Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nanoparticles
2.2.1. Preparation of CS–DS Nanoparticles
2.2.2. Preparation of Whey Protein-Chitosan Nanoparticles
2.3. Characterization of the Nanoparticles
2.3.1. Particle Size Analysis
2.3.2. Zeta Potential Measurements
2.3.3. Transmission Electron Microscopy (TEM)
2.4. Caco-2 Cell Iron Absorption
2.5. Iron Absorption Analysis and Determination of Loading Efficiency
2.6. Statistical Analysis
3. Results
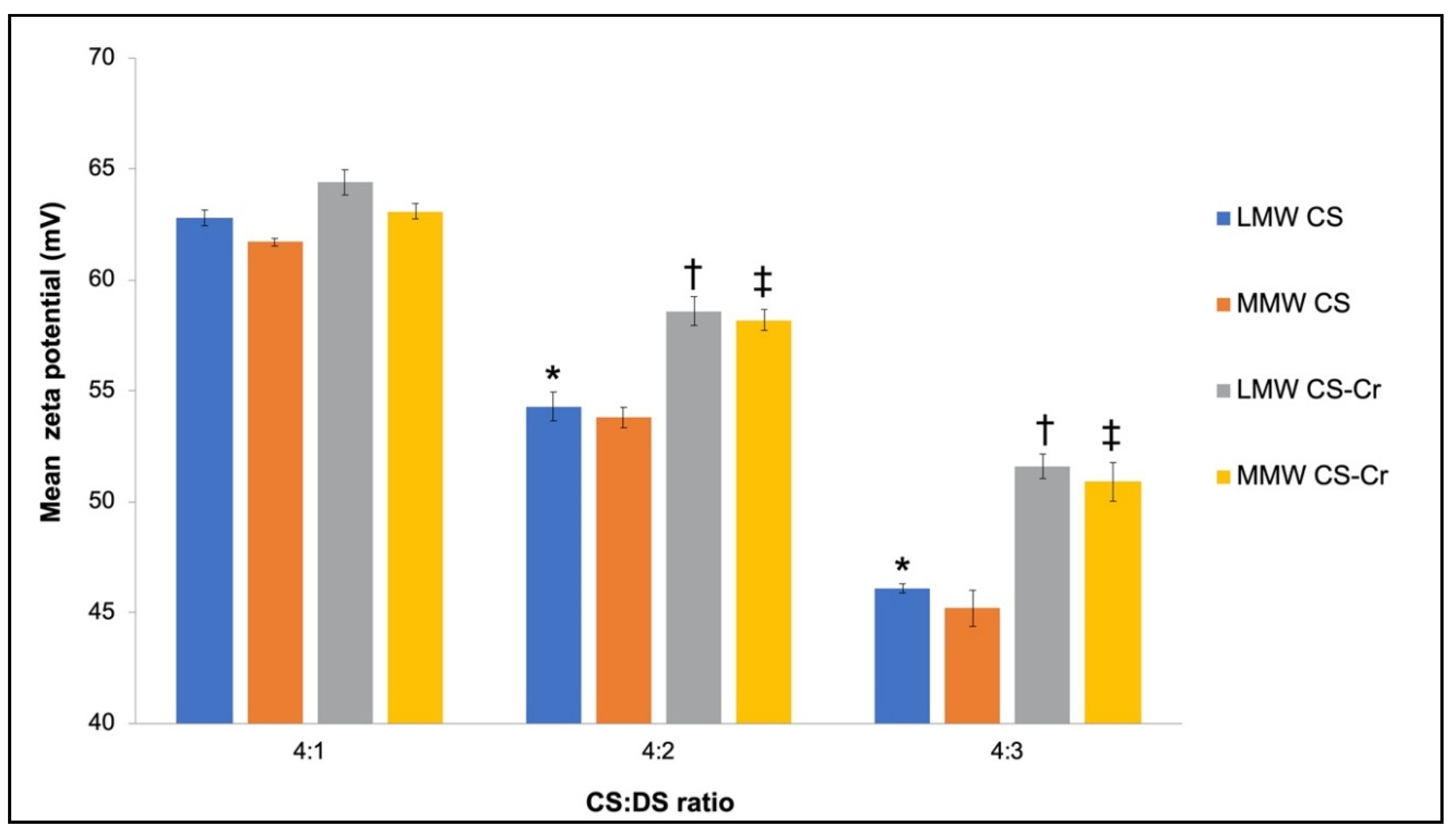
3.1. Physiochemical Characteristics of Chromium-Entrapped CS-DS Nanoparticles
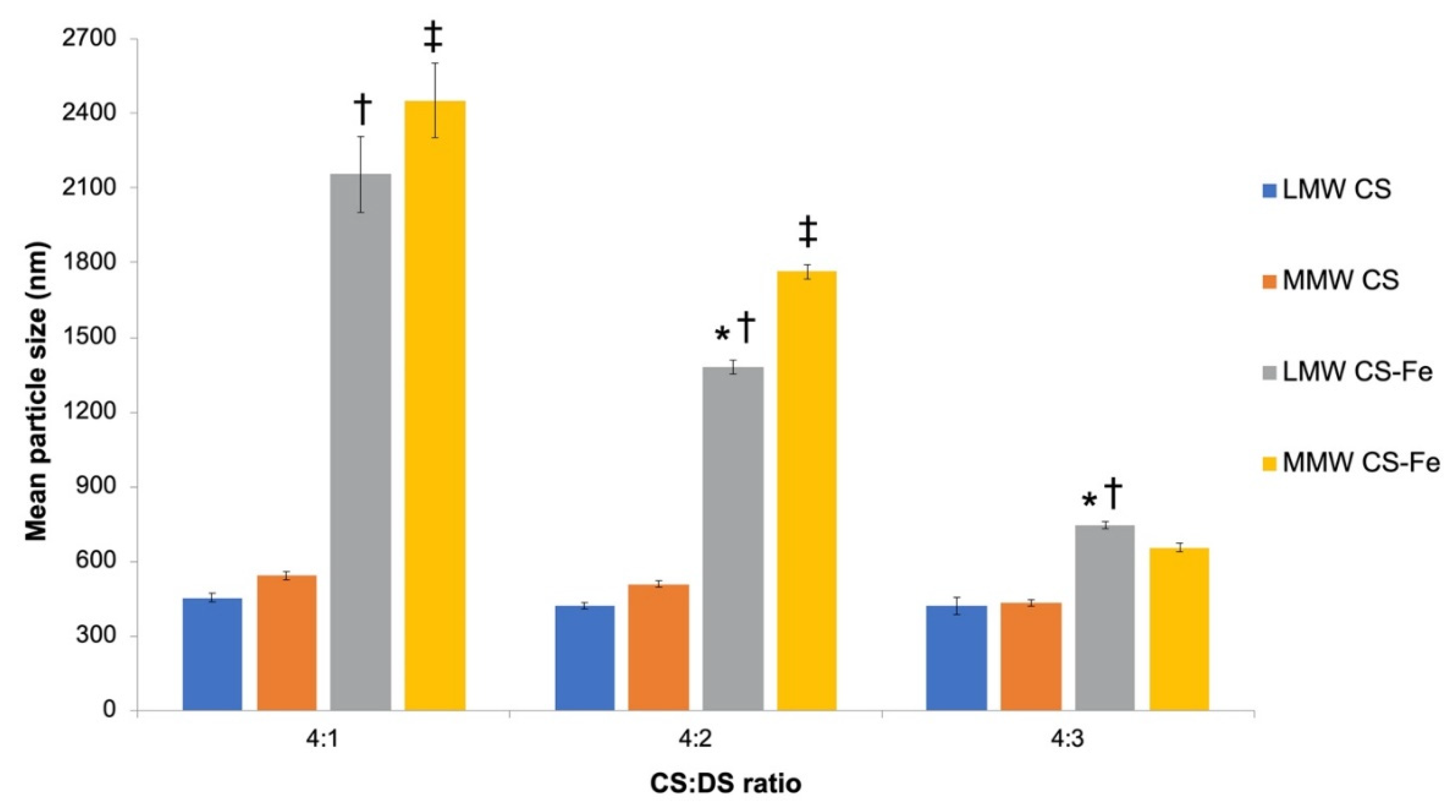
3.2. Physiochemical Characteristics of Iron-Entrapped CS-DS Nanoparticles and Submicron Particles
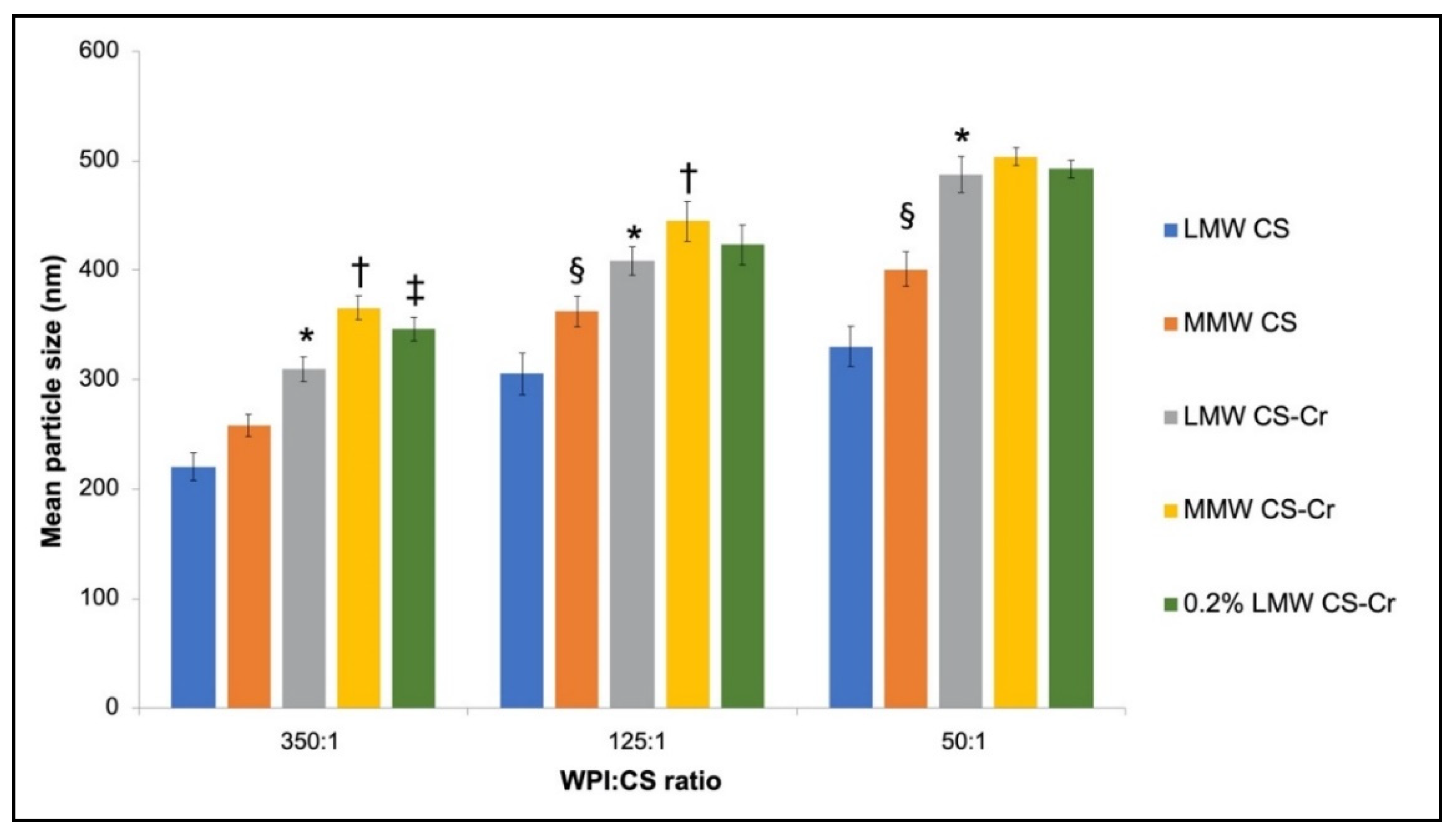
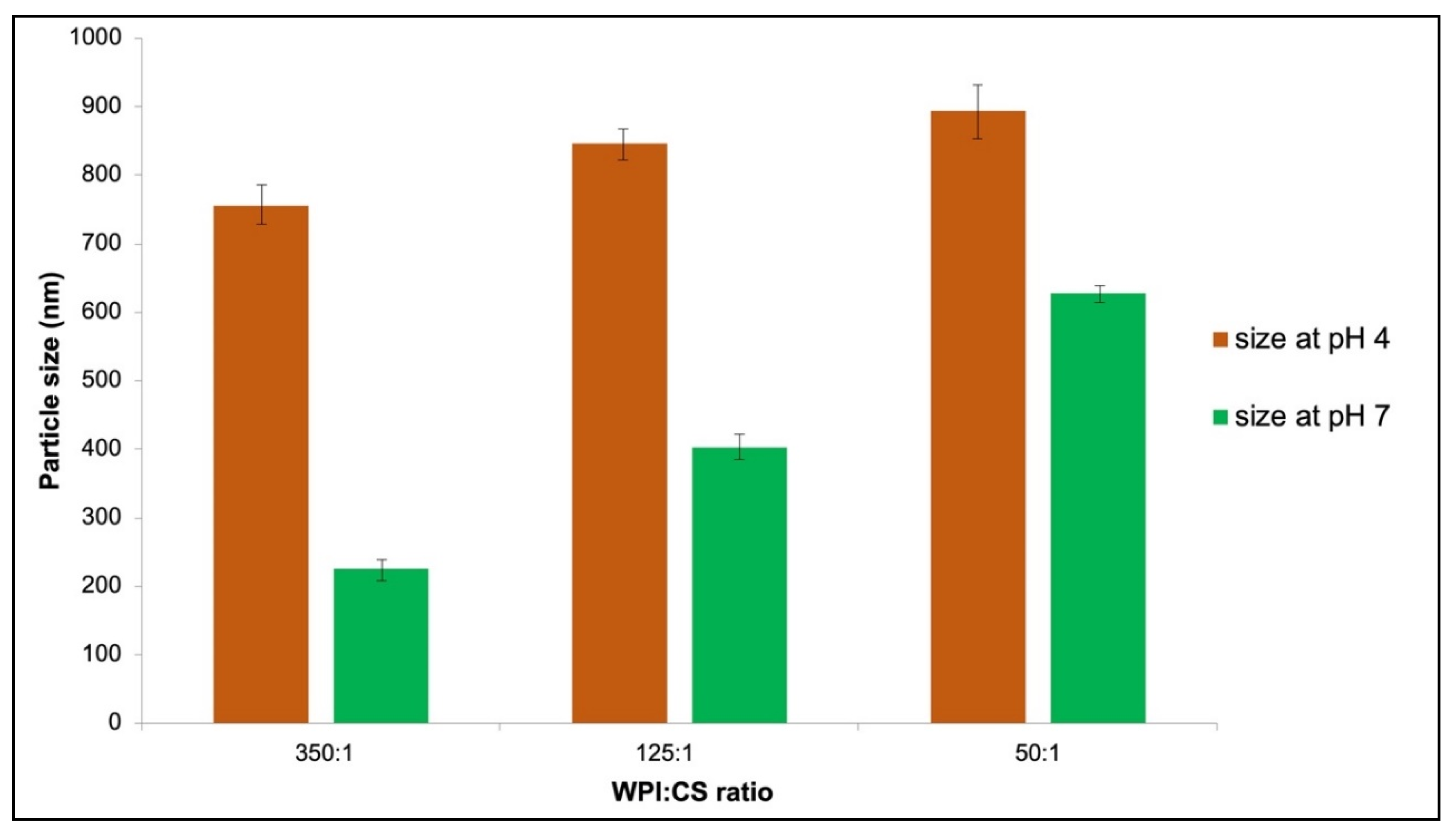
3.3. Physiochemical Characteristics of Chromium-Entrapped WPI-CS Nanoparticles
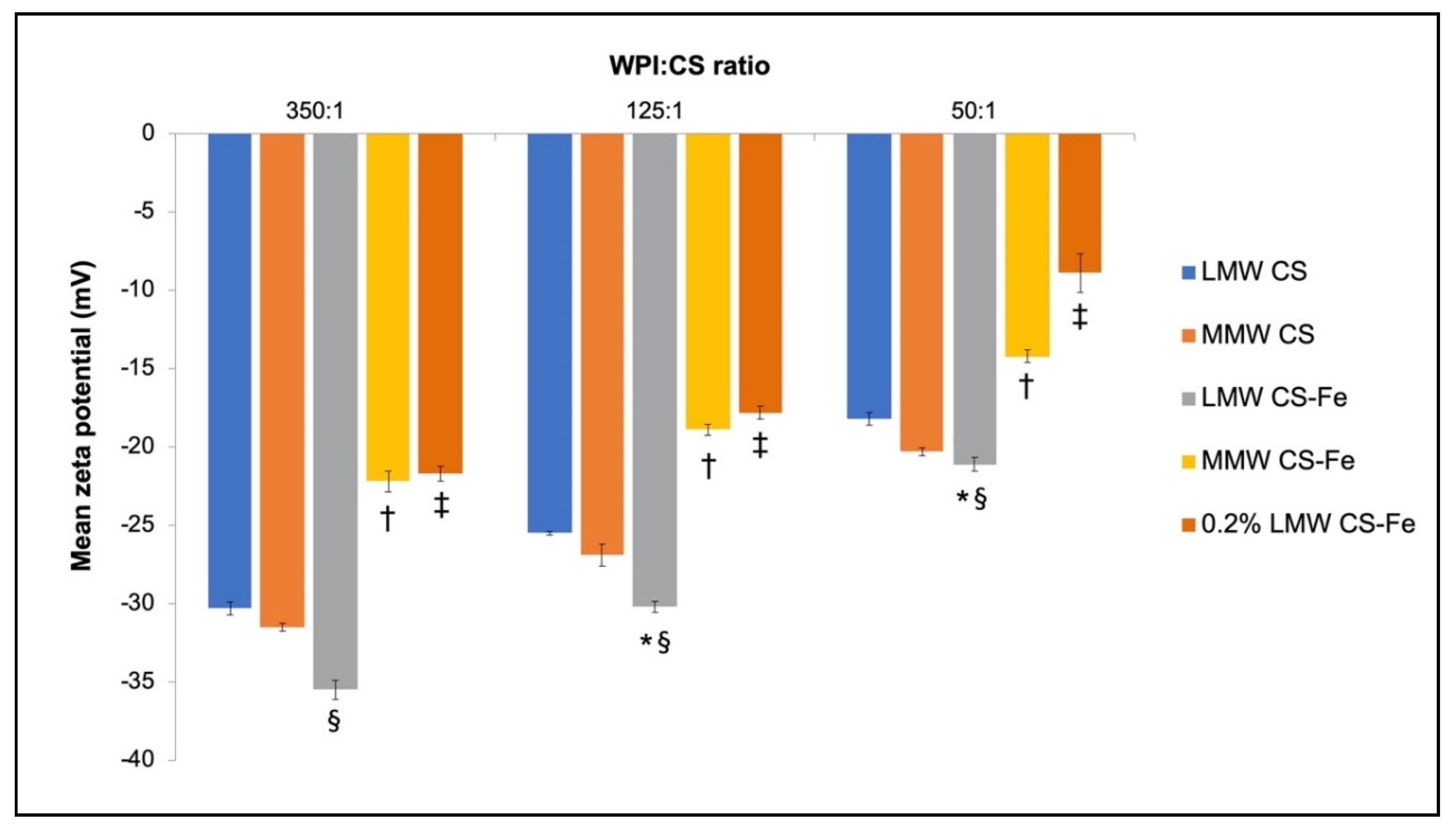
3.4. Physiochemical Characteristics of Iron-Entrapped WPI–CS Nanoparticles
3.5. Caco-2 Cell Iron Permeability Studies
4. Discussion
4.1. Properties of CS–DS Nanoparticles
4.2. Properties of WPI-CS Nanoparticles
