.S.; Tan, O.K. Low-Temperature Growth of SnO2 Nanorod Arrays and Tunable n-p-n Sensing Response of a ZnO/SnO2 Heterojunction for Exclusive Hydrogen Sensors. Adv. Funct. Mater. 2011, 21, 2680–2686. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Hathal, Y.R.; Ibrahim, F.T.; ALI, M.H. Etching effect on sensing behavior of CuO: NiO/PS Hydrogen gas sensor. Int. J. Sci. Eng. Res. 2015, 6, 1664–1668. [Google Scholar]
- Hu, J.; Sun, Y.; Xue, Y.; Zhang, M.; Li, P.; Lian, K.; Zhuiykov, S.; Zhang, W.; Chen, Y. Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sens. Actuators B Chem. 2018, 257, 124–135. [Google Scholar] [CrossRef]
- Motaung, D.E.; Mhlongo, G.; Makgwane, P.R.; Dhonge, B.; Cummings, F.R.; Swart, H.C.; Ray, S.S. Ultra-high sensitive and selective H2 gas sensor manifested by interface of n–n heterostructure of CeO2-SnO2 nanoparticles. Sens. Actuators B Chem. 2018, 254, 984–995. [Google Scholar] [CrossRef]
- Joy, N.A.; Nandasiri, M.I.; Rogers, P.; Jiang, W.; Varga, T.; Kuchibhatla, S.V.N.T.; Thevuthasan, S.; Carpenter, M.A. Selective Plasmonic Gas Sensing: H2, NO2, and CO Spectral Discrimination by a Single Au-CeO2 Nanocomposite Film. Anal. Chem. 2012, 84, 5025–5034. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Kim, D.-H.; Kim, W.-S.; Kang, T.J.; Lee, B.Y.; Hong, S.; Kim, Y.H.; Hong, S.-H. H2 sensing characteristics of SnO2 coated single wall carbon nanotube network sensors. Nanotechnology 2010, 21, 215501. [Google Scholar] [CrossRef]
