A Novel Cysteine-Functionalized MxOy Material as Support for Laccase Immobilization and a Potential Application in Decolorization of Alizarin Red S
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
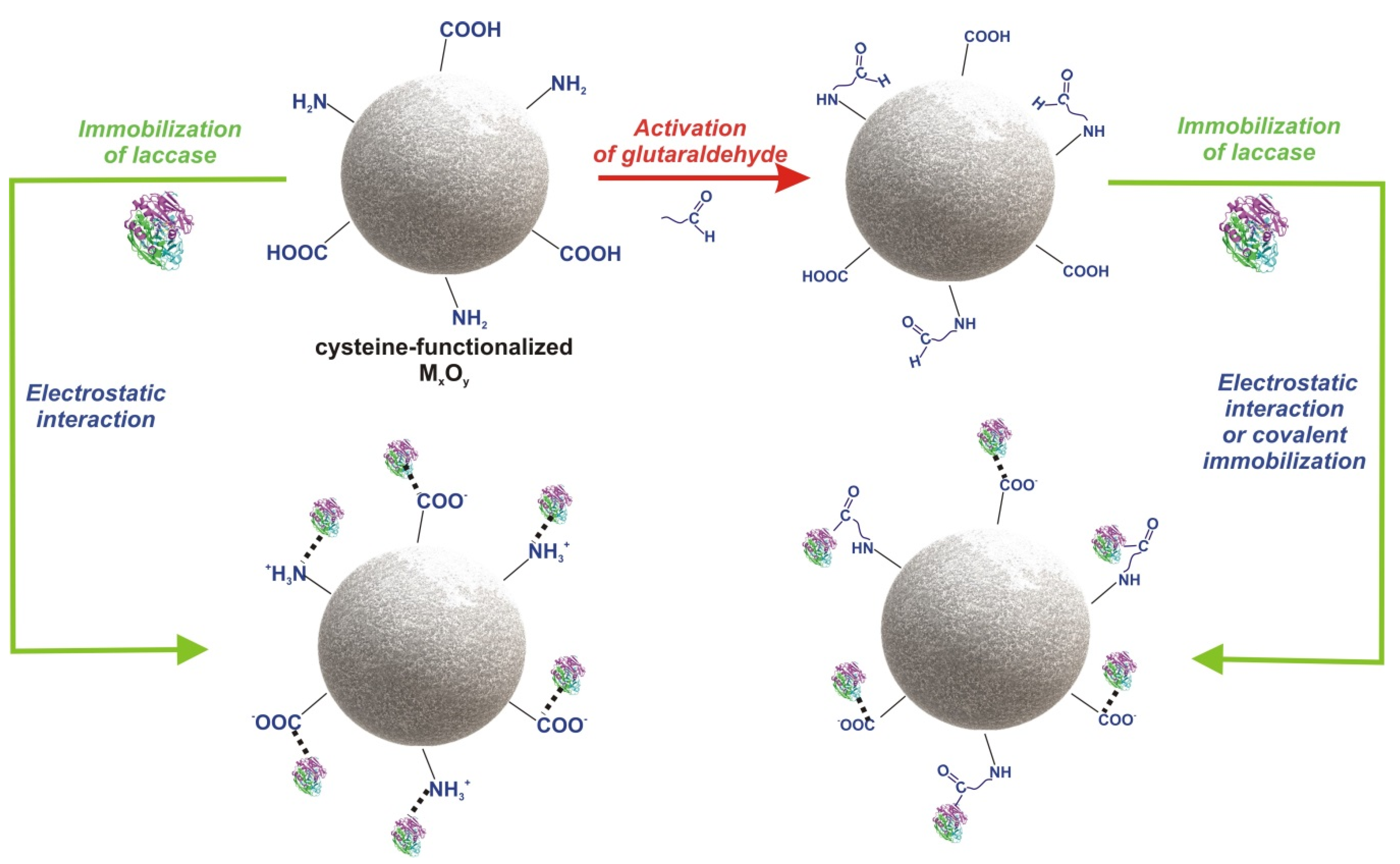
2.2. Synthesis of l-Cysteine-Functionalized MxOy
2.3. Immobilization of Laccase from Trametes Versicolor and Bradford Analysis
2.4. Physicochemical Characterization
2.5. Catalytic Properties
2.6. Decolorization of Alizarin Red S
3. Results
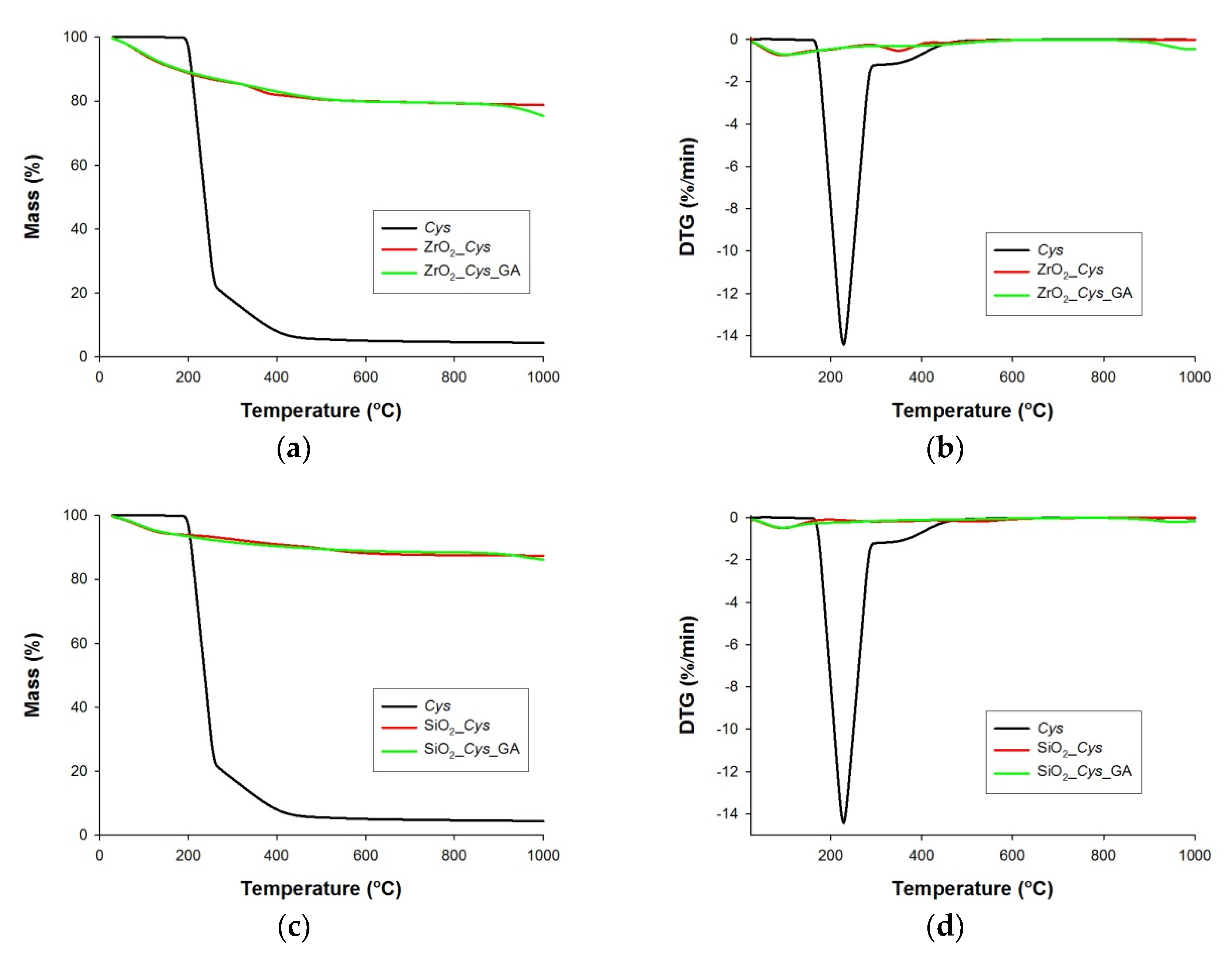
3.1. Thermogravimetric and Elemental Analysis of Pure Supports (ZrO2_Cys, ZrO2_Cys_GA, SiO2_Cys and SiO2_Cys_GA)
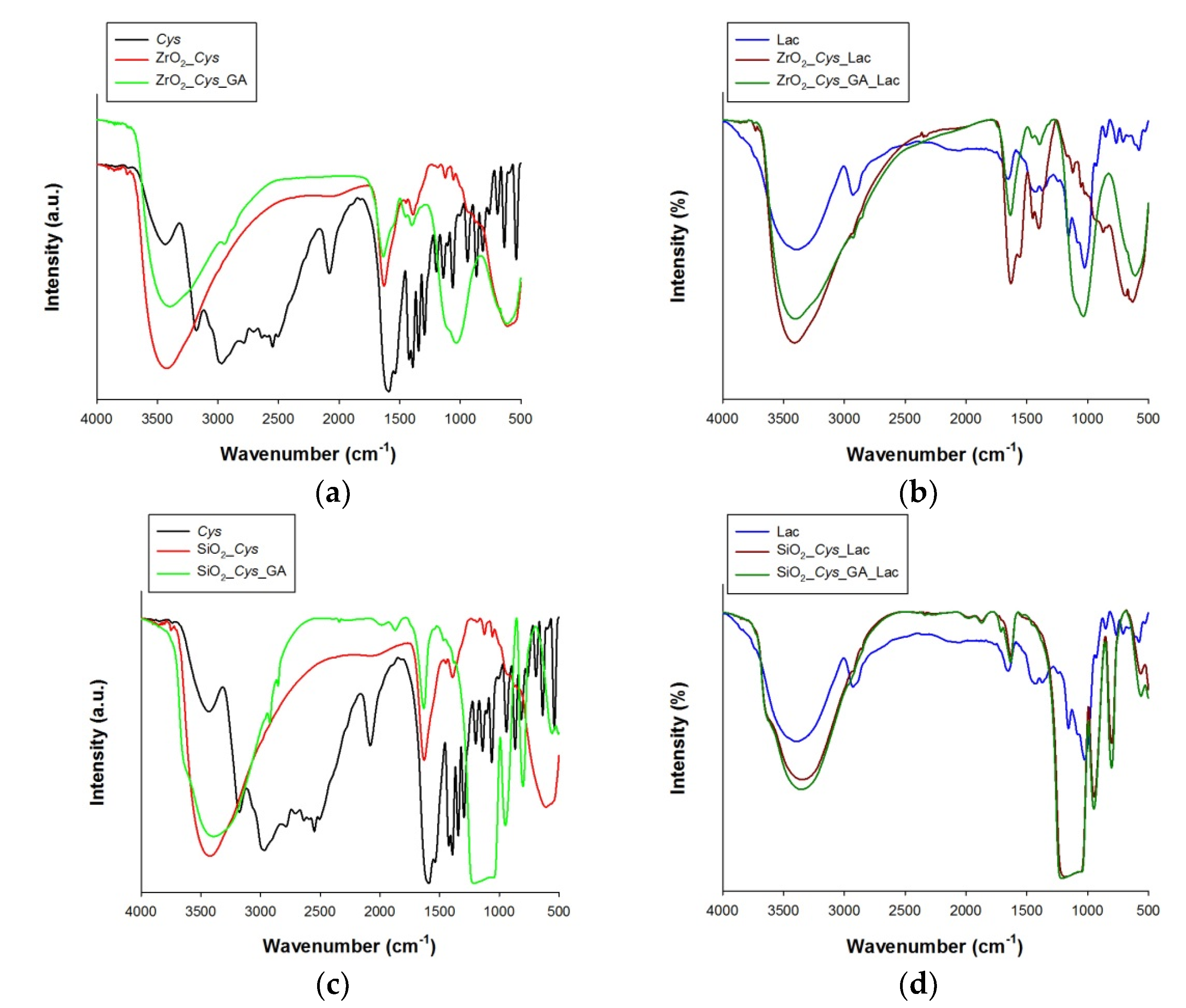
3.2. Spectroscopic, Porous and Zeta Potential Analysis of Samples before and after Immobilization
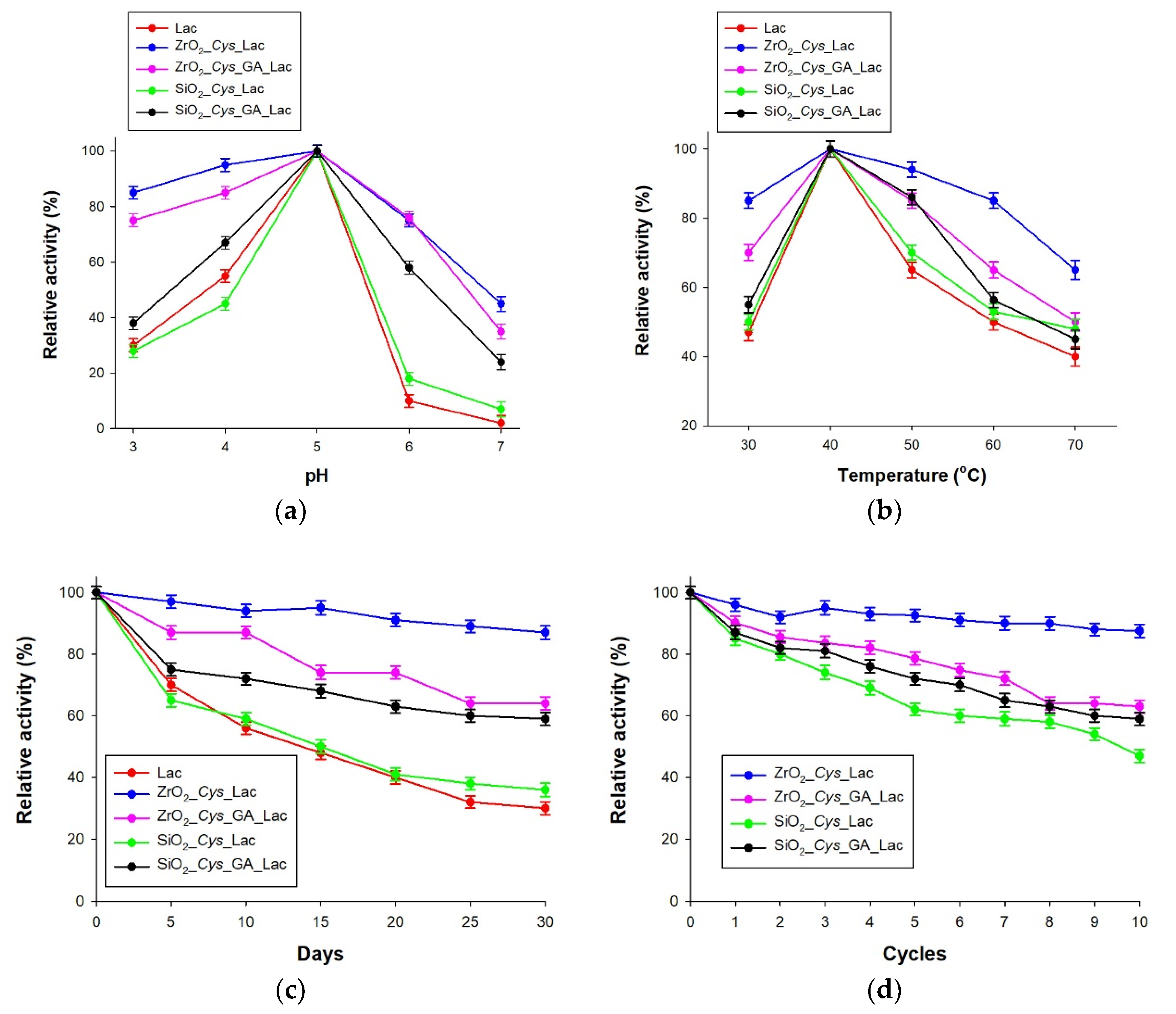
3.3. Catalytic Properties of the Obtained Biocatalytic Systems
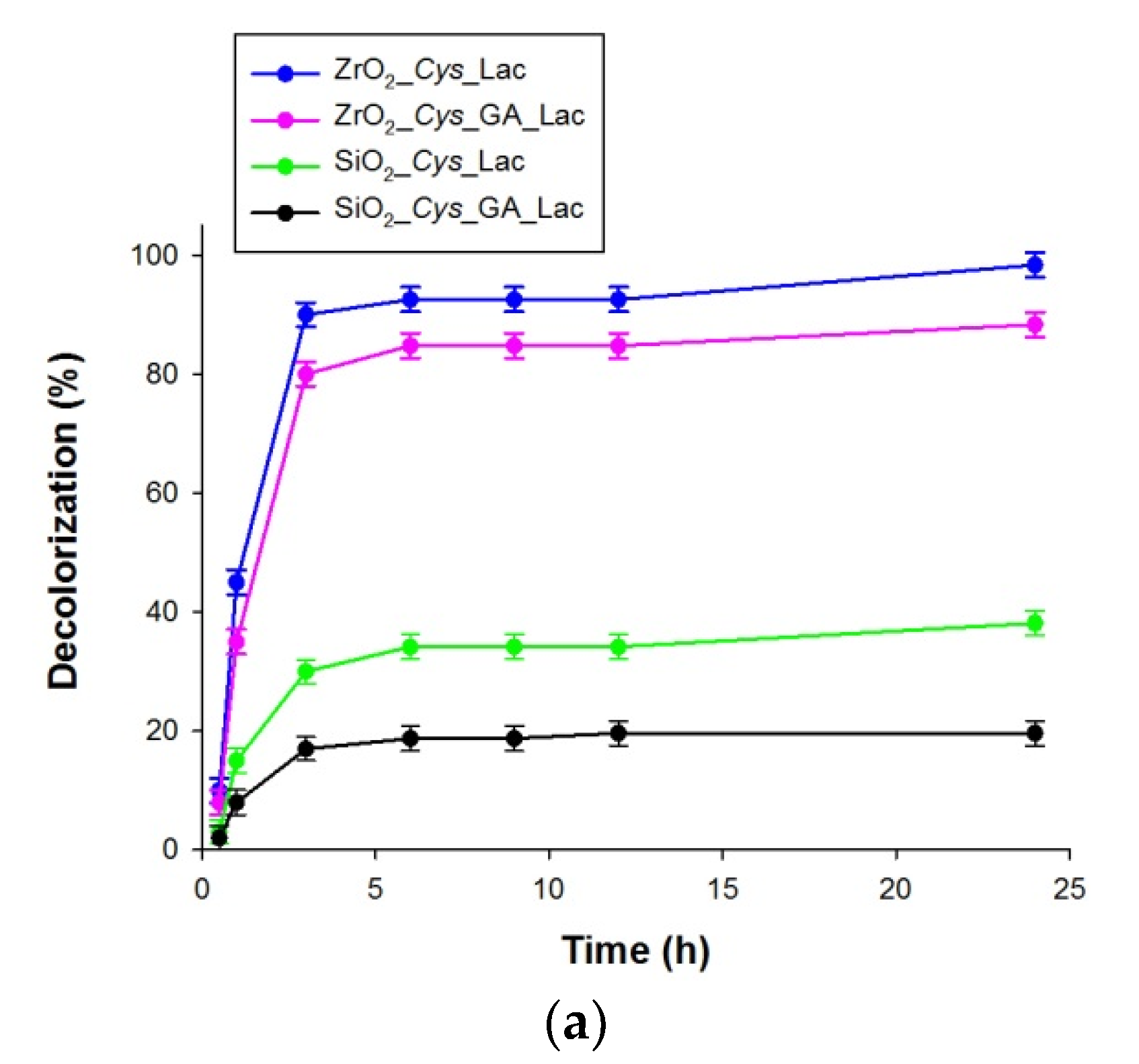
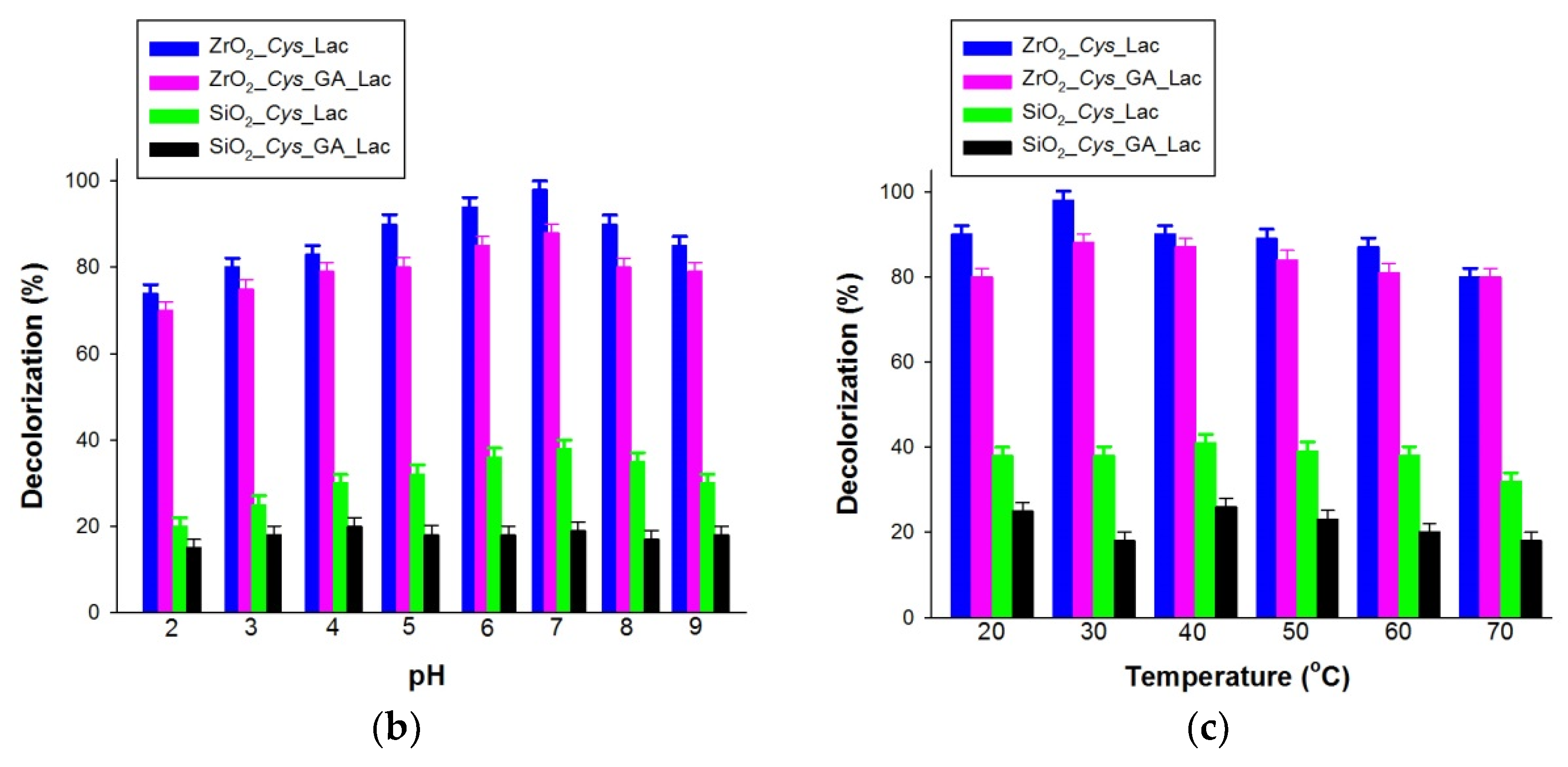
3.4. Decolorization of Alizarin Red S
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Aryal, S.; Remant, B.; Dharmaraj, N.; Bhattarai, N.; Kim, C.H.; Kim, H.Y. Spectroscopic identification of SAu interaction in cysteine capped gold nanoparticles. Spectrochim. Acta A 2006, 63, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.X.; Yang, H.; Zhang, Y.; Yan, X.P. Preparation, characterization and evaluation of water-soluble l-cysteine-capped-CdS nanoparticles as fluorescence probe for detection of Hg (II) in aqueous solution. Anal. Chim. Acta 2006, 559, 234–239. [Google Scholar] [CrossRef]
- Pita, M.; Shleev, S.; Ruzgas, T.; Fernández, V.M.; Yaropolov, A.I.; Gorton, L. Direct heterogeneous electron transfer reactions of fungal laccases at bare and thiol-modified gold electrodes. Electrochem. Commun. 2006, 8, 747–753. [Google Scholar] [CrossRef]
- Almeida, J.S.; Meira, L.A.; Teixeira, L.S.G. Indirect determination of cysteine in pharmaceutical formulations by high-resolution continuum source graphite furnace molecular absorption spectrometry. Microchem. J. 2018, 143, 155–159. [Google Scholar] [CrossRef]
