Production of Liquid Milk Protein Concentrate with Antioxidant Capacity, Angiotensin Converting Enzyme Inhibitory Activity, Antibacterial Activity, and Hypoallergenic Property by Membrane Filtration and Enzymatic Modification of Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Ultra-Heat-Treated Skimmed Cow Milk
2.3. Production of Hypoallergenic Liquid Milk Protein Concentrate with Functional Values
2.3.1. Concentrate Milk Proteins in Ultra-Heat-Treated Skimmed Milk by Membrane Technology
2.3.2. Enzymatic Hydrolysis of Concentrated Proteins in Milk
2.4. Analytical Method
2.4.1. Understanding of Molecular Weight of Proteins in Concentrated Milk
2.4.2. Understanding of Hydrolysis of Liquid Milk Protein Concentrate
2.4.3. Immunoblotting of Concentrated Milk Proteins
2.4.4. Determination of Antioxidant Capacity
2.4.5. Estimation of Angiotensin-Converting-Enzyme Inhibitory Activity
2.4.6. Determination of Protein Concentration
2.4.7. Microbiological Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Concentrate Milk Proteins in Skimmed Milk by Membrane Filtration
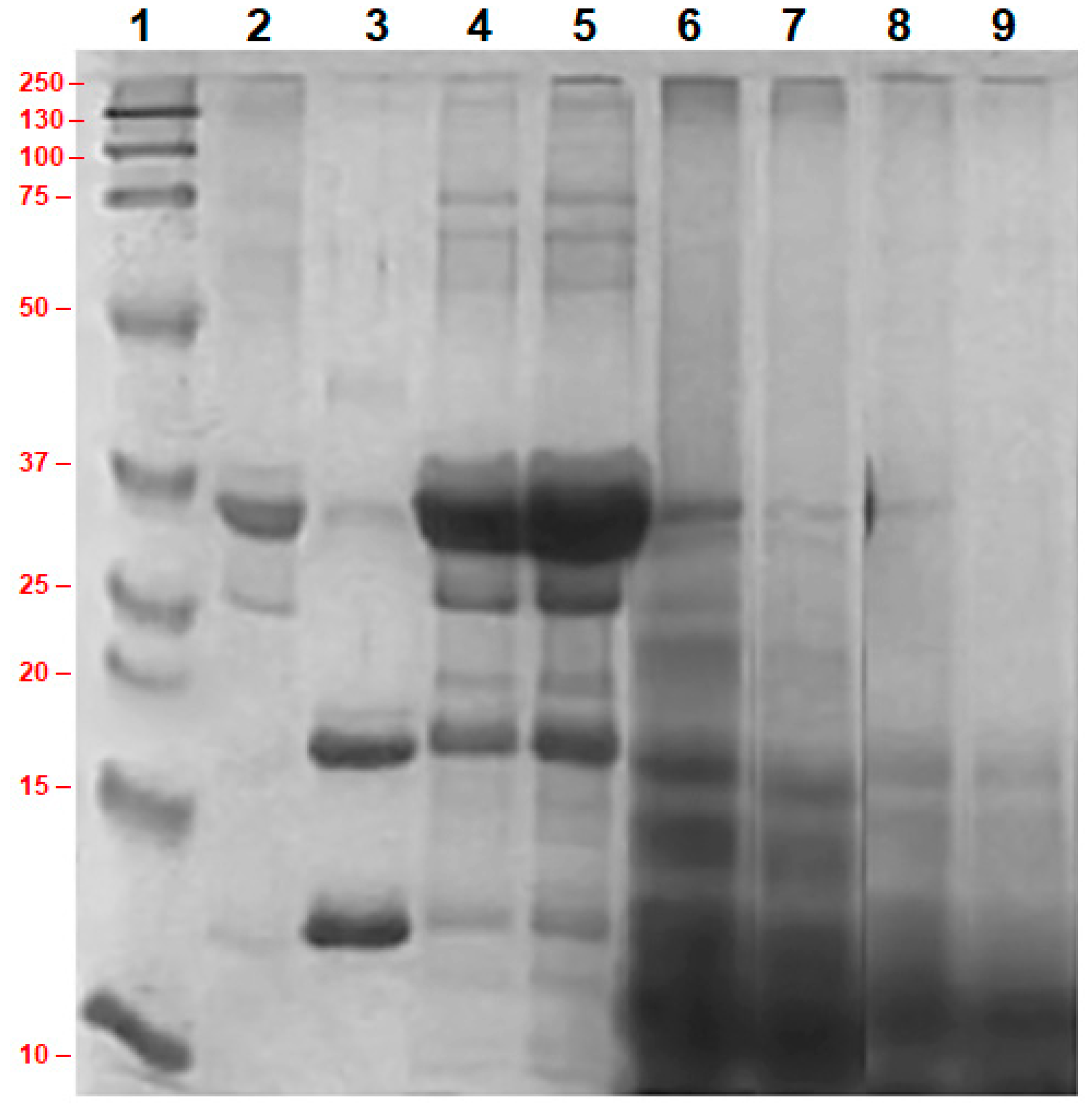
3.2. Molecular Weight of Different Proteins in Concentrated Milk and Their Enzymatic Hydrolysis
3.3. Antioxidant Capacity
3.4. Angiotensin Converting Enzyme-Inhibitory Activity
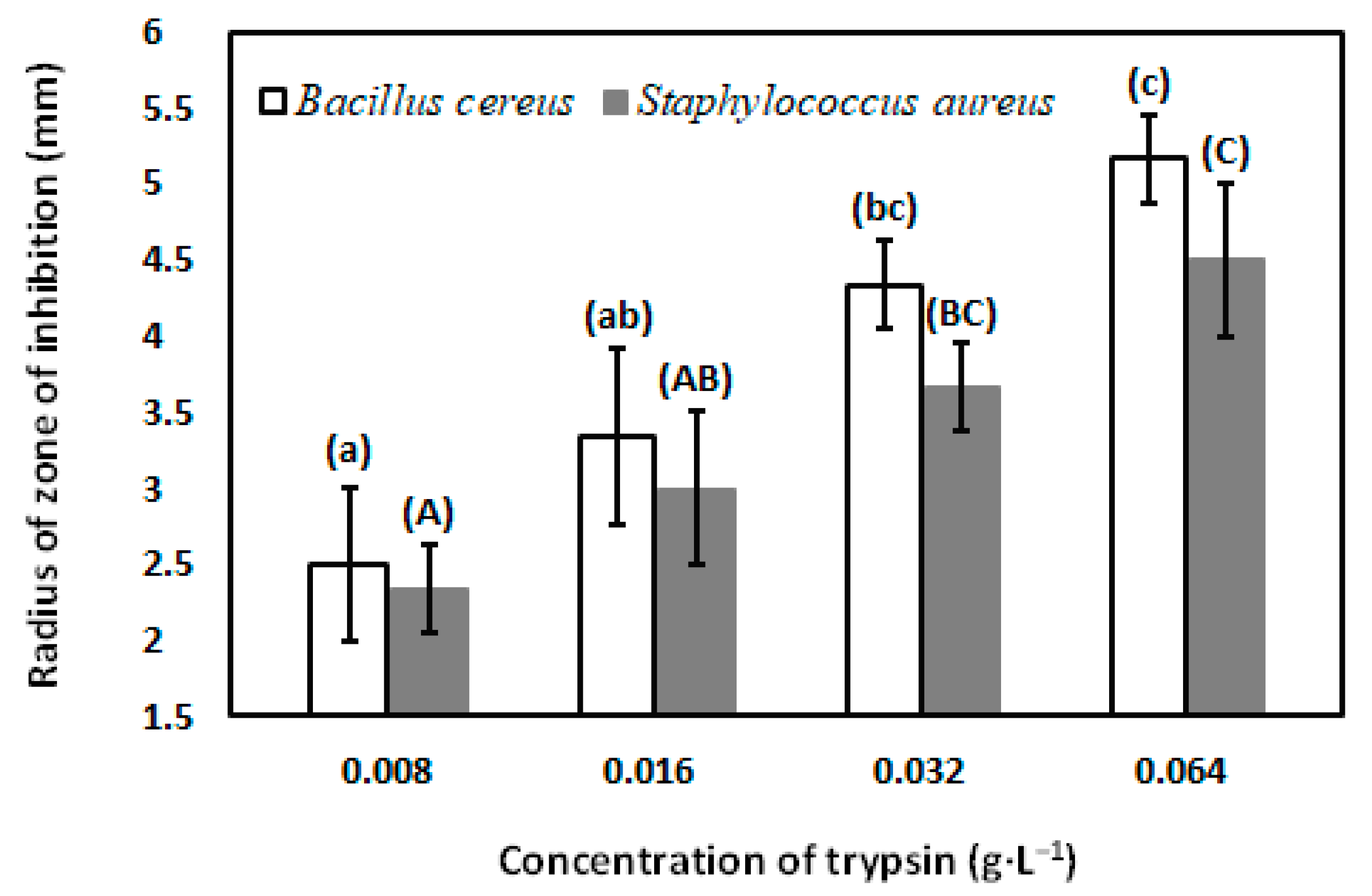
3.5. Antibacterial Activity
3.6. Allergenicity
3.7. Superiority of the Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- St-Onge, M.-P.; Farnworth, E.R.; Jones, P.J. Consumption of fermented and nonfermented dairy products: Effects on cholesterol concentrations and metabolism. Am. J. Clin. Nutr. 2000, 71, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Guinee, T.P.; O’Kennedy, B.T.; Kelly, P.M. Effect of Milk Protein Standardization Using Different Methods on the Composition and Yields of Cheddar Cheese. J. Dairy Sci. 2006, 89, 468–482. [Google Scholar] [CrossRef]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. Int. J. Dairy Technol. 2006, 59, 216–221. [Google Scholar] [CrossRef]
- Schuck, P.; Jeantet, R.; Bhandari, B.; Chen, X.D.; Perrone, Í.T.; De Carvalho, A.F.; Fenelon, M.; Kelly, P. Recent advances in spray drying relevant to the dairy industry: A comprehensive critical review. Dry. Technol. 2016, 34, 1773–1790. [Google Scholar] [CrossRef]
- Ur Rehman, S.; Farkye, N.Y.; Considine, T.; Schaffner, A.; Drake, M.A. Effects of Standardization of Whole Milk with Dry Milk Protein Concentrate on the Yield and Ripening of Reduced-Fat Cheddar Cheese. J. Dairy Sci. 2003, 86, 1608–1615. [Google Scholar] [CrossRef]
- Meena, G.S.; Singh, A.K.; Panjagari, N.R.; Arora, S. Milk protein concentrates: Opportunities and challenges. J. Food Sci. Technol. 2017, 54, 3010–3024. [Google Scholar] [CrossRef]
- Amelia, I.; Barbano, D.M. Production of an 18% protein liquid micellar casein concentrate with a long refrigerated shelf life. J. Dairy Sci. 2013, 96, 3340–3349. [Google Scholar] [CrossRef] [Green Version]
- Henriques, M.H.F.; Gomes, D.M.G.S.; Pereira, C.J.D.; Gil, M.H.M. Effects of Liquid Whey Protein Concentrate on Functional and Sensorial Properties of Set Yogurts and Fresh Cheese. Food Bioprocess Technol. 2013, 6, 952–963. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, M. Current and future applications for membrane processes in the dairy industry. Trends Food Sci. Technol. 1995, 6, 12–19. [Google Scholar] [CrossRef]
- Pouliot, Y. Membrane processes in dairy technology—From a simple idea to worldwide panacea. Int. Dairy J. 2008, 18, 735–740. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, N.; Ranjan, R.; Kumar, S.; Bhat, Z.F.; Jeong, D.K. Perspective of Membrane Technology in Dairy Industry: A Review. Asian Australas. J. Anim. Sci. 2013, 26, 1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Hsu, Y.-C.; Zhang, Z.; Dharsana, N.; Ye, Y.; Chen, V. The influence of milk components on the performance of ultrafiltration/diafiltration of concentrated skim milk. Sep. Sci. Technol. 2017, 52, 381–391. [Google Scholar] [CrossRef]