4.3. Iron Absorption in Caco-2 Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Singer, G.M.; Geohas, J. The effect of chromium picolinate and biotin supplementation on glycemic control in poorly controlled patients with type 2 diabetes mellitus: A placebo-controlled, double-blinded, randomized trial. Diabetes Technol. Ther. 2006, 8, 636–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.A.; Kozlovsky, A.S. Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am. J. Clin. Nutr. 1985, 41, 1177–1183. [Google Scholar] [CrossRef]
- Hex, N.; Bartlett, C.; Wright, D.; Taylor, M.; Varley, D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet. Med. 2012, 29, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Aspects Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Zariwala, M.G.; Somavarapu, S.; Farnaud, S.; Renshaw, D. Comparison study of oral iron preparations using a human intestinal model. Sci. Pharm. 2013, 81, 1123–1139. [Google Scholar] [CrossRef] [Green Version]
- Zariwala, M.G.; Farnaud, S.; Merchant, Z.; Somavarapu, S.; Renshaw, D. Ascorbyl palmitate/DSPE-PEG nanocarriers for oral iron delivery: Preparation, characterisation and in vitro evaluation. Colloids Surf. B Biointerfaces 2014, 115, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Hallberg, L.; Brune, M.; Rossander-Hulthen, L. Is there a physiological role of vitamin C in iron absorption? Ann. N. Y. Acad. Sci. 1987, 498, 324–332. [Google Scholar] [CrossRef]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Jeejeebhoy, K.N. The role of chromium in nutrition and therapeutics and as a potential toxin. Nutr. Rev. 1999, 57, 329–335. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Chen, Y.; Mohanraj, V.J.; Wang, F.; Benson, H.A. Designing chitosan-dextran sulfate nanoparticles using charge ratios. AAPS PharmSciTech 2007, 8, E98. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Gaur, U.; Ghosh, P.C.; Maitra, A.N. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J. Control. Release 2001, 74, 317–323. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Lopez, H.L.; Kedia, A.; Habowski, S.M.; Sandrock, J.E.; Raub, B.; Kerksick, C.M.; Ferrando, A.A. Effects of an amylopectin and chromium complex on the anabolic response to a suboptimal dose of whey protein. J. Int. Soc. Sports Nutr. 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Mohanraj, V.J.; Parkin, J.E. Chitosan-dextran sulfate nanoparticles for delivery of an anti-angiogenesis peptide. Lett. Pept. Sci. 2003, 10, 621–629. [Google Scholar] [CrossRef]