- Nishiuchi, H.; Kohmura, M.; Wakabayashi, H. A rapid and precise method to determine cysteine content in food materials using 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole. Food Sci. Technol. Res. 2011, 17, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Foresti, M.L.; Valle, G.; Bonetto, R.; Ferreira, M.L.; Briand, L.E. FTIR, SEM and fractal dimension characterization of lipase B from Candida antarctica immobilized onto titania at selected conditions. Appl. Surf. Sci. 2010, 256, 1624–1635. [Google Scholar] [CrossRef]
- Valles, D.; Furtado, S.; Villadoniga, C.; Cantera, A.M.B. Adsorption onto alumina and stabilization of cysteine proteinases from crude extract of solanum granuloso-leprosum fruits. Process Biochem. 2011, 46, 592–598. [Google Scholar] [CrossRef]
- Yang, Z.; Si, S.; Zhang, C. Study on the activity and stability of urease immobilized onto nanoporous alumina membranes. Microporous Mesoporous Mater. 2008, 111, 359–366. [Google Scholar] [CrossRef]
- Ataka, K.; Heberle, J. Functional vibrational spectroscopy of a cytochrome c monolayer: SEIDAS probes the interaction with different surface-modified electrodes. J. Am. Chem. Soc. 2004, 126, 9445–9457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Justin, R.; Gooding, J.; Brynn, H.D. Characterization of gold electrodes modified with self-assembled monolayers of l-cysteine for the adsorptive stripping analysis of copper. J. Electroanal. Chem. 2001, 516, 10–16. [Google Scholar] [CrossRef]
- Patil, B.; Kobayashi, Y.; Fujikawa, S.; Okajima, T.; Mao, L.; Ohsaka, T. Direct electrochemistry and intramolecular electron transfer of ascorbate oxidase confined on l-cysteine self-assembled gold electrode. Bioelectrochemistry 2014, 95, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Lata, S.; Devi, R.; Yadav, S.; Pundir, C.S. Fabrication of an amperometric tyramine biosensor based on immobilization of tyramine oxidase on AgNPs/l-Cys-modified Au electrode. J. Solid State Electrochem. 2012, 16, 3869–3876. [Google Scholar] [CrossRef]