- Arunkumar, A.; MR, E. Milk Protein Concentration Using Negatively Charged Ultrafiltration Membranes. Foods 2018, 7, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavazzi-April, C.; Benoit, S.; Doyen, A.; Britten, M.; Pouliot, Y. Preparation of milk protein concentrates by ultrafiltration and continuous diafiltration: Effect of process design on overall efficiency. J. Dairy Sci. 2018, 101, 9670–9679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.S.Y.; Haribabu, M.; Harvie, D.J.E.; Dunstan, D.E.; Martin, G.J.O. Mechanisms of flux decline in skim milk ultrafiltration: A review. J. Membr. Sci. 2017, 523, 144–162. [Google Scholar] [CrossRef]
- Bacchin, P.; Aimar, P.; Field, R.W. Critical and sustainable fluxes: Theory, experiments and applications. J. Membr. Sci. 2006, 281, 42–69. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.D.; Munro, P.A.; Trägårdh, G. The effect of protein fouling in microfiltration and ultrafiltration on permeate flux, protein retention and selectivity: A literature review. Desalination 1993, 91, 65–108. [Google Scholar] [CrossRef]
- Bahnasawy, A.H.; Shenana, M.E. Flux behavior and energy consumption of ultrafiltration (UF) process of milk. Aust. J. Agric. Eng. 2010, 1, 54–65. [Google Scholar]
- Méthot-Hains, S.; Benoit, S.; Bouchard, C.; Doyen, A.; Bazinet, L.; Pouliot, Y. Effect of transmembrane pressure control on energy efficiency during skim milk concentration by ultrafiltration at 10 and 50 °C. J. Dairy Sci. 2016, 99, 8655–8664. [Google Scholar] [CrossRef] [Green Version]
- Guetouache, M.; Guessas, B.; Medjekal, S. Composition and nutritional value of raw milk 2014. Bio. Sci. Pharm. Res. 2014, 2, 115–122. [Google Scholar]
- Walsh, J.; Meyer, R.; Shah, N.; Quekett, J.; Fox, A.T. Differentiating milk allergy (IgE and non-IgE mediated) from lactose intolerance: Understanding the underlying mechanisms and presentations. Br. J. Gen. Pract. 2016, 66, e609–e611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lifschitz, C.; Szajewska, H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur. J. Pediatrics 2015, 174, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA). J. Acad. Nutr. Diet. 2004, 106, 1742–1744. [Google Scholar]
- Sackesen, C.; Altintas, D.U.; Bingol, A.; Bingol, G.; Buyuktiryaki, B.; Demir, E.; Kansu, A.; Kuloglu, Z.; Tamay, Z.; Sekerel, B.E.; et al. Current Trends in Tolerance Induction in Cow’s Milk Allergy: From Passive to Proactive Strategies. Front. Pediatrics 2019, 7, 372. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361. [Google Scholar] [CrossRef] [Green Version]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy Fats and Cardiovascular Disease: Do We Really Need to be Concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.S.S.; Fernandes, C.S.; Vieira, M.N.N.; De Felice, F.G. Insulin Resistance in Alzheimer’s Disease. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Seo, H.I.; Cha, H.Y.; Yang, Y.J.; Kwon, S.H.; Yang, S.J. Diabetes and Alzheimer’s Disease: Mechanisms and Nutritional Aspects. Clin. Nutr. Res. 2018, 7, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Shi, J.; Yao, M.; Jiang, M.; Luo, Y. Effects of heat treatment on the antigenicity of four milk proteins in milk protein concentrates. Food Agric. Immunol. 2016, 27, 401–413. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Mafra, I. Detection and Quantification of Milk Ingredients as Hidden Allergens in Meat Products by a Novel Specific Real-Time PCR Method. Biomolecules 2019, 9, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, C.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Bovine Milk Allergens: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef] [Green Version]
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleber, N.; Maier, S.; Hinrichs, J. Antigenic response of bovine β-lactoglobulin influenced by ultra-high pressure treatment and temperature. Innov. Food Sci. Emerg. Technol. 2007, 8, 39–45. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Alonso, E.; López-Fandiño, R. Immunoreactivity and digestibility of high-pressure-treated whey proteins. Int. Dairy J. 2008, 18, 367–376. [Google Scholar] [CrossRef]