- Ratinac, K.; Yang, W.; Ringer, S.; Braet, F. Toward Ubiquitous Environmental Gas Sensors—Capitalizing on the Promise of Graphene. Environ. Sci. Technol. 2010, 44, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.S.; Balasubramanian, K.; Burghard, M.; Kern, K.; Gómez-Navarro, C. Electrochemical Modification of Graphene. Adv. Mater. 2008, 20, 3050–3053. [Google Scholar] [CrossRef]
- Lu, G.; Park, S.; Yu, K.; Ruoff, R.S.; Ocola, L.E.; Rosenmann, D.; Chen, J. Toward Practical Gas Sensing with Highly Reduced Graphene Oxide: A New Signal Processing Method To Circumvent Run-to-Run and Device-to-Device Variations. ACS Nano 2011, 5, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, X.; Xu, L.; Liao, L.; Liu, W.; Ho, J.C.; Xiao, X.; Jiang, C.; Li, J. Hydrogen gas sensor based on metal oxide nanoparticles decorated graphene transistor. Nanoscale 2015, 7, 10078–10084. [Google Scholar] [CrossRef]
- Munasinghe, M.A.H.M.; Comini, E.; Zappa, D.; Poli, N.; Sberveglieri, G. Low Temperature Gas Sensing Properties of Graphene Oxide/SnO2 Nanowires Composite for H2. Procedia Eng. 2016, 168, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Russo, P.A.; Donato, N.; Leonardi, S.G.; Baek, S.; Conte, D.E.; Neri, G.; Pinna, N. Room-Temperature Hydrogen Sensing with Heteronanostructures Based on Reduced Graphene Oxide and Tin Oxide. Angew. Chem. Int. Ed. 2012, 51, 11053–11057. [Google Scholar] [CrossRef]
- Zhang, M.; Zhen, Y.; Sun, F.; Xu, C. Hydrothermally synthesized SnO2-graphene composites for H2 sensing at low operating temperature. Mater. Sci. Eng. B 2016, 209, 37–44. [Google Scholar] [CrossRef]