- Upadhyay, L.S.B.; Verma, N. Synthesis and characterization of cysteine functionalized silver nanoparticles for biomolecule immobilization. Bioprocess Biosyst. Eng. 2014, 37, 2139–2148. [Google Scholar] [CrossRef]
- Kumar, N.; Upadhyay, L.S.B. Enzyme immobilization over polystyrene surface using cysteine functionalized copper nanoparticle as a linker molecule. Appl. Biochem. Biotechnol. 2020, 191, 1247–1257. [Google Scholar] [CrossRef]
- Sharifia, E.; Shamsa, E.; Salimib, A.; Noorbakhshd, A.; Amini, M.K. Nickel-cysteine nanoparticles: Synthesis, characterization and application for direct electron transfer studies. Colloids Surf. B Biointerfaces 2018, 165, 135–143. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, N.; Upadhyay, L.S.; Sahu, R.; Dutt, A. Fabrication and characterization of cysteine- functionalized zinc oxide nanoparticles for enzyme immobilization. Anal. Lett. 2017, 50, 1839–1850. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, C.; Liu, Y.; Dong, X.; Sun, Y. Cysteine-modified poly(glycidyl methacrylate) grafted onto silica nanoparticles: New supports for significantly enhanced performance of immobilized lipase. Biochem. Eng. J. 2019, 145, 137–144. [Google Scholar] [CrossRef]
- Muthukumar, H.; Malla, S.; Matheswaran, M.; Gummadi, S.N. Immobilization of xylose reductase enzyme on cysteine-functionalized Murraya koenigii mediated magnetite nanoparticles. Mater. Lett. 2020, 261, 127125. [Google Scholar] [CrossRef]
- Bezbradica, D.I.; Mateo, C.; Guisan, J.M. Novel support for enzyme immobilization prepared by chemical activation with cysteine and glutaraldehyde. J. Mol. Catal. B Enzym. 2014, 102, 218–224. [Google Scholar] [CrossRef]
- Monsan, P. Optimization of glutaraldehyde activation of a support for enzyme immobilization. J. Mol. Catal. 1975, 3, 371–384. [Google Scholar] [CrossRef]
- Siara, E.H.; Arana-Pena, S.; Barbosa, O.; Zidoune, M.N.; Fernandez-Lafuente, R. Solid phase chemical modification of agarose glyoxyl-ficin: Improving activity and stability properties by amination and modification with glutaraldehyde. Process Biochem. 2018, 73, 109–116. [Google Scholar] [CrossRef]