- Zamproni, L.N.; Teixeira, D.; Alliegro, A.A.; Maugeri, I.L.; des Rieux, A.; Porcionatto, M.A. Decreased viability and neurite length in neural cells treated with chitosan-dextran sulfate nanocomplexes. Neurotoxicology 2020, 76, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef] [PubMed]
- Giroux., H.J.; Houde, J.; Britten, M. Preparation of nanoparticles from denatured whey protein by pH-cycling treatment. Food Hydrocoll. 2010, 24, 341–346. [Google Scholar] [CrossRef]
- Stekel, A.; Olivares, M.; Pizarro, F.; Chadud, P.; Lopez, I.; Amar, M. Absorption of fortification iron from milk formulas in infants. Am. J. Clin. Nutr. 1986, 43, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Derman, D.P.; Bothwell, T.H.; MacPhail, A.P.; Torrance, J.D.; Bezwoda, W.R.; Charlton, R.W.; Mayet, F.G. Importance of ascorbic acid in the absorption of iron from infant foods. Scand. J. Haematol. 1980, 25, 193–201. [Google Scholar] [CrossRef]
- Maryam, K.; Avadi, M.; Abbaspour, M.; Jahangiri, A.; Boldaji, S. Effect of different molecular weights of chitosan on preparation and characterization of insulin loaded nanoparticles by ion gelation method. Int. J. Drug Dev. Res. 2012, 4, 271–277. [Google Scholar]
- Chaiyasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Mucoadhesive chitosan-dextran sulfate nanoparticles for sustained drug delivery to the ocular surface. J. Ocul. Pharmacol. Ther. 2013, 29, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Anal, A.K.; Tobiassen, A.; Flanagan, J.; Singh, H. Preparation and characterization of nanoparticles formed by chitosan-caseinate interactions. Colloids Surf. B Biointerfaces 2008, 64, 104–110. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzey, D.; McClements, D.J. Characterization of β-lactoglobulin–chitosan interactions in aqueous solutions: A calorimetry, light scattering, electrophoretic mobility and solubility study. Food Hydrocoll. 2006, 20, 124–131. [Google Scholar] [CrossRef]
- Rojas, G.; Silva, J.; Flores, J.; Rodriguez, A.; Ly, M. Adsorption of chromium onto cross-linked chitosan. Sep. Purif. Technol. 2005, 44, 31–36. [Google Scholar] [CrossRef]