- Kleber, N.; Hinrichs, J. Antigenic response of β-lactoglobulin in thermally treated bovine skim milk and sweet whey. Milchwissenschaft 2007, 62, 121–124. [Google Scholar]
- Fiocchi, A.; Restani, P.; Riva, E.; Mirri, G.P.; Santini, I.; Bernardo, L.; Galli, C.L. Heat treatment modifies the allergenicity of beef and bovine serum albumin. Allergy 2008, 53, 798–802. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
- Ismahan, B.D.; Hanane, K.; Omar, K.; Djamel, S. Microwave irradiation of bovine milk reduces allergic response in mouse model of food allergy. Front. Immunol. 2014, 10. [Google Scholar] [CrossRef]
- Cheison, S.C.; Wang, Z.; Xu, S.-Y. Preparation of Whey Protein Hydrolysates Using a Single- and Two-Stage Enzymatic Membrane Reactor and Their Immunological and Antioxidant Properties: Characterization by Multivariate Data Analysis. J. Agric. Food Chem. 2007, 55, 3896–3904. [Google Scholar] [CrossRef]
- Guadix, A.; Camacho, F.; Guadix, E.M. Production of whey protein hydrolysates with reduced allergenicity in a stable membrane reactor. J. Food Eng. 2006, 72, 398–405. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hirano, A.; Ohta, A.; Yoshida, T.; Takahashi, K.; Hattori, M. Reduced Immunogenicity of β-Lactoglobulin by Conjugation with Carboxymethyl Dextran Differing in Molecular Weight. J. Agric. Food Chem. 2001, 49, 823–831. [Google Scholar] [CrossRef]
- Bu, G.; Lu, J.; Zheng, Z.; Luo, Y. Influence of Maillard reaction conditions on the antigenicity of bovine α-lactalbumin using response surface methodology. J. Sci. Food Agric. 2009, 89, 2428–2434. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Lu, J.; Zhang, Y. Reduced antigenicity of β-lactoglobulin by conjugation with glucose through controlled Maillard reaction conditions. Food Agric. Immunol. 2010, 21, 143–156. [Google Scholar] [CrossRef]
- Pahud, J.J.; Monti, J.C.; Jost, R. Allergenicity of whey protein: Its modification by tryptic in vitro hydrolysis of the protein. J. Pediatric Gastroenterol. Nutr. 1985, 4, 408–413. [Google Scholar] [CrossRef]
- Nakamura, T.; Sado, H.; Syukunobe, Y.; Hirota, T. Antigenicity of whey protein hydrolysates prepared by combination of two proteases. Milchwissenschaft 1993, 48, 667–670. [Google Scholar]
- Wroblewska, B.; Karamac, M.; Amarowicz, R.; Szymkiewicz, A.; Troszynska, A.; Kubicka, E. Immunoreactive properties of peptide fractions of cow whey milk proteins after enzymatic hydrolysis. Int. J. Food Sci. Technol. 2004, 39, 839–850. [Google Scholar] [CrossRef]
- Ena, J.M.; Beresteijn, E.C.H.; Robben, A.J.P.M.; Schmidt, D.G. Whey Protein Antigenicity Reduction by Fungal Proteinases and a Pepsin/Pancreatin Combination. J. Food Sci. 1995, 60, 104–110. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Recio, I.; López-Fandiño, R. Influence of high hydrostatic pressure on the proteolysis of β-lactoglobulin A by trypsin. J. Dairy Res. 2006, 73, 121–128. [Google Scholar] [CrossRef]
- Bonomi, F.; Fiocchi, A.; Frøkiær, H.; Gaiaschi, A.; Iametti, S.; Poiesi, C.; Rasmussen, P.; Restani, P.; Rovere, P. Reduction of immunoreactivity of bovine β-lactoglobulin upon combined physical and proteolytic treatment. J. Dairy Res. 2003, 70, 51–59. [Google Scholar] [CrossRef]
- Beran, M.; Klubal, R.; Molik, P.; Strohalm, J.; Urban, M.; Klaudyova, A.A.; Prajzlerova, K. Influence of high-hydrostatic pressure on tryptic and chymotryptic hydrolysis of milk proteins. High Press. Res. 2009, 29, 23–27. [Google Scholar] [CrossRef]
- El Mecherfi, K.E.; Saidi, D.; Kheroua, O.; Boudraa, G.; Touhami, M.; Rouaud, O.; Curet, S.; Choiset, Y.; Rabesona, H.; Chobert, J.-M.; et al. Combined microwave and enzymatic treatments for β-lactoglobulin and bovine whey proteins and their effect on the IgE immunoreactivity. Eur. Food Res. Technol. 2011, 233, 859–867. [Google Scholar] [CrossRef]