- Braham, S.A.; Hussain, F.; Morellon-Sterling, R.; Kamal, S.; Kornecki, J.F.; Barbosa, O.; Kati, D.E.; Fernandez-Lafuente, R. Cooperativity of covalent attachment and ion exchange on alcalase immobilization using glutaraldehyde chemistry: Enzyme stabilization and improved proteolytic activity. Biotechnol. Prog. 2019, 35, e2768. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for one-step immobilization-purification of enyme as industrial biocatalysts. Biotehnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, M.; Batalla, P.; Grazu, V.; Pessela, B.C.C.; Mateo, C.; Montes, T.; Hermoso, J.A.; Guisan, J.M.; Fernandez-Lafuente, R. Mixed ion exchange supports as useful ion exchangers for protein purification: Purification of Penicillin G Acylase from Escherichia coli. Biomacromolecules 2007, 8, 703–707. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Guisan, J.M.; Fernandez-Lafuente, R. Enzyme Stabilization by Glutaraldehyde Crosslinking of Adsorbed Proteins on Aminated Supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatamib, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes—Environmental Impact and Remediation. In Environmental Protection Strategies for Sustainable Development; Springer: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar]
- Salata, R.; Siwinska-Stefanska, K.; Sokołowska, J. Comparative degradation of C. I. Acid Green 25 and C. I. Basic Blue 9 by electrochemical, photoelectrochemical and photocatalytic oxidation methods. Int. J. Electrochem. Sci. 2019, 14, 792–814. [Google Scholar] [CrossRef]
- Ciesielczyk, F.; Bartczak, P.; Zdarta, J.; Jesionowski, T. Active MgO-SiO2 hybrid material for organic dye removal: A mechanism and interaction study of the adsorption of C.I. Acid Blue 29 and C.I. Basic Blue 9. J. Environ. Manag. 2017, 204, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yuan, D.; Liu, Y.; Lining, Z.; Guo, H.; Niu, Q.; Zong, W.; Liu, R. The toxic effects of alizarin red S on catalase at the molecular level. RSC Adv. 2019, 9, 33368–33377. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.S.; Zhang, D.P.; Long, H.Y.; Zhao, G.C. Voltammetric Behavior of the Alizarin Red S Interaction with DNA and Damage to DNA. Electroanalysis 2007, 19, 2577–2582. [Google Scholar] [CrossRef]
- Siwinska-Stefanska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO binary oxide systems: Comprehensive characterization and tests of photocatalytic activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antecka, K.; Zdarta, J.; Siwinska-Stefanska, K.; Sztuk, G.; Jankowska, E.; Oleskowicz-Popiel, P.; Jesionowski, T. Synergistic degradation of dye wastewaters using binary or ternary oxide systems with immobilized laccase. Catalysts 2018, 8, 402. [Google Scholar] [CrossRef] [Green Version]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J. Metal-organic framework as a platform of the enzyme to prepare novel environmentally friendly nanobiocatalyst for degrading pollutant in water. J. Ind. Eng. Chem. 2019, 80, 606–613. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Cao, Z.; Hong, F.F. Enhanced decolourization efficiency of textile dye Reactive Blue 19 in a horizontal rotating reactor using strips of BNC-immobilized laccase: Optimization of conditions and comparison of decolourization efficiency. Biochem. Eng. J. 2020, 156, 107501. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Kijeńska-Gawronska, E.; Biadasz, A.; Jesionowski, T. Electrospun poly(methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation. Environment. Res. 2020, 184, 109332. [Google Scholar] [CrossRef]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Covalently immobilized laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater. J. Mol. Liq. 2019, 276, 153–162. [Google Scholar] [CrossRef]
