MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals
Abstract
:1. Introduction
2. Experimental Part
2.1. Catalysts Synthesis
2.2. Catalysts Characterisation
2.2.1. Nitrogen Physisorption
2.2.2. Temperature Programmed Reduction (TPR)
2.2.3. X-ray Powder Diffraction (XRD)
2.2.4. Raman Spectroscopy
2.2.5. Temperature-programmed Desorption (NH3-TPD)
2.3. Aqueous Phase Transformation Catalytic Tests
3. Result and Discussion
3.1. Catalysts Characterization
3.2. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Xi, J.; Wang, Y. Recent advances in the catalytic production of glucose from lignocellulosic biomass. Green Chem. 2015, 17, 737–751. [Google Scholar] [CrossRef]
- Chen, S.; Maneerung, T.; Tsang, D.C.; Ok, Y.S.; Wang, C.-H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S. Feedstocks for Lignocellulosic Biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Lu, X.; Zhang, S.; Zhang, R.; Wang, X. Kinetic study for Fe (NO3)3 catalyzed hemicellulose hydrolysis of different corn stover silages. Bioresour. Technol. 2011, 102, 2936–2942. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of Lignocellulose; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Karnjanakom, S.; Guan, G.; Asep, B.; Hao, X.; Kongparakul, S.; Samart, C.; Abudula, A. Catalytic Upgrading of Bio-Oil over Cu/MCM-41 and Cu/KIT-6 Prepared by β-Cyclodextrin-Assisted Coimpregnation Method. J. Phys. Chem. C 2016, 120, 3396–3407. [Google Scholar] [CrossRef]
- Qib, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Fast Transformation of Glucose and Di-/Polysaccharides into 5-Hydroxymethylfurfural by Microwave Heating in an Ionic Liquid/Catalyst System. ChemSusChem 2010, 3, 1071–1077. [Google Scholar] [CrossRef]
- Choudhary, V.; Pinar, A.B.; Sandler, S.I.; Vlachos, D.G.; Lobo, R.F. Xylose Isomerization to Xylulose and its Dehydration to Furfural in Aqueous Media. ACS Catal. 2011, 1, 1724–1728. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.Q. Reaction Mechanisms for Renewable Hydrogen from Liquid Phase Reforming of Sugar Compounds. Dev. Chem. Eng. Miner. Process. 2008, 14, 9–18. [Google Scholar] [CrossRef]
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Goto, K.; Tajima, K.; Adschiri, T.; Arai, K. Rapid and selective retro-aldol condensation of glucose to glycolaldehyde in supercritical water. Green Chem. 2002, 4, 285–287. [Google Scholar] [CrossRef]
- Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts 2019, 9, 917. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Zhang, Q.; Wang, Y. Catalytic transformations of cellulose and cellulose-derived carbohydrates into organic acids. Catal. Today 2014, 234, 31–41. [Google Scholar] [CrossRef]