- Izquierdo, F.J.; Alli, I.; Yaylayan, V.; Gomez, R. Microwave-assisted digestion of β-lactoglobulin by pronase, α-chymotrypsin and pepsin. Int. Dairy J. 2007, 17, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, F.J.; Peñas, E.; Baeza, M.L.; Gomez, R. Effects of combined microwave and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. Int. Dairy J. 2008, 18, 918–922. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Tu, Z.; Wang, H.; Zhang, L.; Kaltashov, I.A.; Zhao, Y.; Niu, C.; Yao, H.; Ye, W. The mechanism of reduced IgG/IgE-binding of β-lactoglobulin by pulsed electric field pretreatment combined with glycation revealed by ECD/FTICR-MS. Food Funct. 2018, 9, 417–425. [Google Scholar] [CrossRef]
- Quintieri, L.; Monaci, L.; Baruzzi, F.; Giuffrida, M.G.; De Candia, S.; Caputo, L. Reduction of whey protein concentrate antigenicity by using a combined enzymatic digestion and ultrafiltration approach. J. Food Sci. Technol. 2017, 54, 1910–1916. [Google Scholar] [CrossRef]
- El Mecherfi, K.E.; Curet, S.; Lupi, R.; Larré, C.; Rouaud, O.; Choiset, Y.; Rabesona, H.; Haertlé, T. Combined microwave processing and enzymatic proteolysis of bovine whey proteins: The impact on bovine β-lactoglobulin allergenicity. J. Food Sci. Technol. 2019, 56, 177–186. [Google Scholar] [CrossRef]
- Spies, J.B. Milk Allergy. Milk Food Technol. 1973, 36, 225–231. [Google Scholar] [CrossRef]
- El-Agamy, E.I. The challenge of cow milk protein allergy. Small Rumin. Res. 2007, 68, 64–72. [Google Scholar] [CrossRef]
- Kim, S.B.; Ki, K.S.; Khan, M.A.; Lee, W.S.; Lee, H.J.; Ahn, B.S.; Kim, H.S. Peptic and Tryptic Hydrolysis of Native and Heated Whey Protein to Reduce Its Antigenicity. J. Dairy Sci. 2007, 90, 4043–4050. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Imran, M.; Khan, M.K.; Nisa, M.U. Degree of hydrolysis and antigenicity of buffalo alpha S1 casein and its hydrolysates in children with cow milk allergy. Food Agric. Immunol. 2016, 27, 87–98. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.M.; Sakr, S.S.; El-Dieb, S.M.; Elkashef, H.A.S. Bioactive peptides with ACE-I and antioxidant activity produced from milk proteolysis. Int. J. Food Prop. 2017, 20, 3033–3042. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Pan, K.; Zhong, Q. Physical, chemical and biochemical properties of casein hydrolyzed by three proteases: Partial characterizations. Food Chem. 2014, 155, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Shin, S.H.; Suh, J.-W.; Nimalaratne, C.; Sunwoo, H. Anti-Inflammatory and Antioxidant Properties of Casein Hydrolysate Produced Using High Hydrostatic Pressure Combined with Proteolytic Enzymes. Molecules 2017, 22, 609. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Szécsi, G.; Csehi, B.; Mednyánszky, Z.; Kiskó, G.; Bányai, É.; Dernovics, M.; Koris, A. Production of Hypoallergenic Antibacterial Peptides from Defatted Soybean Meal by a Membrane Associated Bioreactor: A Bioprocess Engineering Study with Comprehensive Product Characterization. Food Technol. Biotechnol. 2017, 55, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Chakraborty, S.; Bhattacharjee, C.; Chowdhury, R. Studies on the separation of proteins and lactose from casein whey by cross-flow ultrafiltration. Desalin. Water Treat. 2015, 54, 481–501. [Google Scholar] [CrossRef]
- Nath, A.; Kailo, G.G.; Mednyánszky, Z.; Kiskó, G.; Csehi, B.; Pásztorné-Huszár, K.; Gerencsér-Berta, R.; Galambos, I.; Pozsgai, E.; Bánvölgyi, S.; et al. Antioxidant and Antibacterial Peptides from Soybean Milk through Enzymatic- and Membrane-Based Technologies. Bioengineering 2019, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel milk protein hydrolysates generated with trypsin. J. Funct. Foods 2017, 34, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Cheison, S.C.; Schmitt, M.; Leeb, E.; Letzel, T.; Kulozik, U. Influence of temperature and degree of hydrolysis on the peptide composition of trypsin hydrolysates of β-lactoglobulin: Analysis by LC–ESI-TOF/MS. Food Chem. 2010, 121, 457–467. [Google Scholar] [CrossRef]
- Elfagm, A.A.; Wheelock, J.V. Interaction of Bovine α-Lactalbumin and β-Lactoglobulin during Heating. J. Dairy Sci. 1978, 61, 28–32. [Google Scholar] [CrossRef]
- De Wit, J.; Klarenbeek, B. Effects of Various Heat Treatments on Structure and Solubility of Whey Proteins. J. Dairy Sci. 1984, 67, 2701–2710. [Google Scholar] [CrossRef]