- Dhall, S.; Kumar, M.; Bhatnagar, M.; Mehta, B. Dual gas sensing properties of graphene-Pd/SnO2 composites for H2 and ethanol: Role of nanoparticles-graphene interface. Int. J. Hydrogen Energy 2018, 43, 17921–17927. [Google Scholar] [CrossRef]
- Esfandiar, A.; Ghasemi, S.; Irajizad, A.; Akhavan, O.; Gholami, M. The decoration of TiO2/reduced graphene oxide by Pd and Pt nanoparticles for hydrogen gas sensing. Int. J. Hydrogen Energy 2012, 37, 15423–15432. [Google Scholar] [CrossRef]
- Esfandiar, A.; Irajizad, A.; Akhavan, O.; Ghasemi, S.; Gholami, M.R. Pd–WO3/reduced graphene oxide hierarchical nanostructures as efficient hydrogen gas sensors. Int. J. Hydrogen Energy 2014, 39, 8169–8179. [Google Scholar] [CrossRef]
- Chen, M.; Zou, L.; Zhang, Z.; Shen, J.; Li, D.; Zong, Q.; Gao, G.; Wu, G.; Zhang, Z. Tandem gasochromic-Pd-WO3/graphene/Si device for room-temperature high-performance optoelectronic hydrogen sensors. Carbon 2018, 130, 281–287. [Google Scholar] [CrossRef]
- Na, C.W.; Woo, H.-S.; Kim, I.-D.; Lee, J.-H. Selective detection of NO2 and C2H5OH using a Co3O4-decorated ZnO nanowire network sensor. Chem. Commun. 2011, 47, 5148. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, H.W.; Kim, S.S. An ultra-sensitive hydrogen gas sensor using reduced graphene oxide-loaded ZnO nanofibers. Chem. Commun. 2015, 51, 15418–15421. [Google Scholar] [CrossRef]
- Singh, G.; Choudhary, A.; Haranath, D.; Joshi, A.G.; Singh, N.; Singh, S.; Pasricha, R. ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon 2012, 50, 385–394. [Google Scholar] [CrossRef]