- Omar Zaki, S.S.; Ibrahim, M.; Katas, H. Particle Size Affects Concentration-Dependent Cytotoxicity of Chitosan Nanoparticles towards Mouse Hematopoietic Stem Cells. J. Nanotechnol. 2015, 2015, 919658. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Lim, L.Y. Uptake of chitosan and associated insulin in Caco-2 cell monolayers: A comparison between chitosan molecules and chitosan nanoparticles. Pharm. Res. 2003, 20, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Hentze, M. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: Implications for regulation and cellular function. Proc. Natl. Acad. Sci. USA 2002, 99, 12345–12350. [Google Scholar] [CrossRef] [Green Version]








| Type of Nanoparticle | CS:DS Ratio | Chitosan Volume (mL) | Dextran Volume (mL) | Chromium Hydrate (mg) | Ferrous Sulfate (mg) | Loading Efficiency (%) |
|---|---|---|---|---|---|---|
| empty | 4:1 | 10 | 2.5 | - | - | - |
| empty | 4:2 | 10 | 5.0 | - | - | - |
| empty | 4:3 | 10 | 7.5 | - | - | - |
| Cr entrapped | 4:1 | 10 | 2.5 | 12.5 | - | ND |
| Cr entrapped | 4:2 | 10 | 5.0 | 15.0 | - | ND |
| Cr entrapped | 4:3 | 10 | 7.5 | 17.5 | - | ND |
| Fe entrapped | 4:1 | 10 | 2.5 | - | 12.5 | 13 ± 2.3 |
| Fe entrapped | 4:2 | 10 | 5.0 | - | 15.0 | 21 ± 4.5 |
| Fe entrapped | 4:3 | 10 | 7.5 | - | 17.5 | 47 ± 5.2 |