- Cassiani, D.M.; Yamul, D.K.; Conforti, P.A.; Pérez, V.A.; Lupano, C.E. Structure and Functionality of Whey Protein Concentrate-Based Products with Different Water Contents. Food Bioprocess Technol. 2013, 6, 217–227. [Google Scholar] [CrossRef]
- Rauh, V.M.; Johansen, L.B.; Bakman, M.; Ipsen, R.; Paulsson, M.; Larsen, L.B.; Hammershøj, M. Protein lactosylation in UHT milk during storage measured by Liquid Chromatography-Mass Spectrometry and quantification of furosine. Int. J. Dairy Technol. 2015, 68, 486–494. [Google Scholar] [CrossRef]
- Csehi, B.; Szerdahelyi, E.; Pásztor-Huszár, K.; Salamon, B.; Tóth, A.; Zeke, I.; Jónás, G.; Friedrich, L. Changes of protein profiles in pork and beef meat caused by high hydrostatic pressure treatment. Acta Aliment. 2016, 45, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Hajós, G.; Polgár, M.; Farkas, J. High-pressure effects on IgE immunoreactivity of proteins in a sausage batter. Innov. Food Sci. Emerg. Technol. 2004, 5, 443–449. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Fagyas, M.; Úri, K.; Siket, I.M.; Daragó, A.; Boczán, J.; Bányai, E.; Édes, I.; Papp, Z.; Tóth, A. New Perspectives in the Renin-Angiotensin-Aldosterone System (RAAS) I: Endogenous Angiotensin Converting Enzyme (ACE) Inhibition. PLoS ONE 2014, 9, e87843. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hussein, K.; Friedrich, L.; Kisko, G.; Ayari, E.; Nemeth, C.; Dalmadi, I. Use of allyl-isothiocyanate and carvacrol to preserve fresh chicken meat during chilling storage. Czech J. Food Sci. 2019, 37, 417–424. [Google Scholar] [CrossRef]
- De Wit, J.N. Nutritional and Functional Characteristics of Whey Proteins in Food Products. J. Dairy Sci. 1998, 81, 597–608. [Google Scholar] [CrossRef]
- Penfield, M.P.; Campbell, A.M.; Penfield, M.P.; Campbell, A.M. Chapter 8—Milk and Milk Products. In Food Science and Technology, 3rd ed.; Penfield, M.P., Campbell, A.M.B.T.-E.F.S., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 162–183. ISBN 978-0-12-157920-3. [Google Scholar]
- Relkin, P.; Mulvihill, D.M. Thermal unfolding of β-lactoglobulin, α-lactalbumin, and bovine serum albumin. A thermodynamic approach. Crit. Rev. Food Sci. Nutr. 1996, 36, 565–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Hong, Y.-H. Electrophoretic Behaviors of α-Lactalbumin and β-Lactoglobulin Mixtures Caused by Heat Treatment. Asian Australas. J. Anim. Sci. 2003, 16, 1041–1045. [Google Scholar] [CrossRef]
- Manderson, G.A.; Hardman, M.J.; Creamer, L.K. Effect of Heat Treatment on the Conformation and Aggregation of β-Lactoglobulin A, B, and C. J. Agric. Food Chem. 1998, 46, 5052–5061. [Google Scholar] [CrossRef]
- Law, A.J.R.; Horne, D.S.; Banks, J.M.; Leaver, J. Heat-induced changes in the whey proteins and caseins. Milchwissenschaft 1994, 49, 125–129. [Google Scholar]
- Dannenberg, F.; Kessler, H.-G. Reaction Kinetics of the Denaturation of Whey Proteins in Milk. J. Food Sci. 1988, 53, 258–263. [Google Scholar] [CrossRef]
- Dannenberg, F.; Kessler, H.G. Thermodynamic approach to kinetics of β-lactoglobulin denaturation in heated skim milk and sweet whey. Milchwissenschaft 1988, 43, 139–142. [Google Scholar]
- Dalgleish, D.G. Denaturation and aggregation of serum proteins and caseins in heated milk. J. Agric. Food Chem. 1990, 38, 1995–1999. [Google Scholar] [CrossRef]
- Gezimati, J.; Singh, H.; Creamer, L.K. Heat-Induced Interactions and Gelation of Mixtures of Bovine β-Lactoglobulin and Serum Albumin. J. Agric. Food Chem. 1996, 44, 804–810. [Google Scholar] [CrossRef]
- Sawyer, W.H. Complex Between β-Lactoglobulin and ĸ-Casein. A Review. J. Dairy Sci. 1969, 52, 1347–1355. [Google Scholar] [CrossRef]
- Sawyer, W.H.; Coulter, S.T.; Jenness, R. Role of Sulfhydryl Groups in the Interaction of κ-Casein and β-Lactoglobulin. J. Dairy Sci. 1963, 46, 564–565. [Google Scholar] [CrossRef]
- Jang, H.; Swaisgood, H. Disulfide Bond Formation Between Thermally Denatured β-Lactoglobulin and κ-Casein in Casein Micelles. J. Dairy Sci. 1990, 73, 900–904. [Google Scholar] [CrossRef]
- Morr, C.V.; Ha, E.Y.W. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef] [PubMed]