- Anand, K.; Singh, R.C.; Singh, M.P.; Kaur, J.; Singh, R.C. Hydrogen sensor based on graphene/ZnO nanocomposite. Sens. Actuators B Chem. 2014, 195, 409–415. [Google Scholar] [CrossRef]
- Tabares, G.; Redondo-Cubero, A.; Vazquez, L.; Revenga, M.; Cortijo-Campos, S.; Lorenzo, E.; De Andrés, A.; Ruiz, E.; Pau, J. A route to detect H2 in ambient conditions using a sensor based on reduced graphene oxide. Sens. Actuators A Phys. 2020, 304, 111884. [Google Scholar] [CrossRef]
- Drmosh, Q.; Hendi, A.; Hossain, M.; Yamani, Z.H.; Moqbel, R.; Hezam, A.; Gondal, M. UV-activated gold decorated rGO/ZnO heterostructured nanocomposite sensor for efficient room temperature H2 detection. Sens. Actuators B Chem. 2019, 290, 666–675. [Google Scholar] [CrossRef]
- Liu, J.W.; Wu, J.; Ahmad, M.Z.; Wlodarski, W. Hybrid aligned zinc oxide nanowires array on CVD graphene for hydrogen sensing. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 194–197. [Google Scholar]
- Chen, H.; Tsai, H.J.; Lin, T.C.; Weng, W.C.; Chang, Y.C.; Chiu, J.L.; Lin, J.J.; Lin, C.-F.; Lin, Y.S.; Chen, H. Incorporation of carbon nanotube and graphene in ZnO nanorods-based hydrogen gas sensor. Ceram. Int. 2018, 44, 12308–12314. [Google Scholar] [CrossRef]
- Dutta, D.; Hazra, S.; Das, J.; Sarkar, C.; Basu, S. Studies on p-TiO2/n-graphene heterojunction for hydrogen detection. Sens. Actuators B Chem. 2015, 212, 84–92. [Google Scholar] [CrossRef]