| Sample | WPI:CS Ratio | Whey Protein (mL) | Chitosan (mL) | Chromium Hydrate (mg) |
|---|---|---|---|---|
| empty | 50:1 | 10.0 | 5.0 | - |
| empty | 125:1 | 12.5 | 2.5 | - |
| empty | 350:1 | 14.0 | 1.0 | - |
| Cr loaded | 50:1 | 10.0 | 5.0 | 5 |
| Cr loaded | 125:1 | 12.5 | 2.5 | 5 |
| Cr loaded | 350:1 | 14.0 | 1.0 | 5 |
| WPI:CS Ratio | Whey Protein (mL) | Chitosan (mL) | Ferrous Sulfate (mg) | Ascorbic Acid (mg) | Loading Efficiency (%) |
|---|---|---|---|---|---|
| 50:1 | 10.0 | 5.0 | 10 | 10 | 29 ± 4.3 |
| 125:1 | 12.5 | 2.5 | 10 | 10 | 38 ± 4.1 |
| 350:1 | 14.0 | 1.0 | 10 | 10 | 54 ± 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, N.; Zariwala, M.G.; Al-Obaidi, H. Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements. Processes 2020, 8, 707. https://doi.org/10.3390/pr8060707
Patel N, Zariwala MG, Al-Obaidi H. Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements. Processes. 2020; 8(6):707. https://doi.org/10.3390/pr8060707
Chicago/Turabian StylePatel, Nishay, Mohammed Gulrez Zariwala, and Hisham Al-Obaidi. 2020. "Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements" Processes 8, no. 6: 707. https://doi.org/10.3390/pr8060707