- Buchert, J.; Ercili Cura, D.; Ma, H.; Gasparetti, C.; Monogioudi, E.; Faccio, G.; Mattinen, M.; Boer, H.; Partanen, R.; Selinheimo, E.; et al. Crosslinking Food Proteins for Improved Functionality. Annu. Rev. Food Sci. Technol. 2010, 1, 113–138. [Google Scholar] [CrossRef]
- Gerrard, J.A. Protein–protein crosslinking in food: Methods, consequences, applications. Trends Food Sci. Technol. 2002, 13, 391–399. [Google Scholar] [CrossRef]
- Singh, H. Modification of food proteins by covalent crosslinking. Trends Food Sci. Technol. 1991, 2, 196–200. [Google Scholar] [CrossRef]
- Miranda, G.; Bianchi, L.; Krupova, Z.; Trossat, P.; Martin, P. An improved LC–MS method to profile molecular diversity and quantify the six main bovine milk proteins, including genetic and splicing variants as well as post-translationally modified isoforms. Food Chem. X 2020, 5, 100080. [Google Scholar] [CrossRef]
- Singh, H. Heat stability of milk. Int. J. Dairy Technol. 2004, 57, 111–119. [Google Scholar] [CrossRef]
- Kastrup Dalsgaard, T.; Holm Nielsen, J.; Bach Larsen, L. Proteolysis of milk proteins lactosylated in model systems. Mol. Nutr. Food Res. 2007, 51, 404–414. [Google Scholar] [CrossRef]
- Czerwenka, C.; Maier, I.; Pittner, F.; Lindner, W. Investigation of the lactosylation of whey proteins by liquid chromatography—Mass spectrometry. J. Agric. Food Chem. 2006, 54, 8874–8882. [Google Scholar] [CrossRef]
- Losito, I.; Carbonara, T.; Monaci, L.; Palmisano, F. Evaluation of the thermal history of bovine milk from the lactosylation of whey proteins: An investigation by liquid chromatography—Electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2007, 389, 2065–2074. [Google Scholar] [CrossRef]
- Almaas, H.; Cases, A.-L.; Devold, T.; Holm, H.; Langsrud, T.; Aabakken, L.; Aadnoey, T.; Vegarud, G. In vitro digestion of bovine and caprine milk by human gastric and duodenal enzymes. Int. Dairy J. 2006, 16, 961–968. [Google Scholar] [CrossRef]
- Costa, F.F.; Vasconcelos Paiva Brito, M.A.; Moreira Furtado, M.A.; Martins, M.F.; Leal de Oliveira, M.A.; Mendonça De Castro Barra, P.; Amigo Garrido, L.; Siqueira De Oliveira Dos Santos, A. Microfluidic chip electrophoresis investigation of major milk proteins: Study of buffer effects and quantitative approaching. Anal. Methods 2014, 6, 1666–1673. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska, B.; Kaliszewska, A. Cow’s Milk Proteins Immunoreactivity and Allergenicity in Processed Food. Czech J. Food Sci. 2012, 30, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.-H.; Creamer, L.K. Changed protein structures of bovine β-lactoglobulin B and α-lactalbumin as a consequence of heat treatment. Int. Dairy J. 2002, 12, 345–359. [Google Scholar] [CrossRef]
- Dunnill, P.; Green, D.W. Sulphydryl groups and the N⇄R conformational change in β-lactoglobulin. J. Mol. Biol. 1966, 15, 147–151. [Google Scholar] [CrossRef]
- Havea, P.; Singh, H.; Creamer, L.K. Characterization of heat-induced aggregates of β-lactoglobulin, α-lactalbumin and bovine serum albumin in a whey protein concentrate environment. J. Dairy Res. 2001, 68, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Raak, N.; Abbate, R.; Lederer, A.; Rohm, H.; Jaros, D. Size Separation Techniques for the Characterisation of Cross-Linked Casein: A Review of Methods and Their Applications. Separations 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Tiambeng, T.N.; Cai, W.; Chen, B.; Lin, Z.; Gregorich, Z.R.; Ge, Y. Impact of Phosphorylation on the Mass Spectrometry Quantification of Intact Phosphoproteins. Anal. Chem. 2018, 90, 4935–4939. [Google Scholar] [CrossRef] [Green Version]
- Bobe, G.; Beitz, D.C.; Freeman, A.E.; Lindberg, G.L. Sample Preparation Affects Separation of Whey Proteins by Reversed-Phase High-Performance Liquid Chromatography. J. Agric. Food Chem. 1998, 46, 1321–1325. [Google Scholar] [CrossRef]
- Cheema, M.; Mohan, M.S.; Campagna, S.R.; Jurat-Fuentes, J.L.; Harte, F.M. The association of low-molecular-weight hydrophobic compounds with native casein micelles in bovine milk. J. Dairy Sci. 2015, 98, 5155–5163. [Google Scholar] [CrossRef] [Green Version]