- Signoretto, M.; Taghavi, S.; Ghedini, E.; Menegazzo, F. Catalytic Production of Levulinic Acid (LA) from Actual Biomass. Molecules 2019, 24, 2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudarsanam, P.; Zhong, R.; Bosch, S.V.D.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, J.; Bhattacharyya, S. Application of Graphene and Graphene-Based Materials in Clean Energy-Related Devices Minghui. Arch. Thermodyn. 2012, 33, 23–40. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Taghavi, S.; Norouzi, O.; Tavasoli, A.; di Maria, F.; Signoretto, M.; Menegazzo, F.; di Michele, A. Catalytic conversion of Venice lagoon brown marine algae for producing hydrogen-rich gas and valuable biochemical using algal biochar and Ni/SBA-15 catalyst. Int. J. Hydrogen Energy 2018, 43, 19918–19929. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, Z.; Tian, Y.; Deng, J.; Shi, J.; Fu, Y. Sn-MCM-41 as Efficient Catalyst for the Conversion of Glucose into 5-Hydroxymethylfurfural in Ionic Liquids. BioResources 2013, 9, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Ya’Aini, N.; Amin, N.A.S.; Endud, S. Characterization and performance of hybrid catalysts for levulinic acid production from glucose. Microporous Mesoporous Mater. 2013, 171, 14–23. [Google Scholar] [CrossRef]
- Ya’Aini, N.; Amin, N.A.S.; Asmadi, M. Optimization of levulinic acid from lignocellulosic biomass using a new hybrid catalyst. Bioresour. Technol. 2012, 116, 58–65. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Chen, W.; Wang, S.; Sun, S. Hydrothermal conversion of xylose, glucose, and cellulose under the catalysis of transition metal sulfates. Carbohydr. Polym. 2015, 118, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ghedini, E.; Menegazzo, F.; Signoretto, M.; Manzoli, M.; Pinna, F.; Strukul, G. Mesoporous silica as supports for Pd-catalyzed H2O2 direct synthesis: Effect of the textural properties of the support on the activity and selectivity. J. Catal. 2010, 273, 266–273. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H. The Use of Low Temperature van der Waals Adsorption Isotherms in Determining the Surface Areas of Various Adsorbents. J. Am. Chem. Soc. 1937, 59, 2682–2689. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area end Porosity; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Union, I.; Pure, O.F.; Chemistry, A. International union of pure commission on colloid and syrface chemistry including catalysis reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity. Area 1985, 57, 603–619. [Google Scholar]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO-Co3O4 crossover using Raman spectroscopy in magnetic octahedron-shaped nanocrystals. J. Raman Spectrosc. 2017, 14, 640–841. [Google Scholar] [CrossRef]