- Kamal, T. High performance NiO decorated graphene as a potential H2 gas sensor. J. Alloy. Compd. 2017, 729, 1058–1063. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, N.; Jiang, C.; Xia, B. Characterization of CuO–reduced graphene oxide sandwiched nanostructure and its hydrogen sensing characteristics. J. Mater. Sci. Mater. Electron. 2016, 28, 2763–2768. [Google Scholar] [CrossRef]
- Arsat, R.; Breedon, M.; Shafiei, M.; Spizziri, P.; Gilje, S.; Kaner, R.; Kalantar-Zadeh, K.; Wlodarski, W. Graphene-like nano-sheets for surface acoustic wave gas sensor applications. Chem. Phys. Lett. 2009, 467, 344–347. [Google Scholar] [CrossRef]
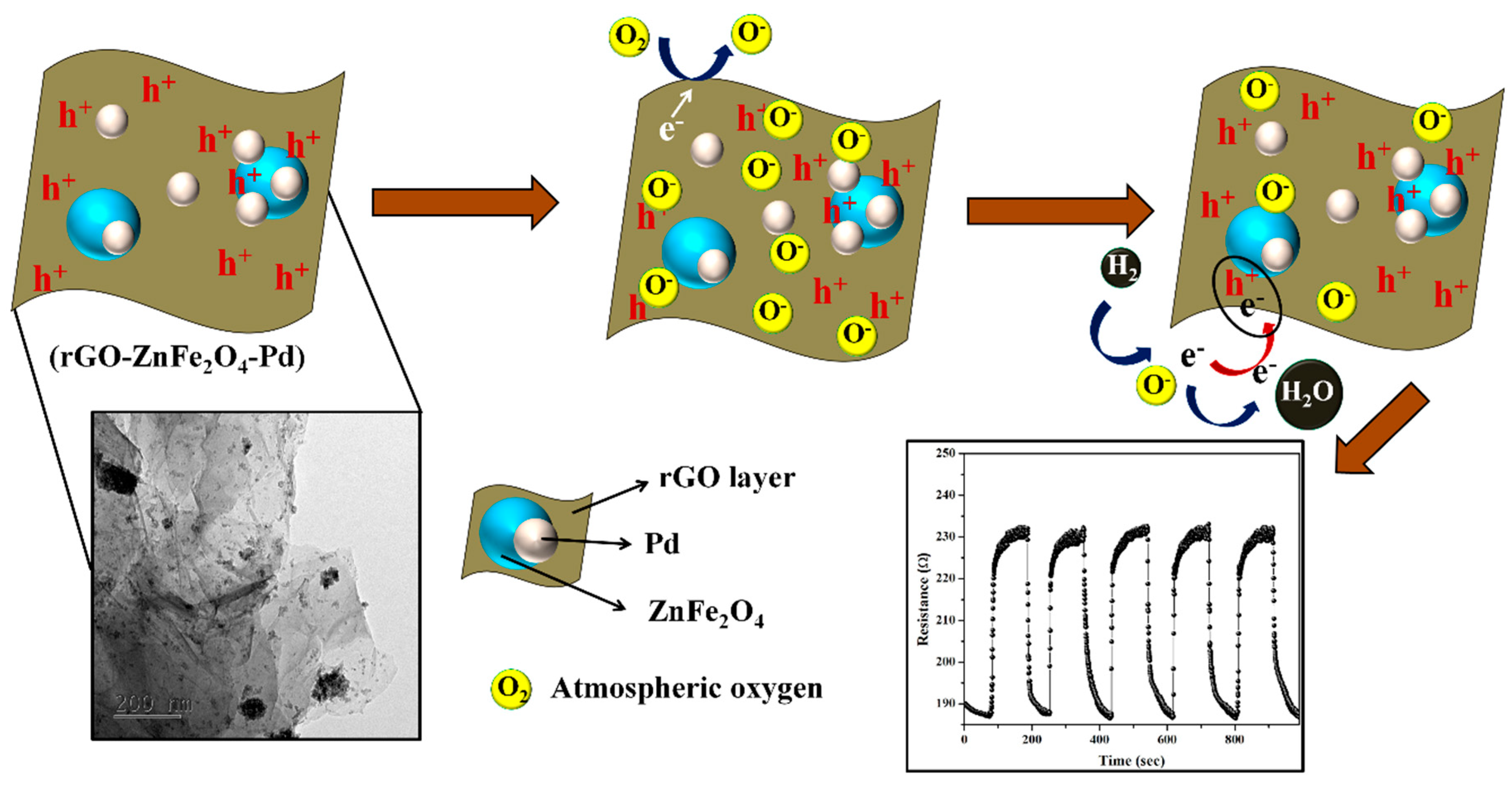
- Achary, L.S.K.; Maji, B.; Kumar, A.; Ghosh, S.P.; Kar, J.P.; Dash, P. Efficient room temperature detection of H2 gas by novel ZnFe2O4–Pd decorated rGO nanocomposite. Int. J. Hydrogen Energy 2020, 45, 5073–5085. [Google Scholar] [CrossRef]









| Sensor Material | Detection Level/Range | Operating Temperature | Response Time | Recovery Time | Ref. |
|---|---|---|---|---|---|
| RGO-PEDOT:PSS | 100 ppm | RT | ~30 s | ~25 s | [68] |
| GR-PANI | 1 vol% | RT | ~25 s | – | [69] |
| RGO-PANI | 1 vol% | RT | 20 s | 50 s | [70] |
| PMMA/Pd NP/SLG | 0.025–2 vol% | RT | 1.81 min | 5.52 min | [71] |
| Sensor Material | Detection Level/Range | Operating Temperature | Response Time | Recovery Time | Ref. |
|---|---|---|---|---|---|
| PdNPs/GR | 0.1–1% | RT | ~30 s | – | [72] |
| Pt/GR | 1% | RT–175 °C | – | – | [73] |
| Pt/RGO | 40–40,000 ppm | RT | – | – | [74] |
| Pt/GR | 1% | RT–100 °C | ~65 s | – | [75] |
| Pt/GR | 0.06–1% | 22–100 °C | – | – | [76] |