- Assem, F.M.; Abd El-Gawad, M.A.M.; Kassem, J.M.; Abd El-Salam, M.H. Proteolysis and antioxidant activity of peptic, tryptic and chymotryptic hydrolysates of cow, buffalo, goat and camel caseins. Int. J. Dairy Technol. 2018, 71, 236–242. [Google Scholar] [CrossRef]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Manso, M.A.; López-Fandiño, R. Angiotensin I converting enzyme-inhibitory activity of bovine, ovine, and caprine kappa-casein macropeptides and their tryptic hydrolysates. J. Food Prot. 2003, 66, 1686–1692. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Decroos, K.; Van Wijmelbeke, L.; Verstraete, W. The Impact of Fermentation and In Vitro Digestion on the Formation of Angiotensin-I-Converting Enzyme Inhibitory Activity from Pea and Whey Protein. J. Dairy Sci. 2003, 86, 429–438. [Google Scholar] [CrossRef]
- Wang, C.; Tu, M.; Wu, D.; Chen, H.; Chen, C.; Wang, Z.; Jiang, L. Identification of an ACE-Inhibitory Peptide from Walnut Protein and Its Evaluation of the Inhibitory Mechanism. Int. J. Mol. Sci. 2018, 19, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pihlanto-Leppälä, A.; Rokka, T.; Korhonen, H. Angiotensin I converting enzyme inhibitory peptides derived from bovine milk proteins. Int. Dairy J. 1998, 8, 325–331. [Google Scholar] [CrossRef]
- Abubakar, A.; Saito, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Structural Analysis of New Antihypertensive Peptides Derived from Cheese Whey Protein by Proteinase K Digestion. J. Dairy Sci. 1998, 81, 3131–3138. [Google Scholar] [CrossRef]
- López-Fandiño, R.; Otte, J.; Van Camp, J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar]
- Medeiros, V.; Rainha, N.; Paiva, L.; Lima, E.; Baptista, J. Bovine Milk Formula Based on Partial Hydrolysis of Caseins by Bromelain Enzyme: Better Digestibility and Angiotensin-Converting Enzyme-Inhibitory Properties. Int. J. Food Prop. 2014, 17, 806–817. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The interaction of antimicrobial peptides with membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Sun, J.; Cao, D.; Tuo, Y.; Jiang, S.; Mu, G. Experimental and Modelling Study of the Denaturation of Milk Protein by Heat Treatment. Korean J. Food Sci. Anim. Resour. 2017, 37, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, S.; Kabashima, K.; Moriyama, T.; Tokura, Y. Food-dependent anaphylaxis with serum IgE immunoreactive to dairy products containing high-molecular-weight proteins. J. Dermatol. Sci. 2010, 57, 137–140. [Google Scholar] [CrossRef]
- Natale, M.; Bisson, C.; Monti, G.; Peltran, A.; Perono Garoffo, L.; Valentini, S.; Fabris, C.; Bertino, E.; Coscia, A.; Conti, A.; et al. Cow’s milk allergens identification by two-dimensional immunoblotting and mass spectrometry. Mol. Nutr. Food Res. 2004, 48, 363–369. [Google Scholar] [CrossRef]
- Meyer, P.; Petermeier, J.; Hartinger, M.; Kulozik, U. Concentration of Skim Milk by a Cascade Comprised of Ultrafiltration and Nanofiltration: Investigation of the Nanofiltration of Skim Milk Ultrafiltration Permeate. Food Bioprocess Technol. 2017, 10, 469–478. [Google Scholar] [CrossRef]
- Mistry, V.V.; Hassan, H.N. Delactosed, High Milk Protein Powder. 1. Manufacture and Composition. J. Dairy Sci. 1991, 74, 1163–1169. [Google Scholar] [CrossRef]
- Luo, X.; Ramchandran, L.; Vasiljevic, T. Lower ultrafiltration temperature improves membrane performance and emulsifying properties of milk protein concentrates. Dairy Sci. Technol. 2015, 95, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Rinaldoni, A.N.; Tarazaga, C.C.; Campderrós, M.E.; Padilla, A.P. Assessing performance of skim milk ultrafiltration by using technical parameters. J. Food Eng. 2009, 92, 226–232. [Google Scholar] [CrossRef]
- Grandison, A.S.; Youravong, W.; Lewis, M.J. Hydrodynamic factors affecting flux and fouling during ultrafiltration of skimmed milk. Le Lait 2000, 80, 165–174. [Google Scholar] [CrossRef]
- Meena, G.S.; Singh, A.K.; Arora, S.; Borad, S.; Sharma, R.; Gupta, V.K. Physico-chemical, functional and rheological properties of milk protein concentrate 60 as affected by disodium phosphate addition, diafiltration and homogenization. J. Food Sci. Technol. 2017, 54, 1678–1688. [Google Scholar] [CrossRef] [Green Version]