- Gouadec, G.; Colomban, P. Raman Spectroscopy of nanomaterials: How spectra relate to disorder, particle size and mechanical properties. Prog. Cryst. Growth Charact. Mater. 2007, 53, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Reddy, G.B. Synthesis, and characterization ofα-MoO3microspheres packed with nanoflakes. J. Phys. D Appl. Phys. 2014, 47, 65305. [Google Scholar] [CrossRef]
- Cherian, C.T.; Reddy, M.V.; Haur, S.C.; Chowdari, B.V.R. Interconnected Network of CoMoO4 Submicrometer Particles As High Capacity Anode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Lorite, I.; Romero, J.J.; Fernández, J.F. Effects of the agglomeration state on the Raman properties of Co3 O4 nanoparticles. J. Raman Spectrosc. 2012, 43, 1443–1448. [Google Scholar] [CrossRef]
- Klissurski, D.G.; Uzunova, E. Cation-deficient nano-dimensional particle size cobalt–manganese spinel mixed oxides. Appl. Surf. Sci. 2003, 214, 370–374. [Google Scholar] [CrossRef]
- Kovanda, F.; Rojka, T.; Dobešová, J.; Machovič, V.; Bezdička, P.; Obalová, L.; Jirátová, K.; Grygar, T.M. Mixed oxides obtained from Co and Mn containing layered double hydroxides: Preparation, characterization, and catalytic properties. J. Solid State Chem. 2006, 179, 812–823. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, B.; Zhang, Y.; Lv, K.; Li, Q.; Ding, Y.; Li, J. Effects of mesoporous structure and Pt promoter on the activity of Co-based catalysts in low-temperature CO2 hydrogenation for higher alcohol synthesis. J. Catal. 2018, 366, 91–97. [Google Scholar] [CrossRef]
- Griboval, A.; Butel, A.; Ordomsky, V.V.; Chernavskii, P.A.; Khodakov, A.Y. Cobalt and iron species in alumina supported bimetallic catalysts for Fischer–Tropsch reaction. Appl. Catal. A Gen. 2014, 481, 116–126. [Google Scholar] [CrossRef]
- Tavasoli, A.; Trépanier, M.; Abbaslou, R.M.M.; Dalai, A.K.; Abatzoglou, N. Fischer–Tropsch synthesis on mono- and bimetallic Co and Fe catalysts supported on carbon nanotubes. Fuel Process. Technol. 2009, 90, 1486–1494. [Google Scholar] [CrossRef]
- Mai, K.; Elder, T.; Groom, L.H.; Spivey, J.J. Fe-based Fischer Tropsch synthesis of biomass-derived syngas: Effect of synthesis method. Catal. Commun. 2015, 65, 76–80. [Google Scholar] [CrossRef]
- Kukushkin, R.; Bulavchenko, O.; Kaichev, V.; Yakovlev, V. Influence of Mo on catalytic activity of Ni-based catalysts in hydrodeoxygenation of esters. Appl. Catal. B Environ. 2015, 163, 531–538. [Google Scholar] [CrossRef]