| Pt/GR | 1% | RT | – | – | [77] |
| Pt/GR | 4 vol% | RT | ~9 min | – | [78] |
| PtNPs/GR | 1.6% | RT | 0.97 s | 0.92 s | [79] |
| Pt/RGO | 0.5% | 50 °C | 63 s | 104 s | [80] |
| Pt/GR | 102–104 ppm | RT, 40 °C | – | – | [81] |
| Pt/GR | 1% | 160 °C | – | – | [82] |
| Pd/GNRs | 1000 ppm | RT | 60 s | 90 s | [83] |
| Pd/GR | 0.5–1% | RT | – | – | [84] |
| Pd/MLGN | 40–8000 ppm | 20–100 °C | 3–8 s | 7–35 s | [85] |
| Pd/SLG | 1000 ppm | RT | – | – | [86] |
| Pt/RGO | 1–100 ppm | RT | – | – | [87] |
| Pd/GR | 0.0025–1% | RT | 213 s | 463 s | [88] |
| Pd/RGO | 3300 ppm | 30–75 °C | 700–1000 s | – | [89] |
| Pd/GR | 1% | RT | – | – | [90] |
| PtNPs/GR | 1–1000 ppm | RT | – | – | [91] |
| Pd/GR | 6–1000 ppm | RT | – | – | [92] |
| Ni-Pd/GR | 1–1000 ppm | RT | – | – | [93] |
| Pd-Ag/GR | 1000 ppm | 70-190 °C | – | – | [94] |
| Pd-ZnO/RGO | 1 ppb–500 ppm | 50 °C | – | – | [96] |
| Pd-WO3/RGO | 50 ppm | 150–350 °C | – | – | [97] |
| Pd/RGO | 0.16% | RT | – | – | [98] |
| Pd/GO | 200–2000 ppm | RT | 10 min | 20 min | [99] |
| Pd/GR | ~20 ppm | RT | ~18 s | – | [100] |
| Pd/GR | 0.1–100 ppm | RT | – | – | [101] |
| Pd-Pt/GR | 2% | −50–100 °C | <2 s | 18 s | [102] |
| Sensor Material | Detection Level/Range | Operating Temperature | Response Time | Recovery Time | Ref. |
|---|---|---|---|---|---|
| SnO2/GR | 100 ppm | 50 °C | – | – | [115] |
| SnO2/GO | 20–100 ppm | 20–150 °C | – | – | [116] |
| Pd-SnO2/RGO | 0.5–3% | RT | 3–7 s | 2–6 s | [117] |
| SnO2/GR | 100% | 150 °C | – | – | [118] |
| Pd-SnO2/GR | 2% | 25–200 °C | – | – | [119] |