- Meena, G.S.; Singh, A.K.; Gupta, V.K.; Borad, S.; Parmar, P.T. Effect of change in pH of skim milk and ultrafiltered/diafiltered retentates on milk protein concentrate (MPC70) powder properties. J. Food Sci. Technol. 2018, 55, 3526–3537. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Mayer, A.; Kulozik, U. High concentration of skim milk proteins by ultrafiltration: Characterisation of a dynamic membrane system with a rotating membrane in comparison with a spiral wound membrane. Int. Dairy J. 2015, 51, 75–83. [Google Scholar] [CrossRef]
- Samuelsson, G.; Dejmek, P.; Trägårdh, G.; Paulsson, M. Minimizing whey protein retention in cross-flow microfiltration of skim milk. Int. Dairy J. 1997, 7, 237–242. [Google Scholar] [CrossRef]
- Pompei, C.; Resmini, P.; Peri, C. Skim Milk Protein Recovery and Purification by Ultrrafiltration Influence of Temperature on Permeation Rate and Retention. J. Food Sci. 1973, 38, 867–870. [Google Scholar] [CrossRef]
- Meena, G.S.; Singh, A.K.; Gupta, V.K.; Borad, S.; Arora, S.; Tomar, S.K. Effect of pH adjustment, homogenization and diafiltration on physicochemical, reconstitution, functional and rheological properties of medium protein milk protein concentrates (MPC70). J. Food Sci. Technol. 2018, 55, 1376–1386. [Google Scholar] [CrossRef]
- Meena, G.S.; Singh, A.K.; Gupta, V.K.; Borad, S.G.; Arora, S.; Tomar, S.K. Alteration in physicochemical, functional, rheological and reconstitution properties of milk protein concentrate powder by pH, homogenization and diafiltration. J. Food Sci. Technol. 2019, 56, 1622–1630. [Google Scholar] [CrossRef]








| TMP (Bar) | RFR (L·h−1) | Without Static | With Static | ||||
|---|---|---|---|---|---|---|---|
| ∆p (Bar) | Jinitial (L·h−1·m−2) | ∆J (%) | ∆p (Bar) | Jinitial (L·h−1·m−2) | ∆J (%) | ||
| 2 | 100 | 0.1 | 8.06 ± 1 a | 41.69 ± 1.27 a | 0.3 | 15.58 ± 1.1 a | 32.33 ± 1.25 a |
| 2 | 200 | 0.1 | 8.2 ± 1.2 a | 37.39 ± 2.35 a b | 0.7 | 15.88 ± 1 a | 31.70 ± 2.5 a |
| 3 | 100 | 0.1 | 13.45 ± 1.1 b | 36.95 ± 1.55 a, b | 0.3 | 34.22 ± 1.08 b | 24.01 ± 1.19 b |
| 3 | 200 | 0.1 | 18 ± 3.9 b | 33.33±2.79 b | 0.7 | 34.55 ± 1.02 b | 23.61 ± 2.31 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, A.; Eren, B.A.; Csighy, A.; Pásztorné-Huszár, K.; Kiskó, G.; Abrankó, L.; Tóth, A.; Szerdahelyi, E.; Kovács, Z.; Koris, A.; et al. Production of Liquid Milk Protein Concentrate with Antioxidant Capacity, Angiotensin Converting Enzyme Inhibitory Activity, Antibacterial Activity, and Hypoallergenic Property by Membrane Filtration and Enzymatic Modification of Proteins. Processes 2020, 8, 871. https://doi.org/10.3390/pr8070871
Nath A, Eren BA, Csighy A, Pásztorné-Huszár K, Kiskó G, Abrankó L, Tóth A, Szerdahelyi E, Kovács Z, Koris A, et al. Production of Liquid Milk Protein Concentrate with Antioxidant Capacity, Angiotensin Converting Enzyme Inhibitory Activity, Antibacterial Activity, and Hypoallergenic Property by Membrane Filtration and Enzymatic Modification of Proteins. Processes. 2020; 8(7):871. https://doi.org/10.3390/pr8070871
Chicago/Turabian StyleNath, Arijit, Burak Atilla Eren, Attila Csighy, Klára Pásztorné-Huszár, Gabriella Kiskó, László Abrankó, Attila Tóth, Emőke Szerdahelyi, Zoltán Kovács, András Koris, and et al. 2020. "Production of Liquid Milk Protein Concentrate with Antioxidant Capacity, Angiotensin Converting Enzyme Inhibitory Activity, Antibacterial Activity, and Hypoallergenic Property by Membrane Filtration and Enzymatic Modification of Proteins" Processes 8, no. 7: 871. https://doi.org/10.3390/pr8070871
APA StyleNath, A., Eren, B. A., Csighy, A., Pásztorné-Huszár, K., Kiskó, G., Abrankó, L., Tóth, A., Szerdahelyi, E., Kovács, Z., Koris, A., & Vatai, G. (2020). Production of Liquid Milk Protein Concentrate with Antioxidant Capacity, Angiotensin Converting Enzyme Inhibitory Activity, Antibacterial Activity, and Hypoallergenic Property by Membrane Filtration and Enzymatic Modification of Proteins. Processes, 8(7), 871. https://doi.org/10.3390/pr8070871