- Saghafi, M.; Heshmati-Manesh, S.; Ataie, A.; Khodadadi, A.A. Synthesis of nanocrystalline molybdenum by hydrogen reduction of mechanically activated MoO3. Int. J. Refract. Met. Hard Mater. 2012, 30, 128–132. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Chaturvedi, S.; Hanson, J.C.; Brito, J.L. Reaction of H2and H2S with CoMoO4and NiMoO4: TPR, XANES, Time-Resolved XRD, and Molecular-Orbital Studies. J. Phys. Chem. B 1999, 103, 770–781. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the Development of Novel Cobalt Fischer−Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Bragança, L.; Ojeda, M.; Fierro, J.L.G.; da Silva, M.P. Bimetallic Co-Fe nanocrystals deposited on SBA-15 and HMS mesoporous silicas as catalysts for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2012, 423, 146–153. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J.K. A versatile bi-metallic copper–cobalt catalyst for liquid phase hydrogenation of furfural to 2-methylfuran. RSC Adv. 2016, 6, 1649–1658. [Google Scholar] [CrossRef]
- Peng, Y.; Chang, H.; Dai, Y.; Li, J. Structural and Surface Effect of MnO2 for Low Temperature Selective Catalytic Reduction of NO with NH3. Procedia Environ. Sci. 2013, 18, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Mosallanejad, S.; Dlugogorski, B.Z.; Kennedy, E.; Stockenhuber, M. On the Chemistry of Iron Oxide Supported on γ-Alumina and Silica Catalysts. ACS Omega 2018, 3, 5362–5374. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Zhai, X.; Wang, X.; Gui, J.Z.; Zhang, C.; Zhu, Y.; Li, Y. Synergistic effect between copper and different metal oxides in the selective hydrogenolysis of glucose. New J. Chem. 2019, 43, 3733–3742. [Google Scholar] [CrossRef]
- Watanabe, M.; Bayer, F.; Kruse, A. Oil formation from glucose with formic acid and cobalt catalyst in hot-compressed water. Carbohydr. Res. 2006, 341, 2891–2900. [Google Scholar] [CrossRef]
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, S.; van Steen, E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 452, 60–67. [Google Scholar] [CrossRef]
- Cheng, Z.; Everhart, J.L.; Tsilomelekis, G.; Nikolakis, V.; Saha, B.; Vlachos, D.G.; Vlachos, D. Structural analysis of humins formed in the Brønsted acid catalyzed dehydration of fructose. Green Chem. 2018, 20, 997–1006. [Google Scholar] [CrossRef]
- Swift, T.D.; Nguyen, H.; Anderko, A.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis/Brønsted homogeneous acid catalysis: Conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium(iii) chloride and hydrochloric acid solution. Green Chem. 2015, 17, 4725–4735. [Google Scholar] [CrossRef] [Green Version]
- Kuninobu, Y.; Uesugi, T.; Kawata, A.; Takai, K. Manganese-Catalyzed Cleavage of a Carbon-Carbon Single Bond between Carbonyl Carbon and α-Carbon Atoms of Ketones. Angew. Chem. Int. Ed. 2011, 50, 10406–10408. [Google Scholar] [CrossRef]





| Catalyst. | Specific Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| SiO2 | 1000 | - | 0.40 |
| 20CoSiO2 | 460 | 1.6 | 0.32 |
| 10Fe10CoSiO2 | 510 | 1.5 | 0.33 |
| 10Mn10CoSiO2 | 403 | 1.6 | 0.30 |
| 10Mo10CoSiO2 | 185 | 2.9 | 0.23 |