| Pt or Pd-TiO2/RGO | 100–10,000 ppm | RT | <1 min | <1 min | [120] |
| Pd-WO3/RGO | 20–10,000 ppm | RT–250 °C | <1 min | <1 min | [121] |
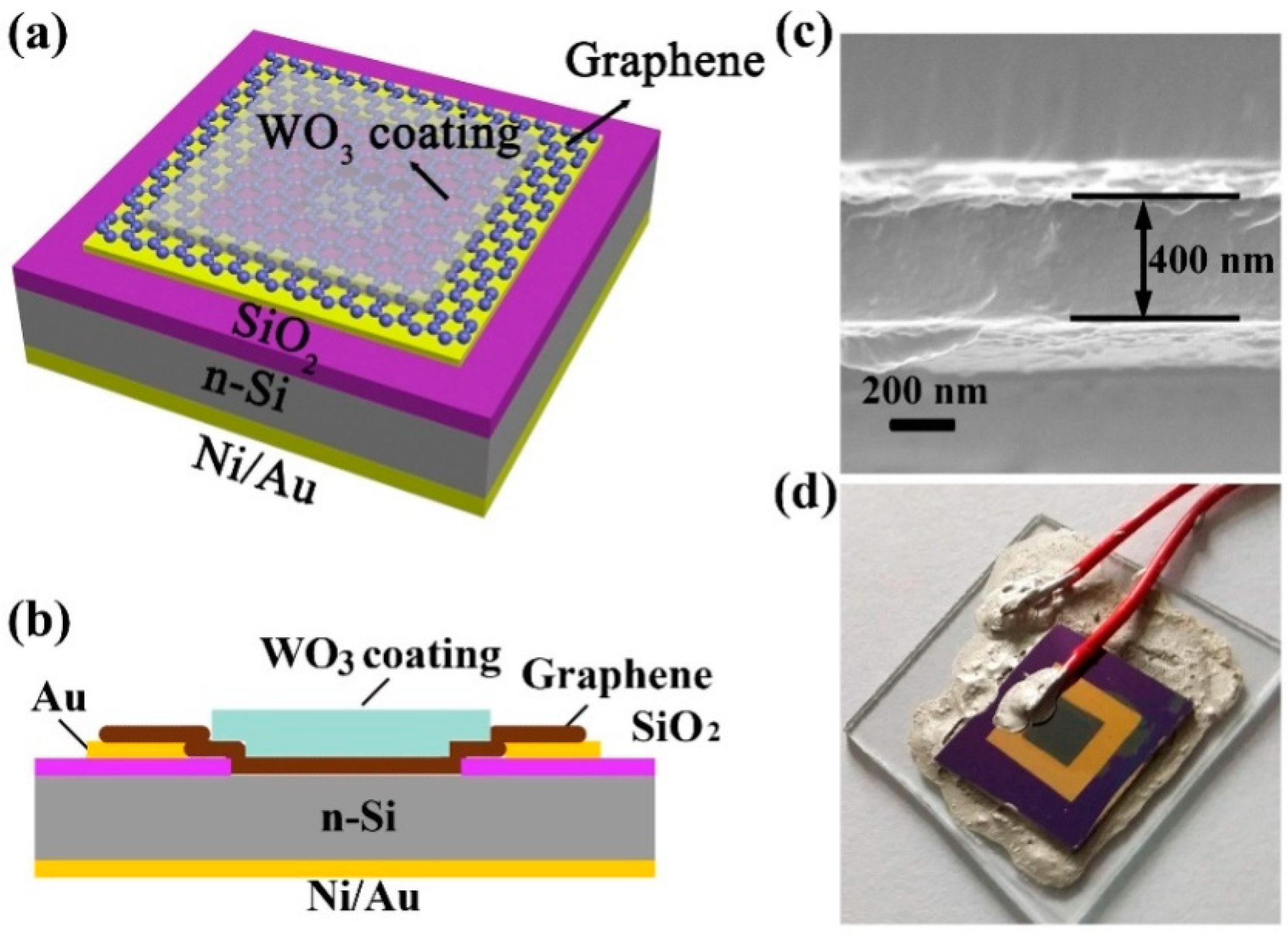
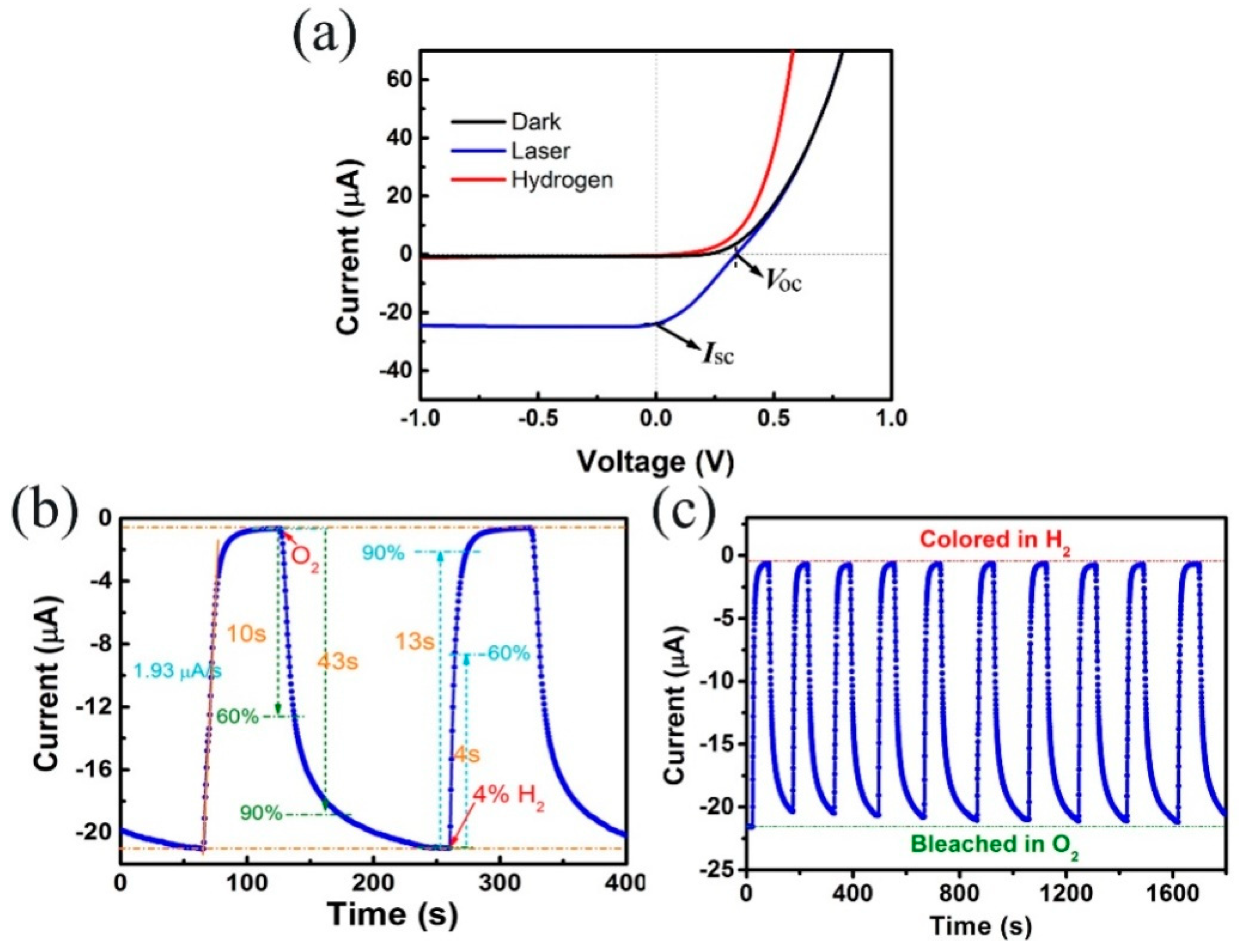
| Pd-WO3/GR | 0.05 vol% | RT | 13 s | 43 s | [122] |
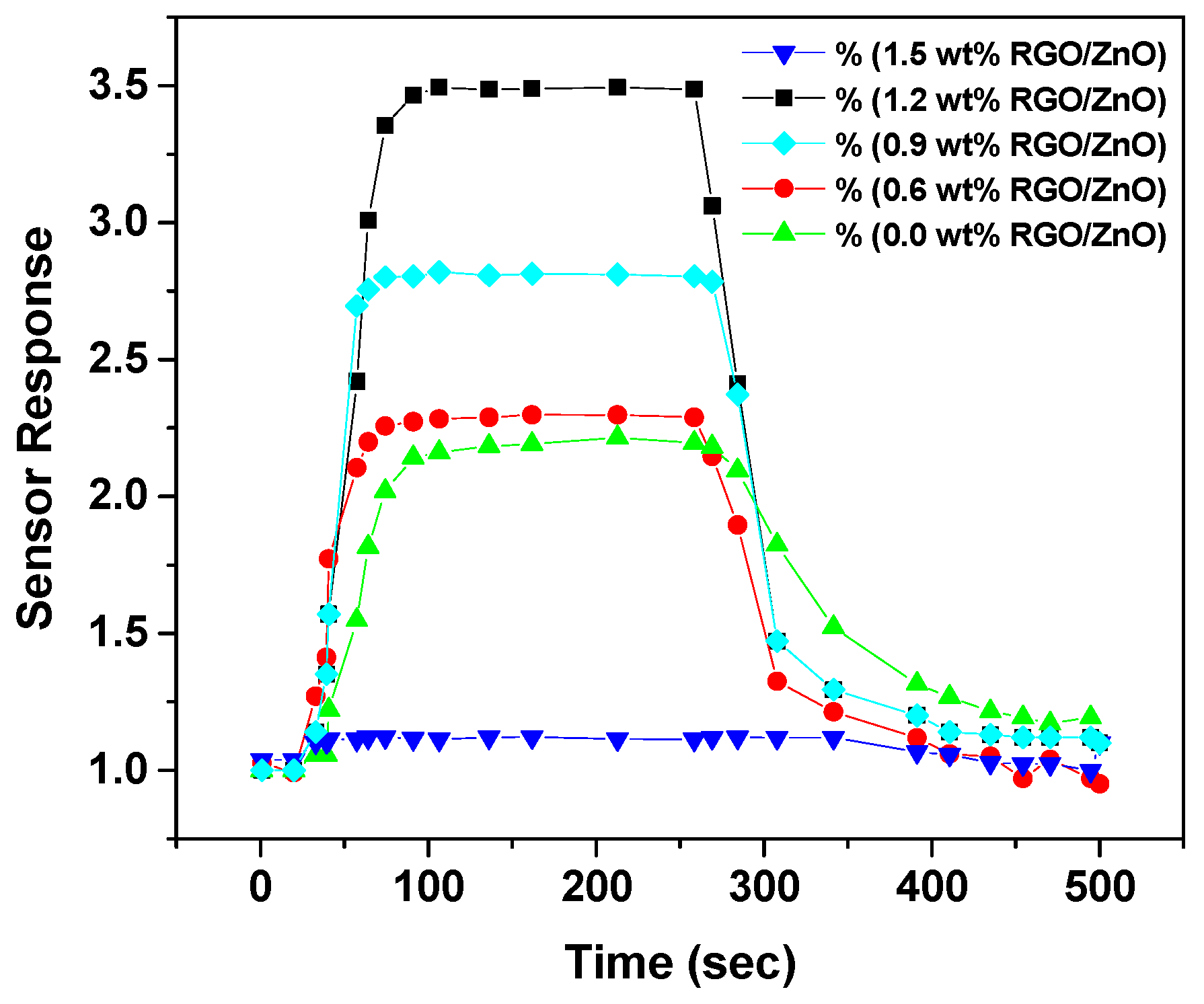
| ZnO/RGO, SnO2/RGO | 10 ppm | 300–450 °C | – | – | [124] |
| ZnO/GR | 200 ppm | 100–150 °C | 22–96 s | 90-190 s | [126] |
| ZnO/RGO | 30–160 ppm | RT | – | – | [127] |
| ZnO/RGO | 500 ppm | RT | 8 s | 612 s | [128] |
| ZnO/GR | 0.06–1% | RT | – | – | [129] |
| ZnO/CNT/GR | 1000 ppm | 300 °C | – | – | [130] |
| TiO2/GR | 0.5% | 75–150 °C | 16 s | 61 s | [131] |
| NiO/GR | 400–2000 ppm | 100–350 °C | – | – | [132] |
| CuO/RGO | 50–1500 ppm | RT | 80 s | 60 s | [133] |
| LiTaO3/GR | 1% | RT–40 °C | <1 min | 10 min | [134] |
| GR | 50–1000 ppm | 25–100 °C | 18 s | 9 s | [135] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilnicka, A.; Lukaszewicz, J.P. Graphene-Based Hydrogen Gas Sensors: A Review. Processes 2020, 8, 633. https://doi.org/10.3390/pr8050633
Ilnicka A, Lukaszewicz JP. Graphene-Based Hydrogen Gas Sensors: A Review. Processes. 2020; 8(5):633. https://doi.org/10.3390/pr8050633
Chicago/Turabian StyleIlnicka, Anna, and Jerzy P. Lukaszewicz. 2020. "Graphene-Based Hydrogen Gas Sensors: A Review" Processes 8, no. 5: 633. https://doi.org/10.3390/pr8050633
APA StyleIlnicka, A., & Lukaszewicz, J. P. (2020). Graphene-Based Hydrogen Gas Sensors: A Review. Processes, 8(5), 633. https://doi.org/10.3390/pr8050633