- Shaheen, R.; Asgher, M.; Hussain, F.; Bhatti, H.N. Immobilized lignin peroxidase from Ganoderma lucidum IBL-05 with improved dye decolorization and cytotoxicity reduction properties. Int. J. Biol. Macromol. 2017, 103, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jin, X.; Jiang, F.; Zhang, R. Immobilization of horseradish peroxidase on ZnO nanowires/macroporous SiO2 composites for the complete decolorization of anthraquinone dyes. Biotechnol. Appl. Biochem. 2018, 65, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, S.; Long, N.; Zhang, R. A robust and stable nano-biocatalyst by co-immobilization of chloroperoxidase and horseradish peroxidase for the decolorization of azo dyes. J. Chem. Technol. Biotechnol. 2018, 93, 489–497. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Liu, D.M.; Dong, C. Recent advances in nano-carrier immobilized enzymes and their applications. Process Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Wisniewska, M.; Ostolska, I.; Szewczuk-Karpisz, K.; Chibowski, S.; Terpiłowski, K.; Gunko, V.M.; Zarko, V.I. Investigation of the polyvinyl alcohol stabilization mechanism and adsorption properties on the surface of ternary mixed nanooxide AST 50 (Al2O3–SiO2–TiO2). J. Nanopart. Res. 2015, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Szewczuk-Karpisz, K.; Wisniewska, M. Lysozyme as a flocculant inducing agent improving the silica removal from aqueous solutions—a turbidimetric study. J. Environ. Manag. 2018, 226, 187–193. [Google Scholar] [CrossRef]
- Qiu, X.; Qin, J.; Xu, M.; Kang, L.; Hu, Y. Organic-inorganic nanocomposites fabricated via functional ionic liquid as the bridging agent for Laccase immobilization and its application in 2,4-dichlorophenol removal. Colloid. Surf. B Biointerfaces 2019, 179, 260–269. [Google Scholar] [CrossRef]
- Zivkovic, L.T.I.; Zivkovic, L.S.; Babicb, B.M.; Kokunesoski, M.J.; Jokic, B.M.; Karadzic, I.M. Immobilization of Candida rugosa lipase by adsorption onto biosafe meso/macroporous silica and zirconia. Biochem. Eng. J. 2015, 93, 73–83. [Google Scholar] [CrossRef]