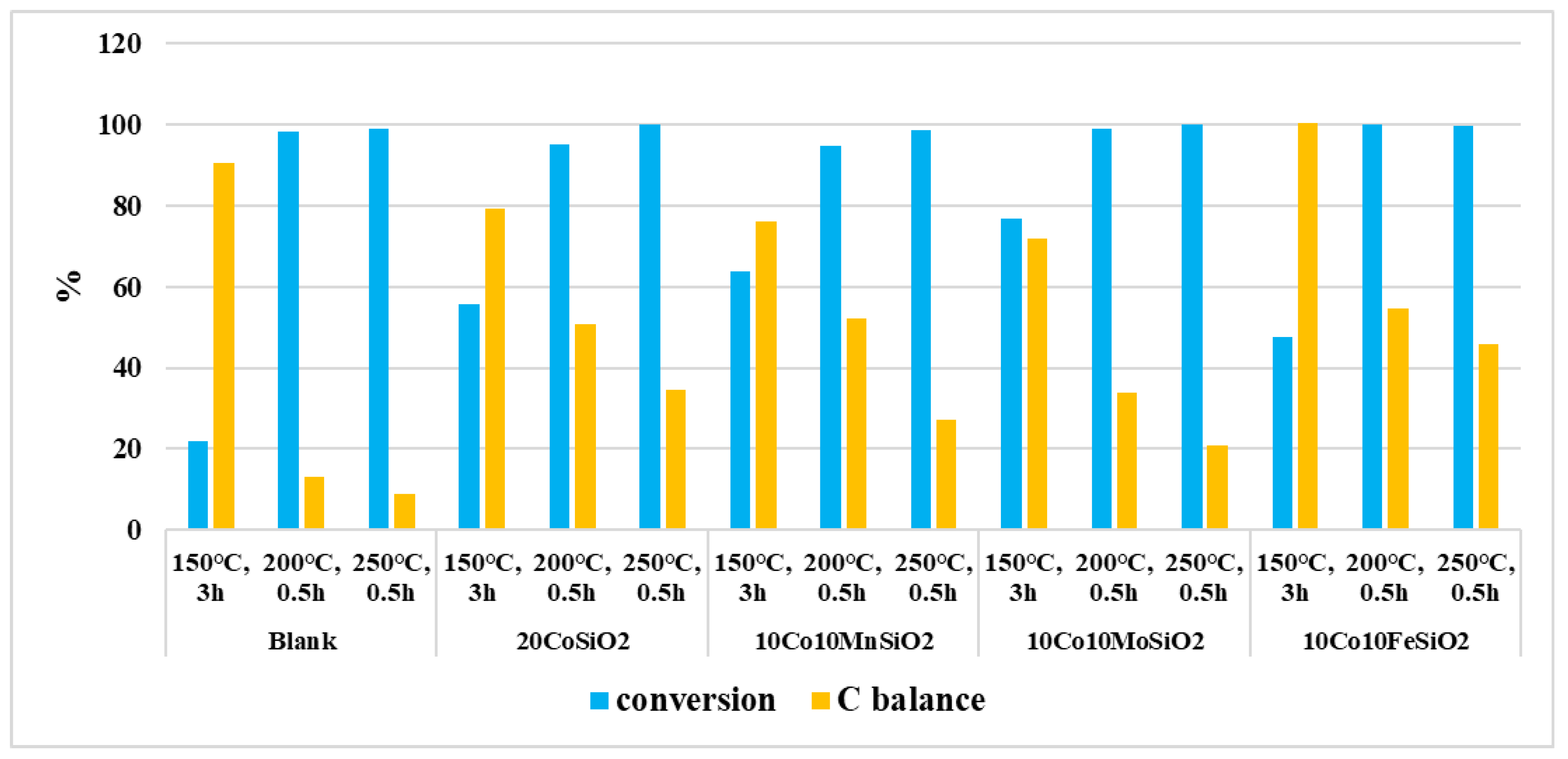
| Catalyst | Reaction Condtions | Conversion, % | Selectivity % | Carbon Balance*, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fructose | C4-5 | Lactic Acid | Acetic Acid | Levulinic Acid | Propiionic Acid | Methanol | Etanol | Glcyerol | Formic Acid | Hydroxyacetone | 5-HMF | ||||
| No cat | 150° C, 3h | 22 | 40 | <1 | 11 | − | − | − | − | − | 5 | − | − | − | 91 |
| 200° C, 0.5h | 98 | 5 | 3 | 2 | − | − | − | − | − | <1 | − | − | − | 13 | |
| 250 °C, 0.5h | 99 | <1 | 4 | <1 | 0.0 | − | − | - | − | <1 | − | − | 1 | 9 | |
| Co | 150 °C, 3 h | 56 | 35 | <1 | 3 | − | − | − | − | − | − | − | − | − | 79 |
| 200 °C, 0.5h | 95 | 2 | 3 | 22 | 1 | − | − | − | − | − | − | 3 | 14 | 51 | |
| 250 °C, 0.5 h | 100 | − | 2 | 11 | <1 | − | − | − | − | − | − | 19 | 3 | 35 | |
| Co-Mn | 150 °C, 3 h | 64 | 25 | 2 | 2 | <1 | − | − | 15 | − | 11 | − | 7 | <1 | 76 |
| 200 °C, 0.5 h | 95 | 2 | 9 | 15 | − | − | <1 | 10 | 4 | 3 | − | 5 | <1 | 52 | |
| 250 °C, 0.5h | 99 | − | 5 | 14 | <1 | − | − | − | 3 | <1 | − | <1 | <1 | 27 | |
| Co-Mo | 150 °C, 3 h | 77 | 27 | − | 7 | − | 25 | − | − | − | − | − | − | − | − |
| 200 °C, 0.5h | 99 | − | 6 | 3 | <1 | 1 | <1 | 5 | 9 | − | 3 | 7 | − | − | |
| 250 °C, 0.5h | 100 | − | 2 | 10 | <1 | <1 | 2 | 3 | 2 | − | − | <1 | − | − | |
| Co-Fe | 150 °C, 3 h | 48 | 39 | 2 | 2 | <1 | 48 | − | − | 1 | <1 | − | − | 5 | 100 |
| 200 °C, 0.5h | 100 | 9 | 1 | 3 | <1 | − | 5 | − | 20 | 1 | - | 14 | <1 | 55 | |
| 250 °C, 0.5 h | 100 | − | 20 | 9 | <1 | − | 2 | − | 11 | − | − | 8 | 12 | 46 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghavi, S.; Ghedini, E.; Menegazzo, F.; Signoretto, M.; Gazzoli, D.; Pietrogiacomi, D.; Matayeva, A.; Fasolini, A.; Vaccari, A.; Basile, F.; et al. MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals. Processes 2020, 8, 843. https://doi.org/10.3390/pr8070843
Taghavi S, Ghedini E, Menegazzo F, Signoretto M, Gazzoli D, Pietrogiacomi D, Matayeva A, Fasolini A, Vaccari A, Basile F, et al. MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals. Processes. 2020; 8(7):843. https://doi.org/10.3390/pr8070843
Chicago/Turabian StyleTaghavi, Somayeh, Elena Ghedini, Federica Menegazzo, Michela Signoretto, Delia Gazzoli, Daniela Pietrogiacomi, Aisha Matayeva, Andrea Fasolini, Angelo Vaccari, Francesco Basile, and et al. 2020. "MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals" Processes 8, no. 7: 843. https://doi.org/10.3390/pr8070843
APA StyleTaghavi, S., Ghedini, E., Menegazzo, F., Signoretto, M., Gazzoli, D., Pietrogiacomi, D., Matayeva, A., Fasolini, A., Vaccari, A., Basile, F., & Fornasari, G. (2020). MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals. Processes, 8(7), 843. https://doi.org/10.3390/pr8070843