- Amirkhani, L.; Moghaddas, J.; Jafarizadeh-Malmiri, H. Candida rugosa lipase immobilization on magnetic silica aerogel nanodispersion. RSC Adv. 2016, 6, 12676–12687. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Liu, Z.; Cui, D.; Bian, X. Properties of immobilized laccase on mesostructured cellular foam silica and its use in dye decolorization. J. Macromol. Sci. Part A 2011, 48, 447–453. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Wang, Y. Immobilization of laccase by alginate–chitosan microcapsules and its use in dye decolorization. World J. Microbiol. Biotechnol. 2007, 23, 159–166. [Google Scholar] [CrossRef]

| ZrO2 | SiO2 |
|---|---|
| Isopropanol—250 mL | Ethanol—100 mL |
| ZIP—60 mL | TEOS—17 mL |
| NH3aq—30 mL | NH3aq—11 mL |
| Sample Name | N | C | H | S |
|---|---|---|---|---|
| (%) | ||||
| ZrO2 | 0.05 | 1.09 | 2.60 | 0.15 |
| ZrO2_Cys | 0.49 | 3.32 | 2.63 | 2.18 |
| ZrO2_Cys_GA | 0.49 | 4.46 | 2.61 | 2.14 |
| SiO2 | 0.04 | 0.15 | 1.67 | 0.08 |
| SiO2_Cys | 0.07 | 1.01 | 1.68 | 0.12 |
| SiO2_Cys_GA | 0.09 | 1.57 | 1.40 | 0.11 |
| Sample | Characteristic Groups | Wavenumber (cm−1) |
|---|---|---|
| Cys | N–H | 3215 |
| S–H | 2550 | |
| C–O | 1585 | |
| Lac | N–H | 3450 |
| C–H | 2960 | |
| Amide I, II and III | 1620, 1480 and 1320 | |
| ZrO2_Cys | N–H, O–H | 3180–3650 |
| C–O | 1610 | |
| Zr–O, Zr–O–Zr | >1000 | |
| ZrO2_Cys_GA | C–H | 2845 |
| ZrO2_Cys | Amide I, II and III | 1615, 1492 and 1334 |
| ZrO2_Cys_GA | Amide I and III | 1616 and 1328 |
| SiO2_Cys | N–H, O–H | 3176–3578 |
| C–O | 1622 | |
| Si–O, Si–O–Si | >1000 | |
| SiO2_Cys_GA | C–H | 2875 |
| SiO2_Cys_Lac | Amide I | 1624 |
| SiO2_Cys_GA_Lac |
| Sample | ABET (m2/g) | Vp (mL/g) | Sp (nm) |
|---|---|---|---|
| ZrO2_Cys | 295 | 0.032 | 1.9 |
| ZrO2_Cys_Lac | 292 | 0.034 | 1.9 |
| ZrO2_Cys_GA | 262 | 0.029 | 1.9 |
| ZrO2_Cys_GA_Lac | 146 | 0.026 | 1.9 |
| SiO2_Cys | 20 | 0.018 | 4.2 |
| SiO2_Cys_Lac | 16 | 0.007 | 2.1 |
| SiO2_Cys_GA | 17 | 0.006 | 2 |
| SiO2_Cys_GA_Lac | 13 | 0.006 | 2.1 |
| Sample | P (mg/g) | IY (%) | AS (U/mg) | AR (%) | KM (mM) | Vmax (mM/s) |
|---|---|---|---|---|---|---|
| Free Lac | - | - | 25 ± 1.6 | - | 0.18 ± 0.024 | 0.027 ± 0.012 |
| ZrO2_Cys_Lac | 250 ± 5.6 | 99.9 ± 0.7 | 19.3 ± 1.5 | 77.2 ± 2.8 | 0.11 ± 0.021 | 0.095 ± 0.011 |
| ZrO2_Cys_GA_Lac | 225 ± 5.4 | 97.5 ± 0.7 | 13.5 ± 1.4 | 53.8 ± 2.5 | 0.14 ± 0.022 | 0.031 ± 0.013 |
| SiO2_Cys_Lac | 216 ± 5.3 | 95.6 ± 0.6 | 0.6 ± 0.3 | 2.6 ± 0.8 | 0.17 ± 0.023 | 0.028 ± 0.010 |
| SiO2_Cys_GA_Lac | 212 ± 5.3 | 94.5 ± 0.6 | 7.1 ± 1.1 | 28.5 ± 1.9 | 0.18 ± 0.022 | 0.029 ± 0.011 |
| Support | Enzyme | As (U/mg) | AR (%) | Number of Cycles; Residual Activity (%) | References |
|---|---|---|---|---|---|
| cysteine-Ag nanoparticles | alkaline phosphatase (AP) | 6.31 | 67 | 7; >60 | [15] |
| cysteine-Cu | urease (Ur) | 45.8 | 72.4 | 10; >80 | [16] |
| cysteine-ZnO | urease (Ur) | 3.42 | 72.5 | - | [18] |
| cysteine-poly(glycidyl methacrylate)-SiO2 | lipase (Lip) | 44.1 | 63.3 | 8; >40 | [19] |
| cysteine-ZrO2 | laccase (Lac) | 19.3 | 77.2 | 10; >90 | This study |
| cysteine-ZrO2-glutaraldehyde | laccase (Lac) | 13.5 | 53.8 | 10; >60 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodziejczak-Radzimska, A.; Jesionowski, T. A Novel Cysteine-Functionalized MxOy Material as Support for Laccase Immobilization and a Potential Application in Decolorization of Alizarin Red S. Processes 2020, 8, 885. https://doi.org/10.3390/pr8080885
Kołodziejczak-Radzimska A, Jesionowski T. A Novel Cysteine-Functionalized MxOy Material as Support for Laccase Immobilization and a Potential Application in Decolorization of Alizarin Red S. Processes. 2020; 8(8):885. https://doi.org/10.3390/pr8080885
Chicago/Turabian StyleKołodziejczak-Radzimska, Agnieszka, and Teofil Jesionowski. 2020. "A Novel Cysteine-Functionalized MxOy Material as Support for Laccase Immobilization and a Potential Application in Decolorization of Alizarin Red S" Processes 8, no. 8: 885. https://doi.org/10.3390/pr8080885
APA StyleKołodziejczak-Radzimska, A., & Jesionowski, T. (2020). A Novel Cysteine-Functionalized MxOy Material as Support for Laccase Immobilization and a Potential Application in Decolorization of Alizarin Red S. Processes, 8(8), 885. https://doi.org/10.3390/pr8080885






